Abstract
Aims
Antibiotic prophylaxis involving timely administration of appropriately dosed antibiotic is considered effective to reduce the risk of surgical site infection (SSI) after total hip and total knee arthroplasty (THA/TKA). Cephalosporins provide effective prophylaxis, although evidence regarding the optimal timing and dosage of prophylactic antibiotics is inconclusive. The aim of this study is to examine the association between cephalosporin prophylaxis dose, timing, and duration, and the risk of SSI after THA/TKA.
Methods
A prospective multicentre cohort study was undertaken in consenting adults with osteoarthritis undergoing elective primary TKA/THA at one of 19 high-volume Australian public/private hospitals. Data were collected prior to and for one-year post surgery. Logistic regression was undertaken to explore associations between dose, timing, and duration of cephalosporin prophylaxis and SSI. Data were analyzed for 1,838 participants. There were 264 SSI comprising 63 deep SSI (defined as requiring intravenous antibiotics, readmission, or reoperation) and 161 superficial SSI (defined as requiring oral antibiotics) experienced by 249 (13.6%) participants within 365 days of surgery.
Results
In adjusted modelling, factors associated with a significant reduction in any SSI and deep SSI included: correct weight-adjusted dose (any SSI; adjusted odds ratio (aOR) 0.68 (95% confidence interval (CI) 0.47 to 0.99); p = 0.045); commencing preoperative cephalosporin within 60 minutes (any SSI, aOR 0.56 (95% CI 0.36 to 0.89); p = 0.012; deep SSI, aOR 0.29 (95% CI 0.15 to 0.59); p < 0.001) or 60 minutes or longer prior to skin incision (aOR 0.35 (95% CI 0.17 to 0.70); p = 0.004; deep SSI, AOR 0.27 (95% CI 0.09 to 0.83); p = 0.022), compared to at or after skin incision. Other factors significantly associated with an increased risk of any SSI, but not deep SSI alone, were receiving a non-cephalosporin antibiotic preoperatively (aOR 1.35 (95% CI 1.01 to 1.81); p = 0.044) and changing cephalosporin dose (aOR 1.76 (95% CI 1.22 to 2.57); p = 0.002). There was no difference in risk of any or deep SSI between the duration of prophylaxis less than or in excess of 24 hours.
Conclusion
Ensuring adequate, weight-adjusted dosing and early, preoperative delivery of prophylactic antibiotics may reduce the risk of SSI in THA/TKA, whereas the duration of prophylaxis beyond 24 hours is unnecessary.
Cite this article: Bone Jt Open 2022;3(3):252–260.
Take home message
Adequate weight-adjusted cephalosporin dosing reduces the risk of surgical site infection (SSI) after total hip arthroplasty (THA) and total knee arthroplasty (TKA).
Commencing antibiotic prophylaxis longer than 60 minutes prior to skin incision was associated with a greater reduction in SSI risk than commencing within 60 minutes. Commencing any time preoperatively was associated with a reduced SSI risk, compared with commencing intraoperatively or postoperatively.
The duration of antibiotic prophylaxis does not appear to be associated with the risk of SSI after THA and TKA.
Introduction
The burden and costs associated with surgical site infection (SSI) after total hip arthroplasty (THA) and total knee joint arthroplasty (TKA) continue to rise, with over half of SSIs considered preventable.1 Antibiotic prophylaxis, involving timely administration of appropriately dosed antibiotic, is considered effective to achieve adequate tissue concentration and reduce the risk of SSI after THA/TKA.2,3 Yet variation in antibiotic prophylaxis persists, with recent systematic reviews suggesting that low-quality evidence means there is a lack of clear evidence to inform care.2,4-6
Research to date has demonstrated no superior antibiotic agent for THA and TKA, with many proven to be effective.2 Clinical guidelines recommend first- and second-generation cephalosporins because they are effective, broad-spectrum, cheap, achieve tissue concentration quickly, and enable more powerful antibiotics to be saved for treatment.6,7 At the time the study commenced, clinical guidelines in Australia and the USA recommended cephazolin as the primary prophylactic antibiotic preoperatively and ceased within 24 hours; the recommended doses were 1 g cephazolin or 2 g for people over 80 kg in Australia and 3 g if over 120 kg in the USA.8,9 A single dose of 1 g to 2 g of cephalosporin is usually recommended, although evidence from heterogenous studies has yielded inconsistent results.7,10 Recommendations for commencing prophylaxis vary from any time preoperatively11 to within 60 minutes12 or 120 minutes prior to skin incision.7,13,14 Evidence is inconsistent regarding the need for weight-based dosing for obese patients, and an intraoperative second dose within two half-lives of first administration.7,15 Systematic reviews reported no benefit of multiple doses or prolonged duration, but potential for increased risk of adverse events including antimicrobial resistance.10,14
The persisting controversy regarding the evidence for antibiotic prophylaxis raises questions about current recommendations for antibiotic prophylaxis in THA/TKA and may be contributing to widespread clinical variation and suboptimal outcomes.5,6,14 Using a prospective cohort of participants who underwent elective TKA or THA, this study aims to examine the association between cephalosporin prophylaxis dose, timing, and duration, and the risk of SSI in patients undergoing primary THA/TKA.
Methods
Registration and data collection
This is a secondary data analysis collected from a prospective observational cohort study of people undergoing elective primary THA/TKA for osteoarthritis (OA) to examine the relationship between non-compliance with infection and venous thromboembolism (VTE) prevention guidelines, and patient outcomes. Eligible sites included 19 private and public Australian hospitals with high annual surgical volume (> 275 cases) of THA/TKA surgery. Inclusion criteria for participants in the study were: consenting adults (aged over 18 years) with a primary diagnosis of OA undergoing primary THA/TKA; sufficient English to comprehend the protocol; and available to participate in follow-up. The study protocol was registered prior to commencement16 and ethical approval was obtained from the nine relevant human research ethics committees.
Data were collected prospectively between June 2013 and January 2015 prior to surgery, during the acute admission and via telephone follow-up at approximately 35, 90, and 365 days post-surgery. Participants provided sociodemographic information, past medical history, indications, and contraindications for antimicrobial prophylaxis. Details regarding prophylaxis SSI were obtained from sites and participants at follow-up.
The primary outcome included any SSI, defined as requiring oral or intravenous (IV) antibiotics, readmission, or reoperation occurring within 365 days of surgery. The secondary outcome was deep SSI, defined as requiring IV antibiotics, readmission, or reoperation occurring within 365 days of surgery. Reoperation was defined as any unplanned return to theatre to address the SSI. The accuracy of antibiotic prophylaxis details, acute complications, and all SSIs requiring treatment with readmission or reoperation was verified by medical record audits at all sites and by contacting surgeons, primary care physicians, and other hospitals. Any reported SSIs were coded as a dichotomous variable to indicate whether the participant did or did not experience the complication.
Sample ascertainment
Overall, 77% (2,529/3,285) of all patients screened were eligible for participation (Figure 1). Of these, 2,143 provided consent preoperatively, and data were received for 1,905 (88.9%) consenting participants, as some did not proceed to surgery, or no acute data were received by investigators. After excluding 30 (1.6%) patients without any post-acute follow-up, and 37 who did not receive a cephalosporin, 1,838 patients were included in the analyses. Missing data for each variable was less than 2% for all variables except ASA grade (2.3%).
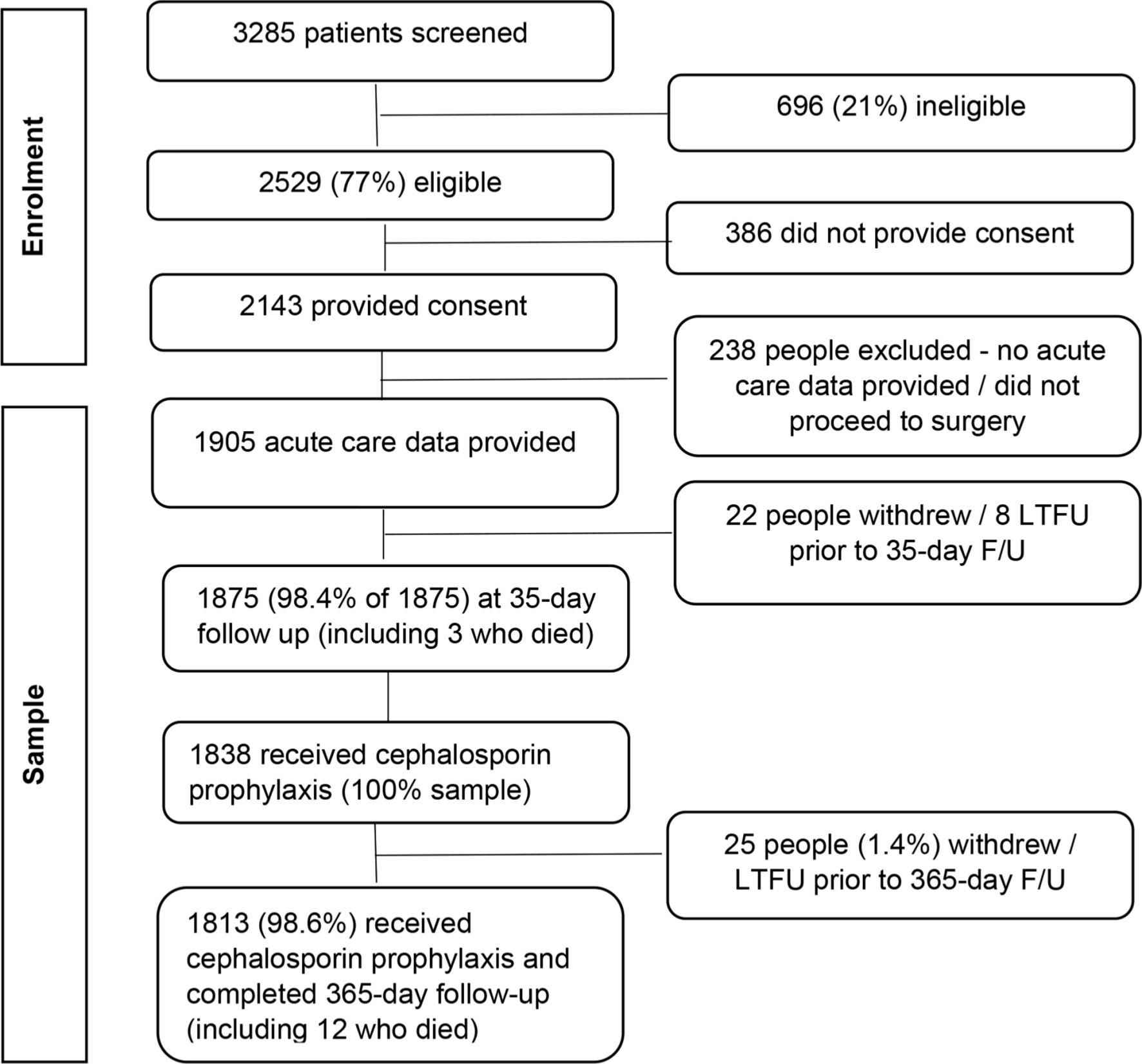
Fig. 1
Participant recruitment, eligibility, and participation results. F/U, follow-up; LTFU, lost to follow-up.
Site, surgeon, and participant characteristics
Site, surgeon, and participant characteristics are provided in Table I. A total of 19 sites from five Australian states included ten public hospitals that completed 45.9% of surgical procedures. The number of procedures ranged from 1 to 125 per surgeon.
Table I.
Site, surgeon, and participant characteristics.
| Site and surgeon characteristics | Results |
|---|---|
| Site, n (%) | |
| Public | 10 (54) |
| Private | 9 (46) |
| Surgeons, n | 118 |
| Median participants, n (IQR) | |
| Per surgeon | 7 (2 to 18) |
| Per site | 82 (51 to 139) |
| Median length of stay, days (IQR) | 5 (1.22 to 1.95) |
| Participant characteristics | |
| Joint (all surgeries), n (%) | |
| Hip | 802 (43.6) |
| Knee | 1,036 (56.4) |
| Bilateral joint arthroplasty, n | 90 |
| Public hospital, n (%) | 844 (45.9) |
| Median duration of surgery, hrs (IQR) (n = 1,837) | 1.62 (1.2 to 2.0) |
| Median age, yrs (IQR) | 67.6 (61 to 74) |
| Female sex, n (%) | 994 (54.1) |
| Insurance status, n (%) | |
| Public | 804 (43.7) |
| Private health insurance | 961 (52.3) |
| Self-funded (private) | 29 (1.6) |
| Other insurance/compensation | 15 (0.8) |
| Department of Veterans Affairs | 28 (1.5) |
| Post-school education status (n = 1,866), n (%) | |
| Up to school completion | 874 (47.8) |
| Post school qualification | 955 (52.2) |
| Median BMI, kg/m2 (IQR) | 29.7 (26.3 to 34.2) |
| Current smoker at the time of recruitment (n = 1,829), n (%) | |
| No | 1,678 (91.7) |
| Yes | 151 (8.3) |
| Comorbid conditions, n (%) | |
| Heart disease | 459 (25.0) |
| History of stroke | 110 (6.0) |
| Bleeding disorder | 19 (1.0) |
| Previous VTE (n = 1,873) | 147 (8.0) |
| Diabetes | 295 (16.1) |
| Hypertension | 1,120 (60.9) |
| High cholesterol | 683 (37.2) |
| Kidney disease | 61 (3.3) |
| Liver disease | 46 (2.5) |
| Current cancer (any type) | 39 (2.1) |
| History of cancer (any type) (n = 1,873) | 214 (11.7) |
| Lung disease | 330 (18.0) |
| Anxiety/depression | 343 (18.7) |
| GORD | 477 (26.0) |
| Sleep apnoea | 128 (7.0) |
| Neurological conditions | 53 (2.9) |
| Musculoskeletal conditions (n = 1,873) | 887 (48.3) |
| Any other comorbid conditions | 710 (38.6) |
| Previous arthroplasty, n (%) | |
| Hip | 239 (13.0) |
| Knee | 300 (16.3) |
| Medications taken for osteoarthritis, n (%) * | |
| Paracetamol | 1,067 (58.1) |
| NSAIDs | 511 (27.8) |
| Opioids | 378 (20.6) |
| Antidepressant/antiepileptics | 26 (2.0) |
| Steroids | 5 (0.3) |
| Any indications or contraindication for antibiotics, n (%) | 225 (12.4) |
| History of antibiotic resistant infection/swab MRSA | 82 (4.5) |
| Gram-negative infection | 1 (0.05) |
| Self-reported allergy to penicillin, cephalosporin, or all beta-lactam Abs | 194 (10.6) |
| Hospital admission with LOS > 5 days within 3 months of THA or TKA | 14 (0.8) |
| ASA grade (n = 1,833), n (%) | |
| 1 or 2 | 1,231 (68.5) |
| 3 or 4 | 656 (31.5) |
| Acute processes of care, n (%) | |
| Routine doppler performed (n = 1,847) | 147 (8.0) |
| Cement fixation used (n = 1,837) | 1,178 (64.1) |
| Antibiotic cement | 1,113 (60.8) |
| Tranexamic acid (n = 1,831) | 1,107 (60.5) |
| Neuraxial anaesthesia (n = 1,837) | 1,160 (63.1) |
| Intra-articular drain (n = 18) | 809 (44.2) |
| Tourniquet (only used for TKA) | 887 (48.3) |
| Blood transfusion (n = 1,831) | 327 (17.9) |
| Indwelling catheter | 1,434 (78.1) |
| Skin incision, n (%) | |
| Hip | |
| Posterior/posterolateral | 494 (26.9) |
| Anterolateral/anterior | 175 (9.5) |
| Other | 129 (16.2) |
| Knee | |
| Medial parapatellar | 939 (92.7) |
| Midvastus | 61 (6.0) |
| Minimally invasive | 6 (0.6) |
| Lateral | 7 (0.7) |
-
*
Medications not exclusive, people may have been taking multiple medications for pain.
-
ASA, American Society of Anesthesiologists; GORD, gastrointestinal reflux disorder; IQR, interquartile range; LOS, length of stay; MRSA, Methicillin-resistant Staphylococcus aureus; NSAIDs, non-steroidal anti-inflammatory drugs; THA, total hip arthroplasty; TKA, total knee arthroplasty; VTE, venous thromboembolism.
Statistical analysis
This is a secondary data analysis, and the sample size was based on the expected event rates and effect size of the primary study.16 All data were entered into a REDCap database,17,18 and data analyses were undertaken using R environment for statistical computing (R Foundation for Statistical Computing, Austria). Descriptive statistics were calculated to profile site-level and participant-level characteristics. Continuous variables are presented as median and interquartile range.
Some variables (bilateral joint, smoking status, American Society of Anesthesiologists (ASA)19 grade, education, and neuraxial anaesthesia) were collapsed to allow for adequately sized and clinically meaningful groups to be included in analyses. The study population was restricted to cases where cephalosporin prophylaxis was used. Appropriate doses for weight included cephazolin (1 g if < 120 kg, 2 g if > 120 kg), cefotaxime (1 g), and ceftriaxone (2 g); as cephalothin is not recommended, no dosage was considered recommended.8 Due to low numbers of non-cephalosporin antibiotic use, the specific features of each non-cephalosporin prophylaxis were categorized into preoperative use, number of non-cephalosporin agents received, and duration longer than 24 hours.
The duration for each cephalosporin agent from time of skin incision was calculated based on the actual duration minus number of preoperative minutes for cephalosporins given preoperatively, or the number of minutes calculated from the start time of the first antibiotic (for antibiotics commenced after skin incision) and surgical start time added to the actual antibiotic duration. Continuous data for time and duration were not normally distributed, so duration was categorized as less than or greater than 24 hours from the time of skin incision, and preoperative timing was categorized into within or longer than 60 minutes prior to skin incision, intraoperative or postoperative. The Fishers Exact test was used to analyze the association between categorical elements of cephalosporin prophylaxis and SSI.
We conducted univariate and multiple logistic regression analyses to explore associations between the development of any postoperative SSI and features of cephalosporin prophylaxis including preoperative timing, first dose strength, and duration. Patient and care factors known to increase the risk of SSI were considered as potential confounders.20-22 Factors identified on univariate analysis with a p-value < 0.25 were entered into a backwards, stepwise multivariate logistic regression model using the Akaike information criterion (AIC) to identify independent risk factors associated with a higher risk of SSI. Factors included in the initial model included eligible antibiotic factors, and surgical, care, and patient factors (Supplementary Table i). A second logistic regression model was also run with deep infection as the outcome.
Missing data were assessed and imputed using multivariate imputation by chained equations (MICE). Model selection was performed using one of the imputed datasets. The final model was run with each of the five imputed datasets. Effect estimates were taken from the pooled estimates using the five imputed datasets. We tested the model with two-way interaction terms with primary predictors. Sensitivity analyses were performed using complete case analysis and using Bayesian information criterion (BIC) instead of AIC. Analysis of variance was used to compare model results and differences in effect estimates, confidence intervals (CIs), and p-values calculated; p-values of < 0.05 were considered statistically significant. Interaction terms for the main predictors retained in the final model (weight adjusted dose, dose changed, and preoperative timing) against each other variable were tested. The data and full R code for all analyses are available online.23,24
Results
All infection outcomes up to one year
There were 264 SSI events experienced by 249 (13.6%) participants. The majority of SSIs occurred within 35 days of surgery (203 (11.0%)), although just over half (10/18) deep SSIs requiring reoperation occurred between 36 and 365 days (Table II and Supplementary Table ii).
Table II.
Surgical site infection outcomes and oozy wounds by follow-up timeframe.
| Type of infection | Participants (n = 1,838), n (%) |
SSIs for each follow-up timeframe, n (%)* | ||||
|---|---|---|---|---|---|---|
| Day 0 - Acute D/C | Acute D/C - 35 days | 36 to 90 days | 91 to 365 days | Total | ||
| Superficial | 186 (10.12) | 70 (3.81) | 87 (4.73) | 40 (2.18) | 2 (0.11) | 199 (10.83) |
| Deep SSI: required IV AB or readmission | 45 (2.45) | 21 (1.14) | 16 (0.87) | 8 (0.44) | 1 (0.05) | 46 (2.50) |
| Deep SSI: required reoperation | 18 (0.98) | 3 (0.16) | 6 (0.33) | 5 (0.27) | 5 (0.27) | 19 (1.03) |
| Total SSIs | 249 (13.5) | 94 (5.11) | 109 (5.93) | 53 (2.88) | 8 (0.44) | 264 (14.36) |
| All oozy wounds (including with and without antibiotic treatment) | 165 (9.0) | 151 (8.2) | 15 (0.8) | 0 | 0 | 0 |
| Experienced oozy wounds and any SSI | 68† (3.7) | 62 (2.1) | 7 (0.2) | 0 | 0 | 0 |
-
*
249 participants experienced 264 surgical site infections.
-
†
Percentage based on calculation for all participants (n = 1,838).
-
AB, antibiotics; D/C, discharge; IV, intravenous; SSI, surgical site infection.
Cephalosporin prophylaxis
In unadjusted univariate analyses, the cephalosporin prophylaxis factors associated with an increased risk of any SSI included appropriate first dose for weight, (6.6% vs 6.9%; p = 0.013, Fisher’s exact test), dose change after the initial dose (7.1% vs 6.4%; p = 0.040, Fisher’s exact test), the number of additional non-cephalosporin prophylactic antibiotic agents received (at any time including preoperatively, intraoperatively, or postoperatively) (no additional antibiotic, vs one or two additional antibiotics, 7.8% vs 0.7%; p < 0.001, Fisher’s exact test) and receiving a preoperative non-cephalosporin antibiotic (6.8% vs 6.7%; p = 0.002, Fisher’s exact test) (Table III and Supplementary Table ii). There were no differences in outcomes based on the duration of cephalosporin prophylaxis, noting over half of the sample received antibiotic prophylaxis beyond 24 hours from the time of skin incision (56.5%, 1,039/1,838).
Table III.
Unadjusted association between joint and features of antibiotic prophylaxis and risk of any and deep surgical site infection at one year compared to no surgical site infection.
| Cephalosporin prophylaxis | Total (n = 1,838) |
No SSI, n (%) (n = 1,589) | SSI, n (%) (n = 249) |
p-value (Fisher’s Exact test) | Deep SSI, n (%) (n = 63) |
p-value (Fisher’s Exact test) |
|---|---|---|---|---|---|---|
| Appropriate first dose for weight, n (%) 25 | 0.013 | 0.242 | ||||
| Yes | 1,073 (58.4) | 946 (51.5) | 122 (6.6) | 32 (1.7) | ||
| No | 765 (41.6) | 643 (35.0) | 127 (6.9) | 31 (1.7) | ||
| Dose changed after first dose, n (%) | 0.040 | 0.247 | ||||
| Yes | 985 (53.6) | 722 (39.3) | 131 (7.1) | 34 (1.8) | ||
| No | 853 (46.4) | 867 (47.4) | 118 (6.4) | 29 (1.6) | ||
| Preoperative timing, n (%) | 0.147 | 0.050 | ||||
| Not received preoperatively | 201 (10.9) | 168 (9.1) | 33 (1.8) | 13 (0.7) | ||
| Received first dose within 60 mins prior to skin incision | 1,486 (80.8) | 1,284 (70.0) | 202 (11.1) | 45 (2.4) | ||
| Received first dose 60 mins or longer prior to skin incision | 151 (8.2) | 137 (7.5) | 14 (0.8) | 5 (0.3) | ||
| Duration from time of skin incision, n (%) | 0.131 | 0.301 | ||||
| < 24 hrs | 797 (43.4) | 700 (38.1) | 97 (5.3) | 23 (1.3) | ||
| ≥ 24 hours | 1,039 (56.5) | 887 (48.3) | 152 (8.3) | 40 (2.2) | ||
| Cephalosporin prophylaxis ceased prior to end of surgery, n (%) | 0.143 | N/A* | ||||
| Yes | 51 (2.8) | 48 (2.6) | 3 (0.2) | 0 | ||
| No | 1,784 (97.2) | 1,538 (83.8) | 246 (13.4) | 63 (3.4) | ||
| Joint and other antibiotic prophylaxis, n (%) | < 0.001 | < 0.001 | ||||
| Hip | 802 (43.6) | 739 (40.2) | 63 (3.4) | 12 (0.7) | ||
| Knee | 1,036 (56.4) | 850 (46.2) | 186 (10.1) | 51 (2.8) | ||
| Non-cephalosporin prophylactic antibiotic agents received, n (%) | < 0.001 | < 0.001 | ||||
| None | 900 (49.0) | 808 (40.4) | 92 (0.5) | 24 (1.3) | ||
| One | 835 (45.5) | 691 (37.6) | 144 (7.8) | 37 (2.0) | ||
| Two | 103 (5.6) | 90 (4.9) | 13 (0.7) | 2 (0.1) | ||
| Received a non-cephalosporin antibiotic preoperatively, n (%) | 0.002 | 0.795 | ||||
| Yes | 756 (41.1) | 631 (34.3) | 125 (6.8) | 27 (1.5) | ||
| No | 1,082 (58.9) | 958 (52.1) | 124 (6.7) | 36 (2.0) | ||
| Received a non-cephalosporin preoperatively, n (%) | 0.723 | 0.733 | ||||
| < 24 hrs | 71 (3.9) | 1,526 (83.0) | 241 (13.1) | 3 (0.2) | ||
| ≥ 24 hrs | 1,767 (96.1%) | 63 (3.4%) | 8 (0.4%) | 60 (3.3%) | ||
| Received oral prophylactic antibiotics, n (%) | 0.116 | 0.805 | ||||
| Yes | 135 (7.3) | 123 (6.7) | 12 (0.7) | 5 (0.3) | ||
| No | 1,703 (92.7) | 1,466 (79.8) | 237 (12.9) | 58 (3.2) |
-
*
No test of association completed due to cell count < 5.
-
N/A, not applicable; SSI, surgical site infection.
Association between SSI and features of cephalosporin prophylaxis
In adjusted modelling exploring the association between antibiotic prophylaxis and risk of SSI, factors associated with a statistically significant reduction in risk of any SSI included receiving the correct dose for weight (aOR 0.68 (95% CI 0.47 to 0.99); p = 0.045, chi-squared test),25 commencing preoperative cephalosporin within 60 minutes (aOR 0.56 (95% CI 0.36 to 0.89); p = 0.003, chi-squared test), and commencing 60 minutes or longer prior to skin incision (aOR 0.35 (95% CI 0.17 to 0.70); p = 0.004, chi-squared test) compared to intraoperative or postoperative commencement (Table IV). Receiving a non-cephalosporin antibiotic preoperatively (aOR 1.35 (95% CI 1.01 to 1.81); p = 0.044, chi-squared test) and changing cephazolin dose after the initial dose (aOR 1.76 (95% CI 1.22 to 2.57); p = 0.002, chi-squared test) were associated with a higher risk of SSI. The dose was reduced for all but ten of the 853 participants who had a change in dose, and the initial dose was adequate for weight for 88% of people whose dose was subsequently changed (n = 748). The duration of cephalosporin was not retained in the final model as a significant factor associated with the risk of SSI.
Table IV.
Association between elements of cephalosporin prophylaxis and any surgical site infection outcome in adjusted modelling.
| Variables in the final model | Adjusted OR (95% CI) | p-value* |
|---|---|---|
| Preoperatively taking antidepressant/anticonvulsant (e.g. tricyclics, pregabalin) medication for arthritis pain | 2.42 (1.06 to 5.18) | 0.031 |
| Total knee arthroplasty | 2.24 (1.64 to 3.09) | < 0.001 |
| Comorbid neurological conditions | 2.19 (1.08 to 4.19) | 0.025 |
| Cephalosporin dose changed after initial dose | 1.76 (1.22 to 2.57) | 0.002 |
| Longer surgical duration | 1.48 (1.14 to 1.92) | 0.003 |
| Received a non-cephalosporin antibiotic preoperatively | 1.35 (1.01 to 1.81) | 0.044 |
| Higher BMI | 1.05 (1.03 to 1.07) | < 0.001 |
| Received correct first dose of a cephalosporin for weight | 0.68 (0.47 to 0.99) | 0.045 |
| Preoperative cephalosporin commenced within 60 mins of skin incision | 0.56 (0.36 to 0.89) | 0.003 |
| Bilateral THA/TKA | 0.37 (0.15 to 0.80) | 0.018 |
| Preoperative cephalosporin commenced 60 mins or longer prior to skin incision | 0.35 (0.17 to 0.70) | 0.004 |
| Received rivaroxaban for VTE prophylaxis | 0.35 (0.14 to 0.72) | 0.009 |
| History of stroke | 1.59 (0.93 to 2.62) | 0.081 |
-
*
Chi-squared test calculated during logistic regression modelling.
-
CI, confidence interval; OR, odds ratio; THA, total hip arthroplasty; TKA, total knee arthroplasty; VTE, venous thromboembolism.
A second regression model was completed with deep infection only as the outcome. Factors associated with a reduction in risk of deep SSI included preoperative cephalosporin commenced within 60 minutes of skin incision (OR 0.29 (95% CI 0.15 to 0.59); p < 0.001) and preoperative cephalosporin commenced 60 minutes or longer prior to skin incision (OR 0.27 (95% CI 0.09 to 0.83); p = 0.002) (Table V). Factors associated with an increased risk of deep SSI included total knee arthroplasty (OR 3.04 (95% CI 1.58 to 5.86); p < 0.001), current smoker (OR 2.73 (95% CI 1.36 to 5.49); p = 0.005), history of premorbid stroke (OR 2.41 (95% CI 1.03 to 5.60); p = 0.041), and higher BMI (OR 1.06 (95% CI 1.03 to 1.10); p < 0.001). Change in dose was not associated with the risk of deep SSI (aOR 1.53 (95% CI 0.88 to 2.67); p = 0.133).
Table V.
Association between elements of cephalosporin prophylaxis and deep surgical site infection outcome in adjusted modelling.
| Variables in the final model | Adjusted OR (95% CI) | p-value* |
|---|---|---|
| Preoperative cephalosporin commenced within 60 mins of skin incision | 0.29 (0.15 to 0.59) | < 0.001 |
| Preoperative cephalosporin commenced 60 mins or longer prior to skin incision | 0.27 (0.09 to 0.83) | 0.022 |
| Total knee arthroplasty | 2.25 (1.58 to 5.86) | < 0.001 |
| Current smoker | 2.73 (1.36 to 5.49) | 0.005 |
| History of stroke | 2.41 (1.03 to 5.60) | 0.041 |
| Higher BMI | 1.06 (1.02 to 1.10) | < 0.001 |
| Cephalosporin dose changed after initial dose | 1.53 (0.88 to 2.67) | 0.133 |
| Sleep apnoea | 1.83 (0.87 to 3.84) | 0.112 |
| Preoperatively taking NSAIDs | 0.67 (0.40 to 1.12) | 0.126 |
-
*
Chi-squared test calculated during logistic regression modelling.
-
CI, confidence interval; NSAID, non-steroidal inflammatory drugs; OR, odds ratio.
Discussion
Cephalosporin prophylaxis commenced any time preoperatively was associated with a lower rate of any SSI and deep SSI than starting intraoperatively or postoperatively. Starting 60 minutes or more preoperatively was associated with a greater reduction in risk than starting within 60 minutes of skin incision. Previous research has supported the protective benefits of commencing antibiotic prophylaxis within 120 minutes of skin incision but found no difference in SSI risk between 0 and 60, and 60 to 120 minutes,26 or 0 and 30, and 30 to 60 minutes.25 However, clinical guideline recommendations vary, and this may contribute to variation; some recommend administration of the first dose within 60 minutes,27 and others within 120 minutes, of skin incision.28
Most of the participants received an adequate initial dose, although this was not associated with the risk of any SSI or deep SSI, despite this being considered crucial to achieving an adequate tissue concentration.7 Almost all (99% (975/985) changes in dose involved a dose reduction, and this was associated with a significant increase in the risk of any SSI in adjusted and unadjusted modelling, but was not associated with the risk of deep SSI. The initial dose was adequate for weight for 88% of people whose dose was subsequently changed. In contrast to current recommendations,28 over half of the sample received multiple different antibiotic agents and using additional antibiotic agents preoperatively was also associated with a statistically significant increased risk of any SSI.
TKA was associated with a much higher risk of any SSI and deep SSI than THA, and it may be important to communicate this with prospective patients. Consistent with previous literature, there was no difference in the risk of SSI between duration of prophylaxis less than or greater than 24 hours,10,14,29 however the fact that duration of prophylaxis exceeded 24 hours for two-thirds of participants remains a concern, due to the increased risk of antimicrobial resistance.7 Our study reported an unexpectedly high level of clinical variation, despite inappropriate antimicrobial prophylaxis demonstrated to increase the risk of SSI and antimicrobial resistance.6,14
A strength of the study lies in the prospectively collected data in this large multicentre study, and the accurate recording of antimicrobial prophylaxis regimens and surgical complications through robust audit of medical records and verification processes.30 We collected more detailed data regarding the antibiotic prophylaxis, and surgical and acute care processes, than captured in administrative data. We explored multiple aspects of cephalosporin prophylaxis as clinical guidelines recommend that dose, timing, and duration are all important, and a recent systematic review reported that variation in the timing can influence the impact of different antibiotic durations.14
There are several limitations to this study. The study was observational, and while efforts were made to adjust for the variation in antibiotic prophylaxis and the impact of known patient and surgical risk factors, we may not have captured all confounding factors.2 Decisions to add or change antibiotic agents, dose, or duration may be based on patient-specific indications, although we did not have access to data regarding clinical reasoning. The majority of agent and dose changes happened after the initial dose was given in the operating theatre. Given the large variation in additional antibiotics used, data were insufficient to account for differences in all aspects of the antibiotic regimens. We defined SSI based on medical management, including reoperation, but were unable to access pathology or use more stringent criteria to identify infections.31 Although higher cost and burden are associated with deep SSI,32,33 most people who experienced superficial SSI had signs and symptoms of infection,34 and many of these people required intensive health service intervention, and possibly increased costs and poorer patient experience.35,36 The study may have been underpowered to detect association with deep SSI due to the low event rate. The timeframe for the primary outcome was longer than typically recommended,28 but the experience of complications affects patient satisfaction in the longer term.37
Further high-quality research is needed regarding the most efficacious timing for the first preoperative dose of antibiotics, given this study has highlighted potential inconsistencies between evidence and contemporaneous clinical guideline recommendations.7 A randomized clinical trial is underway to explore dual routine use of prophylactic cephazolin and vancomycin, given increasing concern about antibiotic-resistant SSI.38 Further research is recommended, including economic evaluation due to the high cost and burden of SSI,32 and to explore the reduction in SSI risk we observed with the use of rivaroxaban for VTE prophylaxis.23
This study has provided evidence of the association between cephalosporin dosing and timing, and the risk of SSI. While the event rate of deep SSI is low, higher-quality evidence addressing modifiable risk factors associated with the risk of SSI is needed to maximize the value and outcomes after THA and TKA.3,20
References
1. Fields AC , Pradarelli JC , Itani KMF . Preventing surgical site infections: looking beyond the current guidelines . JAMA . 2020 ; 323 ( 11 ): 1087 – 1088 . Crossref PubMed Google Scholar
2. Siddiqi A , Forte SA , Docter S , Bryant D , Sheth NP , Chen AF . Perioperative antibiotic prophylaxis in total joint arthroplasty . J Bone Joint Surg Am . 2019 ; 101-A ( 9 ): 828 – 842 . Crossref PubMed Google Scholar
3. Rezapoor M , Parvizi J . Prevention of periprosthetic joint infection . J Arthroplasty . 2015 ; 30 ( 6 ): 902 – 907 . Crossref PubMed Google Scholar
4. Voigt J , Mosier M , Darouiche R . Antibiotics and antiseptics for preventing infection in people receiving revision total hip and knee prostheses: a systematic review of randomized controlled trials . BMC Infect Dis . 2016 ; 16 ( 1 ): 749 . Crossref PubMed Google Scholar
5. Gouvêa M , Novaes C de O , Pereira DMT , Iglesias AC . Adherence to guidelines for surgical antibiotic prophylaxis: a review . Braz J Infect Dis . 2015 ; 19 ( 5 ): 517 – 524 . Crossref PubMed Google Scholar
6. Ierano C , Peel T , Ayton D , Rajkhowa A , Marshall C , Thursky K . Surgical antibiotic prophylaxis – The evidence and understanding its impact on consensus guidelines . Infection, Disease & Health . 2018 ; 23 ( 3 ): 179 – 188 . Crossref Google Scholar
7. Parvizi J , Shohat N , Gehrke T . Prevention of periprosthetic joint infection . Bone Joint J . 2017 ; 99-B ( 4_Supple_B ): 3 – 10 . Crossref PubMed Google Scholar
8. Bratzler DW , Dellinger EP , Olsen KM , et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery . Am J Health Syst Pharm . 2013 ; 70 ( 3 ): 195 – 283 . Crossref PubMed Google Scholar
9. No authors listed . Therapeutic Guidelines – Antibiotic . Therapeutic Guidelines . 2010 . https://tgldcdp.tg.org.au/etgcomplete ( date last accessed 14 February 2022 ). Google Scholar
10. Ryan SP , Kildow BJ , Tan TL , et al. Is there a difference in infection risk between single and multiple doses of prophylactic antibiotics? A meta-analysis . Clin Orthop Relat Res . 2019 ; 477 ( 7 ): 1577 – 1590 . Crossref PubMed Google Scholar
11. Berríos-Torres SI , Umscheid CA , Bratzler DW , et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017 . JAMA Surg . 2017 ; 152 ( 8 ): 784 . Crossref PubMed Google Scholar
12. Branch-Elliman W , O’Brien W , Strymish J , Itani K , Wyatt C , Gupta K . Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events . JAMA Surg . 2019 ; 154 ( 7 ): 590 – 598 . Crossref PubMed Google Scholar
13. Allegranzi B , Zayed B , Bischoff P , et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective . Lancet Infect Dis . 2016 ; 16 ( 12 ): e288 – e303 . Crossref PubMed Google Scholar
14. de Jonge SW , Boldingh QJJ , Solomkin JS , et al. Effect of postoperative continuation of antibiotic prophylaxis on the incidence of surgical site infection: a systematic review and meta-analysis . Lancet Infect Dis . 2020 ; 20 ( 10 ): 1182 – 1192 . Crossref PubMed Google Scholar
15. Blum S , Cunha CB , Cunha BA . Lack of pharmacokinetic basis of weight-based dosing and intra-operative re-dosing with cefazolin surgical prophylaxis in obese patients: implications for antibiotic stewardship . Surg Infect (Larchmt) . 2019 ; 20 ( 6 ): 439 – 443 . Crossref PubMed Google Scholar
16. No authors listed . ClinicalTrials.gov Identifier: NCT01899443: U.S. National Library of Medicine . 2013 . https://www.clinicaltrials.gov/ct2/show/NCT01899443?term=NCT01899443&draw=2&rank=1 ( date last accessed 17 January 2022 ). Google Scholar
17. Harris PA , Taylor R , Minor BL , et al. The REDCap consortium: Building an international community of software platform partners . J Biomed Inform . 2019 ; 95 : 103208 . Crossref PubMed Google Scholar
18. No authors listed . Research electronic data capture (REDcap) software . 2017 . https://projectredcap.org/ ( date last accessed 17 January 2022 ). Google Scholar
19. Saklad M . Grading of patients for surgical procedures . Anesthesiol . 1941 ; 2 ( 5 ): 281 – 284 . Crossref Google Scholar
20. Alamanda VK , Springer BD . The prevention of infection: 12 modifiable risk factors . Bone Joint J . 2019 ; 101-B ( 1_Supple_A ): 3 – 9 . Crossref PubMed Google Scholar
21. Korol E , Johnston K , Waser N , et al. A systematic review of risk factors associated with surgical site infections among surgical patients . PLoS ONE . 2013 ; 8 ( 12 ): e83743 . Crossref PubMed Google Scholar
22. Aggarwal VK , Weintraub S , Klock J , et al. 2019 Frank Stinchfield Award: A comparison of prosthetic joint infection rates between direct anterior and non-anterior approach total hip arthroplasty . Bone Joint J . 2019 ; 101-B ( 6_Supple_B ): 2 – 8 . Crossref PubMed Google Scholar
23. Wang Z , Anderson FA , Ward M , Bhattacharyya T . Surgical site infections and other postoperative complications following prophylactic anticoagulation in total joint arthroplasty . PloS ONE . 2014 ; 9 ( 4 ): e91755 . Crossref PubMed Google Scholar
24. Harris I , Badge H , Xuan W , Naylor J . Evidence-based Processes and Outcomes of Care (EPOC): Improving services and outcomes for joint replacement patients. UNSW Sydney . https://repository.unsworks.unsw.edu.au/entities/dataset/2eda46eb-f709-48c1-933f-9b5d00f340bd ( date last accessed 17 January 2022 ). Google Scholar
25. Weber WP , Mujagic E , Zwahlen M , et al. Timing of surgical antimicrobial prophylaxis: a phase 3 randomised controlled trial . Lancet Infect Dis . 2017 ; 17 ( 6 ): 605 – 614 . Crossref PubMed Google Scholar
26. de Jonge SW , Gans SL , Atema JJ , Solomkin JS , Dellinger PE , Boermeester MA . Timing of preoperative antibiotic prophylaxis in 54,552 patients and the risk of surgical site infection . Medicine (Baltimore) . 2017 ; 96 ( 29 ): e6903 . Crossref PubMed Google Scholar
27. No authors listed . Therapeutic Guidelines - Antibiotic . Therapeutic Guidelines Ltd , 2010 . Google Scholar
28. No authors listed . Global Guidelines for the Prevention of Surgical Site Infection . World Health Organisation . 2016 . https://www.who.int/gpsc/global-guidelines-web.pdf?ua=1 ( date last accessed 14 February 2022 ). Google Scholar
29. Tan TL , Shohat N , Rondon AJ , et al. Perioperative antibiotic prophylaxis in total joint arthroplasty: a single dose is as effective as multiple doses . J Bone Joint Surg Am . 2019 ; 101-A ( 5 ): 429 – 437 . Crossref PubMed Google Scholar
30. Dang KLT , Badge H , Harris IA . Validity of patient-reported complications after total hip and knee arthroplasty . J Orthop Surg . 2018 ; 26 ( 3 ). Crossref PubMed Google Scholar
31. Parvizi J , Tan TL , Goswami K , et al. The 2018 definition of periprosthetic hip and knee: an evidence-based and validated criteria . J Arthroplasty . 2018 ; 33 ( 5 ): 1309 – 1314 . Crossref Google Scholar
32. Adeyemi A , Trueman P . Economic burden of surgical site infections within the episode of care following joint replacement . J Orthop Surg Res . 2019 ; 14 ( 1 ): 196 . Crossref PubMed Google Scholar
33. Peel TN , Cheng AC , Liew D , et al. Direct hospital cost determinants following hip and knee arthroplasty . Arthritis Care Res (Hoboken) . 2015 ; 67 ( 6 ): 782 – 790 . Crossref PubMed Google Scholar
34. No authors listed . Surgical Site Infections: Prevention and Treatment, NICE Guideline NG125 . NICE . 2020 . https://www.nice.org.uk/guidance/NG125 ( date last accessed 14 February 2022 ). Google Scholar
35. Smith TO , Sexton D , Mann C , Donell S . Sutures versus staples for skin closure in orthopaedic surgery: meta-analysis . BMJ . 2010 ; 340 : c1199 . Crossref PubMed Google Scholar
36. Carroll K , Dowsey M , Choong P , Peel T . Risk factors for superficial wound complications in hip and knee arthroplasty . Clin Microbiol Infect . 2014 ; 20 ( 2 ): 130 – 135 . Crossref PubMed Google Scholar
37. Khatib Y , Badge H , Xuan W , Naylor JM , Harris IA . Patient satisfaction and perception of success after total knee arthroplasty are more strongly associated with patient factors and complications than surgical or anaesthetic factors . Knee Surg Sports Traumatol Arthrosc . 2020 ; 28 ( 10 ): 3156 – 3163 . Crossref PubMed Google Scholar
38. Peel T , Astbury S , Cheng AC , et al. Multicentre randomised double-blind placebo controlled trial of combination vancomycin and cefazolin surgical antibiotic prophylaxis: the Australian surgical antibiotic prophylaxis (ASAP) trial . BMJ Open . 2019 ; 9 ( 11 ): e033718 . Crossref PubMed Google Scholar
Author contributions
H. Badge: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft.
T. Churches: Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing.
W. Xuan: Formal analysis, Software, Supervision, Validation, Writing – review & editing.
J. M. Naylor: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
I. A. Harris: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writer – review and editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: a grant through the HCF Health and Medical Research Foundation.
ICMJE COI statement
H. Badge is supported by an Australian Government Research Training Program Scholarship.
Acknowledgements
Thanks to Dr Luan Dang, Shirley Cross and Carolyn Gray-Robens for verifying the accuracy of patient reported complications. The authors would like to acknowledge the contribution of the following research assistants who supported data collection and data entry including Carolyn Gray-Robens, Shirley Cross, Marg Easterbrook, Michelle Jones, Nidhi Jain, Catherine Belousoff, Kelly Wheeler and others. In memory of Jo.
Ethical review statement
Ethical approval was obtained from nine ethics committees: Austin Health Human Research Ethics Committee (EC00204) – Approval LNR/14/Austin/208; Barwon Health Human Research Ethics Committee (EC00208) - Approval LR13/82; Calvary Health Care Adelaide Human Research Ethics Committee (EC00302) - Approval 13_CHREC-E007; Calvary Health Care Tasmania Clinical and Research Ethics Committee - Approval 2:05:13 and 5:13:12; Epworth HealthCare Human Research Ethics Committee (EC00217) Approval LR138- 13; Hunter New England Human Research Ethics Committee (EC00403) Approval LNR/HNE/390; St Vincent's Health and Aged Care HREC (EC00324) Approval HREC #13/10; Sydney Adventist Hospital Group Human Research Ethics Committee (EC00141) – Approval 2013-016; The Prince Charles Hospital HREC (EC00168) Approval HREC 13-141. Prior to data collection informed written consent was obtained from eligible participants and the signed consent form was witnessed by the site coordinator.
Open access funding
The open access funding for this study was obtained through the HCF Health and Medical Research Foundation (Grant number IHIIAMR2012073043).
Follow H. Badge @helen_badge
Follow T. Churches @timchurches
Follow I. A. Harris @doctordoubter
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/









