Abstract
Osteoarthritis is extremely common and many different causes for it have been described. One such cause is abnormal morphology of the affected joint, the hip being a good example of this. For those joints with femoroacetabular impingement (FAI) or developmental dysplasia of the hip (DDH), a link with subsequent osteoarthritis seems clear. However, far from being abnormal, these variants may be explained by evolution, certainly so for FAI, and may actually be normal rather than representing deformity or disease. The animal equivalent of FAI is coxa recta, commonly found in species that run and jump. It is rarely found in animals that climb and swim. In contrast are the animals with coxa rotunda, a perfectly spherical femoral head, and more in keeping with the coxa profunda of mankind. This article describes the evolutionary process of the human hip and its link to FAI and DDH. Do we need to worry after all?
Osteoarthritis (OA) is extraordinarily common and dominates the lives of so many patients and surgeons. Of the many causes suggested for OA, abnormal morphology of a joint is a recurring theme in the literature. The hip is a good example of this with both femoroacetabular impingement (FAI) and developmental dysplasia of the hip (DDH) being widely regarded as predisposing to eventual osteoarthritis. Yet do we really need to worry? Probably yes say some, probably no say others. Could it actually be beneficial to have one of these morphological variants? Perhaps the answer can be found many millions of years ago. The development of mankind tells us a great deal.
Unlike subjects such as particle physics, biology has its grand unified theory - evolution. This is the framework that explains all biological morphology, from protein structure and function to the macroscopic form of living organisms.1,2 Journals continue to publish a steady flow of inspiring papers on all aspects of evolution. In a recent study of beetles, van de Kamp et al3 described a previously unknown type of joint. At half a millimetre in size, the hip of the Papuan weevil functions as a nut and bolt3 (Fig. 1). The common ancestor to such beetles and humans can be dated to approximately 590 million years ago, a time when one of the major splits in evolution occurred, between the deuterostomes (much later giving rise to primates including man) and protostomes (giving rise to a vast number of species, including one of the largest groups, the insects).4

Fig. 1
Papuan weevils (a) have a hip joint that functions as a bolt - the trochanteric or leg portion (c) screwing into a nut, the coxa or trunk portion (b). Reconstructions made from micro CT scans (from van de Kamp et al3., with permission from American Association for the Advancement of Science).
Evolution can be a difficult concept to grasp as its vast time span, similar to the huge distances in astronomy, are beyond the realms of regular human experience or understanding. Two fundamental principles of evolution are “blindness” and “the good enough principle”. Blindness refers to the “blind watchmaker”,5 and describes evolution as lacking purpose and direction. Good enough means just good enough to spawn the next generation. Evolution works more like a tinkerer than a perfectionist engineer. Nature is awash with examples where a fresh design from scratch could vastly improve performance.6 Indeed, many so-called perfect designs of Nature are in reality quite the opposite.
For Homo sapiens, the female pelvis is the single skeletal element that conveys information about the two most peculiar traits of human evolution. These are upright gait and an ultra-large brain. It shows both the adaptations that occurred to facilitate a permanent bipedal gait, and at the same time the adjustments required to accommodate the birth of a large-brained foetus.7,8 Using such an evolutionary perspective, two human hip disorders can be considered – FAI and DDH. Both feature frequently in current orthopaedic practice.
The Prologue
DNA evidence dates the shared ancestor of chimpanzees and humans to approximately between six and seven million years ago.9 Since then extensive changes have occurred in the pelvis (Fig. 2) and, by comparison, the morphological changes in the hip have been quite minor. The last 50 years have yielded spectacular fossil finds that have helped map hominid evolution. The restructuring of the pelvis is best described as a compacting of the pelvis, with transition from a nearly two-dimensional to three-dimensional form.
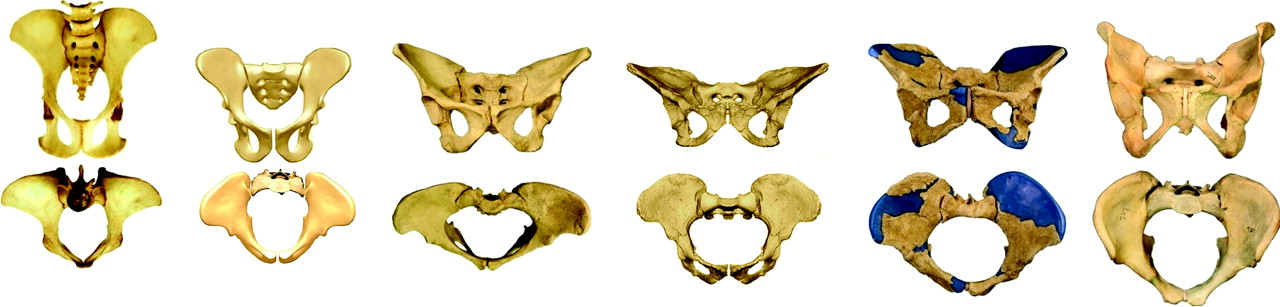
Fig. 2
Pelves in anteroposterior (top row) and axial views (bottom row). From left to right: Chimpanzee (Pan troglodytes), Ardi (Ardipithecusramidus, 4.4 million years ago), Lucy (Australopithecus afarensis, 3.2 million years ago), Australopithecus africanus (2.7 million years ago), Homo erectus (1.5 million years ago) and Homo sapiens. Note the birth canal first widens transversely but from Au. afarensis to H. sapiens only anteroposterior deepening occurs (adapted from Bergé and Goularas40, Lovejoy et al41 and Simpson et al42, with permission). In Darwin’s day, only the specimens far right and far left were known.
The main feature of this compacting8 has been a marked shortening of the ilium, while the sacrum enlarged in all dimensions and came lower to lie opposite the pubis. The result has been a bony birth canal that can cause trouble during childbirth. In addition, the sacrum moved forward (ventrally) and tilted, while the lumbar spine lengthened. The number of lumbar vertebrae increased, from three or four in the chimpanzee to five, sometimes six, in Homo sapiens.This facilitated the development of a lumbar lordosis, thereby positioning the spine more centrally and bringing the centre of gravity of the upper body closer to the hip joints in the sagittal (lateral) plane.
The human ilium may have become shorter, but it also arches further forward (ventrally), creating prominent anterior superior iliac spines. This forward-arching ilium repositions the gluteal muscles over the hip joint. In the large apes (orang-utan, chimpanzee, gorilla) these muscles are almost entirely posterior to the hip joint, which is why they function mainly as hip extensors. Meanwhile, human gluteal muscles are posterior, directly above and anterior to the hip joint, making them true hip abductors.
Early human ancestors (hominids) first began walking upright and only later developed a large brain. Evidence for this comes from Australopithecus afarensis of 3.2 million years ago, that was well-adapted to a permanent upright gait11 but still had a body and brain size similar to a chimpanzee.7 In the subsequent three million years, body size approximately doubled while brain size tripled. This brain enlargement thus happened when the pelvis, in evolutionary terms, had already undergone extensive restructuring to facilitate a true upright gait. There had also been a remarkable elongation of the lower limbs.
From approximately three to 0.5 million years ago only anteroposterior deepening of the pelvis appears to have taken place through relative growth of the pubic bones while the relative width of the pelvis decreased. This may be because of the importance of an efficient abductor mechanism for the now permanent bipedal gait of early humans. To keep required abductor work within limits, the lever arm of bodyweight should also be kept within limits.12 Indeed, the distance between the midline of the pelvis and the centre of the femoral head has been said to be larger for human females than males.13
A large foetal brain and long legs may present serious problems at childbirth. Today’s rate of Caesarean section is approximately 20% in developed countries.14 Meanwhile, obstetric problems have never been documented in the large apes.15 Nevertheless, difficulties with childbirth are not exclusive to humans, as bovids and smaller primates such as the macaque are known to have birthing problems.
Developmental dysplasia of the hip (DDH)
In the early twentieth century, Pierre Le Damany, a surgeon from Brittany, France, treated many infants with DDH, a condition that occurred more commonly in his part of the world. In comparative anatomical studies, Le Damany16 noticed a marked difference in uterine space available for the human foetus compared with quadrupeds. He postulated that the growing human foetus, with its ultra-large head and long legs, was progressively forced into a “position pénible” with the hips in hyperflexion, a position already depicted by Leonardo da Vinci (Fig. 3). Hyperflexion levers the long femur against the prominent anterosuperior iliac spine, which is far less prominent in the apes.
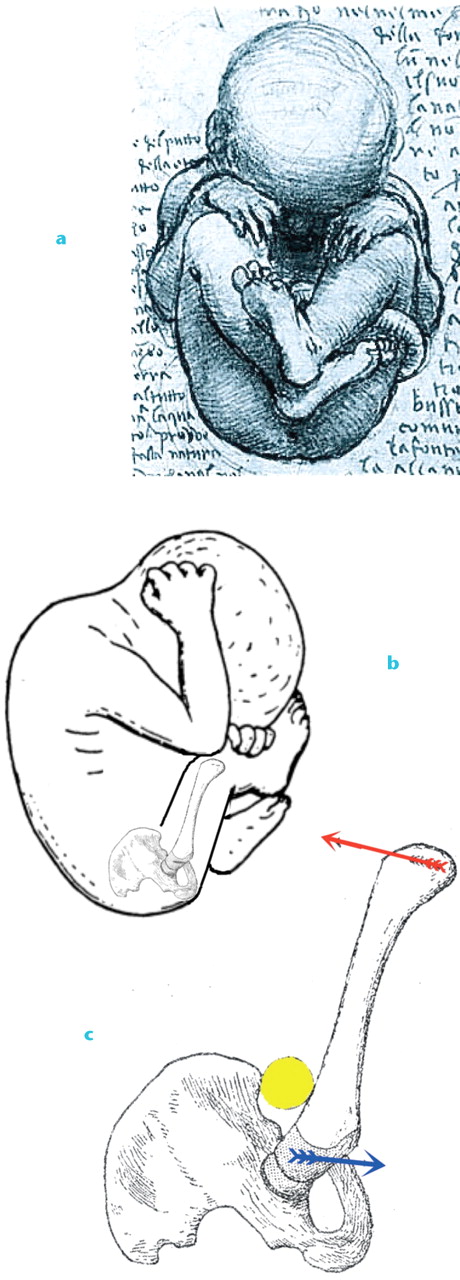
Fig. 3
Leonardo da Vinci’s’s depiction (a & b) of the “position pénible” and c) the levering of the femur against the anterosuperior iliac spine. The yellow ball depicts interposed soft tissue and the red arrow depicts pressure from the uterine wall. The blue arrow shows the torsional moment that may increase anteversion while the femoral head is levered out of the acetabulum (modified fromLe Damany P.La Luxation Congenitale de la Hanche. Paris: Masson, 1923.16)
This hyperflexed position has two effects. First, it tends to lever the femoral head out of the acetabulum. Secondly, however, it also creates a torsional moment on the femur that may increase anteversion. Le Damany16 thus surmised that as the growing human foetus has progressively less space available to it in utero, the acetabulum becomes shallower because of the reduced pressure created by the femoral head; meanwhile anteversion of the femoral neck increases. His studies demonstrated that the human, and not the quadrupedal acetabulum, gradually became shallower as birth approached. This, and increasing anteversion during the last trimester of foetal growth, were subsequently confirmed by others. 17-19 As a consequence, Le Damany treated DDH with a hip-positioning device similar to those used today.
Femoroacetabular impingement (FAI)
There were five publications citing FAI in their title in 2001, 100 in 2010 and 78 in the first half of 2011. Hip morphological variants such as cam deformities and coxa profunda, the mechanism and problems created by FAI, gender differences, and possible arthritic sequelae are now well documented.20 These morphological variants appear to develop during adolescence as they are unknown in childhood21 and are related to loading history in adolescence, for example during sports.22,23 However, why these variants develop is yet to be explained.
Examining mammalian hips, Hogervorst, Bouma and de Vos24 found severe impingement morphology to be quite common (Fig. 4). Indeed, round femoral heads were the exception rather than the rule. They proposed the terms coxa recta and coxa rotunda to conceptualise mammalian hip morphology in relation to hip function and movement. Coxa recta is an aspherical femoral head, which may be seen in running and jumping mammals. In human terms this is a cam hip.25 Coxa rotunda, a spherical femoral head, is a hip seen in climbing and swimming mammals. The human equivalent is coxa profunda.

Fig. 4
Coxa recta in a horse (Equus caballus) (a) and coxa rotunda in a walrus (Odobenus rosmarus) (b). The horse coxa recta has a straight section of the femoral head superodorsally and an asymmetrical position of the femoral head relative to the neck, resulting in a shallow concavity on one side of the femoral head/neck junction. The coxa rotunda of the walrus demonstrates a round femoral head, positioned more symmetrically on a longer femoral neck and resulting in deeper concavity all round.
Coxa recta
A coxa recta is a hip with limited or no concavity of the head-neck junction. The amount of concavity determines the range of impingement-free movement of the hip, in conjunction with acetabular morphology. Concavity reflects both the sphericity of the femoral head and the position of the femoral head on the femoral neck. This is clear when mammalian hips are studied (Fig. 4) but is often more subtly present in human hips. In coxa recta the centre of the femoral head is not in line with the anatomical axis of the femoral neck, but is displaced, decreasing concavity of the head-neck junction opposite the direction of shift.
Most mammals have a coxa recta. Jumpers (lemur), hoppers (kangaroo), and runners (horse) with high hip-loading may benefit from a sturdy hip with a short, thick neck and an aspherical femoral head. Most species of mammal do not appear to need a large range of hip rotation - coxa recta appears to be Nature’s default hip.
A human cam-type hip is essentially a coxa recta. Hogervorst et al24 did not find a single example of coxa recta when examining over 200 ape femora in the chimpanzee, bonobo and gorilla. It thus appears that coxa recta in humans is an adaptation in the evolution of a running ape,26 which might require a sturdy hip without the need for a large range of rotational movement.
When comparing coxa recta between quadrupeds and humans, the non-spherical section of the femoral head is at different locations. In quadrupedal mammals it is located posterosuperiorly (Fig. 4), while in humans the cam is positioned anterosuperiorly. On the basis that the recta section relates to the highest tensile stresses seen at the head-neck junction during hoof or heel strike, this difference appears to be the result of a horizontal (quadrupeds) versus vertical (human) trunk axis during locomotion.27
Coxa rotunda
A coxa rotunda is a hip with a round femoral head positioned more centrally on a relatively long femoral neck. This creates an obvious circumferential concavity (Fig. 4). Few mammals have a coxa rotunda. Examples of those that do are the large apes (orang-utan, chimpanzee and gorilla). Swimming mammals such as the sea otter, walrus and seal also have a coxa rotunda, although it is less pronounced than for the large apes. In a climber, a hip with a round femoral head and high concavity allows rotation, increasing the arc within which surfaces can be grabbed. Likewise, in a swimmer, rotation of the hip increases the thrust generation of a flipper.
Looking at the female hip from an evolutionary perspective, it may be that coxa profunda is an adaptation to the widening of the female birth canal.27 In turn, a profunda acetabulum is associated with a round femoral head – coxa rotunda – because of the reciprocal development of the femoral head and acetabulum.21,28
Currently, the human femur is interpreted as normal if a coxa rotunda is present. A human cam-type hip, a coxa recta, is regarded as abnormal. Whether evolution would agree with this is a different matter.
Discussion
An evolutionary perspective can offer an elegant explanation for two common hip disorders, DDH and FAI. Is FAI truly a disorder or could it simply be an evolutionary variant?
Both morphological variants share a role played by mechanical loading - hyperflexion for DDH and a high loading history for FAI. Both also share a marked disparity in prevalence across race and gender.29-32 This suggests that neither condition is created solely by mechanical forces acting on the developing hip.
For DDH, the uterus represents a unique, largely constant mechanical environment. Yet marked variation is still seen in the rate of DDH among different populations.33-35
For FAI, two studies point to a relationship between a history of hip loading during sport in adolescence and morphology of the adult femoral head.21,22 However, other evidence points to a distinctly genetic component.36 In addition, racial and gender disparities support the notion that loading is not the only issue. For example, Asian hips rarely have aspherical femoral heads29,37 meanwhile gender disparities are evident.30,31
This ultimately leads to a core question of evolutionary biology.1,2,38 Is the palette of possible forms truly unlimited and actually governed by genetic variation? Or, do physical and chemical parameters play an important role?
From an evolutionary viewpoint and, for that matter, in the orthopaedic consulting room, it appears that so-called disorders of the hip joint may be no more than morphological variants. For example, a cam hip can easily be interpreted as a coxa recta, a variant that has appeared during the evolution of a running ape. It is because of the advanced age that can be reached by Homo sapiens that this variant may cause trouble in the form of OA. Indeed, as OA is primarily a disease of advanced age, i.e. after reproduction, it is hardly important in evolutionary selection.
It is also possible that morphology of the hip joint has nothing to do with the development of OA at all. If one considers OA at a molecular level only, it can be regarded as being created by loss of the protein-signalling pathways that protect articular cartilage during reproductive life. These pathways decline after reproductive age as there is no pressure for evolution to maintain them after this point. For example, changes in chondrocyte Transforming Growth Factor Beta (TGF-β) signalling, may lead to chondrocyte differentiation after reproductive age.39 This causes cartilage degeneration and eventual OA as the ageing process continues.
Consequently, an evolutionary perspective to the human hip joint can help orthopaedic surgeons explain the joint’s morphology and its variants. Not every deformity may lead to disease and not all disease is a result of deformity. Indeed, during the reproductive years, some deformities may actually confer an advantage.
1 Ball P. Shapes: nature's patterns: a tapestry in three parts. Oxford: Oxford University Press, 2009. Google Scholar
2 Carroll SB. Endless forms most beautiful: the new science of evo devo. New York: W. W. Norton and Company, 2005. Google Scholar
3 van de Kamp T , VagovičP, BaumbachT, RiedelA. A biological screw in a beetle's leg. Science2011;333:52.CrossrefPubMed Google Scholar
4 Dawkins R. The ancestor's tale: a pilgrimage to the dawn of evolution. London: Weidenfeld & Nicholson, 2004. Google Scholar
5 Dawkins R. The blind watchmaker: why the evidence of evolution reveals a universe without design. London: Penguin Science, 1986. Google Scholar
6 Shubin NH. Your inner fish: a journey into the 3.5-billion-year history of the human body. New York: Pantheon Books, 2008. Google Scholar
7 Johanson D, Elgar B. From Lucy to Language. London: Cassell, 2001. Google Scholar
8 Lovejoy CO . The natural history of human gait and posture: part 1: spine and pelvis. Gait Posture2005;21:95–112. Google Scholar
9 Sibley CG , AhlquistJE. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol1987;26:99–121.CrossrefPubMed Google Scholar
10 Schultz AH. The life of primates. New York: Universe books, 1969. Google Scholar
11 Lovejoy CO , HeipleKG, BursteinAH. The gait of Australopithecus. Am J Phys Anthropol1973;38:757–779.CrossrefPubMed Google Scholar
12 Inman VT . Functional aspects of the abductor muscles of the hip. J Bone and Joint Surg [Am]1947;29-A:607–619.PubMed Google Scholar
13 Tague RG . Commonalities in dimorphism and variability in the anthropoid pelvis, with implications for the fossil record. J Hum Evol1991;21:153–176. Google Scholar
14 Althabe F , SosaC, BelizánJM, GibbonsL, JacqueriozF, BergelE. Cesarean section rates and maternal and neonatal mortality in low-, medium-, and high-income countries: an ecological study. Birth2006;33:270–277.CrossrefPubMed Google Scholar
15 Abitbol MM. Birth and human evolution. anatomical and obstetrical mechanics in primates. Westport: Bergin and Garvey, 1996. Google Scholar
16 Le Damany P. La Luxation Congénitale de la hanche. Paris: Masson, 1923. Google Scholar
17 Rális Z , McKibbinB. Changes in shape of the human hip joint during its development and their relation to its stability. J Bone Joint Surg [Br]1973;55-B:780–785.PubMed Google Scholar
18 Walker JM , GoldsmithCH. Morphometric study of the fetal development of the human hip joint: significance for congenital hip disease. Yale J Biol Med1981;54:411–437.PubMed Google Scholar
19 Bonneau N , SimonisC, SeringeR, TardieuC. Study of femoral torsion during prenatal growth: interpretations associated with the effects of intrauterine pressure. Am J Phys Anthropol2011;145:438–445.CrossrefPubMed Google Scholar
20 Ganz R , LeunigM, Leunig-GanzK, HarrisWH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop2008;466:264–272.CrossrefPubMed Google Scholar
21 Ogden JA. Development and growth of the hip. In: Katz JF, Siffert RS, eds. Management of hip disorders in children. Philadelphia: JB Lippincott, 1983:1-32. Google Scholar
22 Murray RO , DuncanC. Athletic activity in adolescence as an etiological factor in degenerative hip disease. J Bone Joint Surg [Br]1971;53-B:406–419.PubMed Google Scholar
23 Siebenrock KA , FernerF, NoblePC, et al.The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop2011;469:3229–3240.CrossrefPubMed Google Scholar
24 Hogervorst T , BoumaHW, de VosJ. Evolution of the hip and pelvis. Acta Orthopaedica2009;80(Suppl):1–39.CrossrefPubMed Google Scholar
25 Beck M , KalhorM, LeunigM, GanzR. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg [Br]2005;87-B:1012–1018.CrossrefPubMed Google Scholar
26 Bramble DM , LiebermanDE. Endurance running and the evolution of Homo. Nature2004;432:345–352.CrossrefPubMed Google Scholar
27 Hogervorst T , BoumaHW, Boer deSF, Vos deJ. Human hip impingement morphology: an evolutionary explanation. J Bone Joint Surg [Br]2011;93-B:769–776.CrossrefPubMed Google Scholar
28 Steppacher SD , TannastM, WerlenS, SiebenrockKA. Femoral morphology differs between deficient and excessive acetabular coverage. Clin Orthop2008;466:782–790.CrossrefPubMed Google Scholar
29 Takeyama A , NaitoM, ShiramizuK, KiyamaT. Prevalence of femoroacetabular impingement in Asian patients with osteoarthritis of the hip. Int Orthop2009;33:1229–1232.CrossrefPubMed Google Scholar
30 Gosvig KK , JacobsenS, Sonne-HolmS, PalmH, TroelsenA. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg [Am]2010;92-A:1162–1169.CrossrefPubMed Google Scholar
31 Hack K , Di PrimioG, RakhraK, BeauléPE. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg [Am]2010;92-A:2436–2444.CrossrefPubMed Google Scholar
32 Dudda M , KimYJ, ZhangY, et al.Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis?Arthritis Rheum2011;63:2992–2999.CrossrefPubMed Google Scholar
33 Dezateux C , RosendahlK. Developmental dysplasia of the hip. Lancet2007;369:1541–1552.CrossrefPubMed Google Scholar
34 Inoue K , WicartP, KawasakiT, et al.Prevalence of hip osteoarthritis and acetabular dysplasia in french and japanese adults. Rheumatology (Oxford)2000;39:745–748.CrossrefPubMed Google Scholar
35 Yoshimura N , CampbellL, HashimotoT, et al.Acetabular dysplasia and hip osteoarthritis in Britain and Japan. Br J Rheumatol1998;37:1193–1197.CrossrefPubMed Google Scholar
36 Pollard TC , VillarRN, NortonMR, et al.Genetic influences in the aetiology of femoroacetabular impingement: a sibling study. J Bone Joint Surg [Br]2010;92-B:209–216.CrossrefPubMed Google Scholar
37 Hoaglund FT , SteinbachLS. Primary osteoarthritis of the hip: etiology and epidemiology. J Am Acad Orthop Surg2001;9:320–327.CrossrefPubMed Google Scholar
38 Thompson DA. On growth and form. Cambridge: Cambridge University Press, 1917. Google Scholar
39 van der Kraan PM , van den BergWB. Osteoarthritis in the context of ageing and evolution: loss of chondrocyte differentiation block during ageing. Ageing Res Rev2008;7:106–113. Google Scholar
40 Bergé C , GoularasD. A new reconstruction of Sts 14 pelvis (Australopithecus africanus) from computed tomography and three-dimensional modeling techniques. J Hum Evol2010;58:262–272.CrossrefPubMed Google Scholar
41 Lovejoy CO , SuwaG, SpurlockL, AsfawB, WhiteTD. The pelvis and femur of Ardipithecus ramidus: the emergence of upright walking. Science2009;326:71.PubMed Google Scholar
42 Simpson SW , QuadeJ, LevinNE, et al.A female Homo erectus pelvis from Gona, Ethiopia. Science2008;322:1089–1092.CrossrefPubMed Google Scholar









