Abstract
Aims
The anterior cruciate ligament (ACL) is known to have a poor wound healing capacity, whereas other ligaments outside of the knee joint capsule such as the medial collateral ligament (MCL) apparently heal more easily. Plasmin has been identified as a major component in the synovial fluid that varies among patients. The aim of this study was to test whether plasmin, a component of synovial fluid, could be a main factor responsible for the poor wound healing capacity of the ACL.
Methods
The effects of increasing concentrations of plasmin (0, 0.1, 1, 10, and 50 µg/ml) onto the wound closing speed (WCS) of primary ACL-derived ligamentocytes (ACL-LCs) were tested using wound scratch assay and time-lapse phase-contrast microscopy. Additionally, relative expression changes (quantitative PCR (qPCR)) of major LC-relevant genes and catabolic genes were investigated. The positive controls were 10% fetal calf serum (FCS) and platelet-derived growth factor (PDGF).
Results
WCS did not differ significantly among no plasmin versus each of the tested concentrations (six donors). The positive controls with PDGF and with FCS differed significantly from the negative controls. However, we found a trend demonstrating that higher plasmin concentrations up-regulate the expression of matrix metalloproteinase 13 (MMP13), 3 (MMP3), and tenomodulin (TNMD).
Conclusion
The clinical relevance of this study is the possibility that it is not solely the plasmin, but also additional factors in the synovial fluid of the knee, that may be responsible for the poor healing capacity of the ACL.
Cite this article: Bone Joint Res 2020;9(9):543–553.
Article focus
-
This hypothesis was tested using primary ACL-derived ligamentocytes (ACL-LCs) in wound scratch assays using real-time microscopy and quantitative PCR (qPCR) at genes relevant for the ligament.
Key messages
-
Platelet-derived growth factor (PDGF) and fetal calf serum (FCS) controls markedly boosted the wound closure speed (WCS).
-
It could be demonstrated that increasing plasmin concentration had a notable effect on the wound closing rate (WCR) of ACL-LCs in vitro in four out of the six analyzed donors, in particular increasing the plasmin concentration to 50 µg/ml, which is equivalent value of plasmin concentration in the synovial fluids.
-
Relative gene expression revealed that MMP13 expression seemed to be dependent on increasing plasmin concentrations.
Strengths and limitations
-
The article tests for the first time the importance of plasmin in the synovial fluid as a possible player for inhibition of wound healing in the ACL.
-
The article reports on a newly established image processing chain using real-time microscopy phase-contrast images, which are able to accurately segment wound scratch assays using primary ACL-LCs.
-
Although up to 50 µg/ml of plasmin was tested no inhibiting effect could be demonstrated across all six human donors. Possibly other unknown factors in the synovial fluid may be responsible for the observed low wound healing potential of the ACL.
Introduction
Rupture of the ACL is a widespread knee injury. The annual incidence in the USA is estimated to lie between 100,000 to 200,000 cases.1,2 ACL tears mostly occur as a result of rotational forces alongside varus/valgus stresses acting on the knee. Furthermore, about 80% of all ACL incidences are so-called ‘non-contact’ injuries that happen due to unfortunate knee positioning and a strong unopposed quadriceps contraction.3,4 It is generally well-accepted that ACL ruptures have a very poor self-healing capacity. As for the regeneration of this tissue, tendon-specific stem cells might become an option in the future. However, more evidence on the safe application of these cell populations and their fate would be required for clinical translation.5,6
In 2014 Kiapour and Murray3 raised an interesting theory to explain the relatively poor wound healing potential of the ACL compared with other ligaments such as the medial collateral ligament (MCL)3 (see Figure 1). They questioned whether the poor healing capacity of the ACL was due to the presence or absence of scar formation, which might be directly linked to the presence or absence of fibrin blood clot formation during the healing process.3,7 It would be important to know the composition of the synovial fluid in such a case. The main constituents of the synovial fluid are hyaluronic acid, collagen, and fibronectin. A deficiency in the lubrication system may contribute to erosion of cartilage surfaces and can lead to arthritis. Several studies have reported on the composition of synovial fluid and demonstrated increased concentrations of plasmin.8,9 It has been shown in synovial fluid of patients with knee trauma, versus healthy control patients, that there is an increase of proteolytic enzymes such as elastase, collagenase, and cathepsin G, which could also potentially threaten structural elements of the enclosed cartilage.10 Furthermore, it was argued that this might be due to the presence of plasmin and/or plasmin activator in the synovial fluid (see Figure 1).11 Indeed, it could be the synovial fluid, also known as diarthrosis, that might be responsible for the delayed wound healing response.12 Biochemically it is an ultrafiltrate of plasma across the synovial membrane enriched with various compounds produced by ‘synoviocytes’ (the total of cells in and around the synovium). Here, the effects of different plasmin concentrations on the wound closing rate (WCR) of primary ligamentocytes (LCs) isolated from primary patient-derived human ACLs (referred as ACL-LCs) were tested experimentally in a simplified ‘wound’ using scratch wound assays. In injured knees, the plasmin concentrations were determined to lie between 5 µg/ml and 50 µg/ml.13,14 Therefore, this concentration range should be considered when testing the influence of plasmin on ACL-LCs.
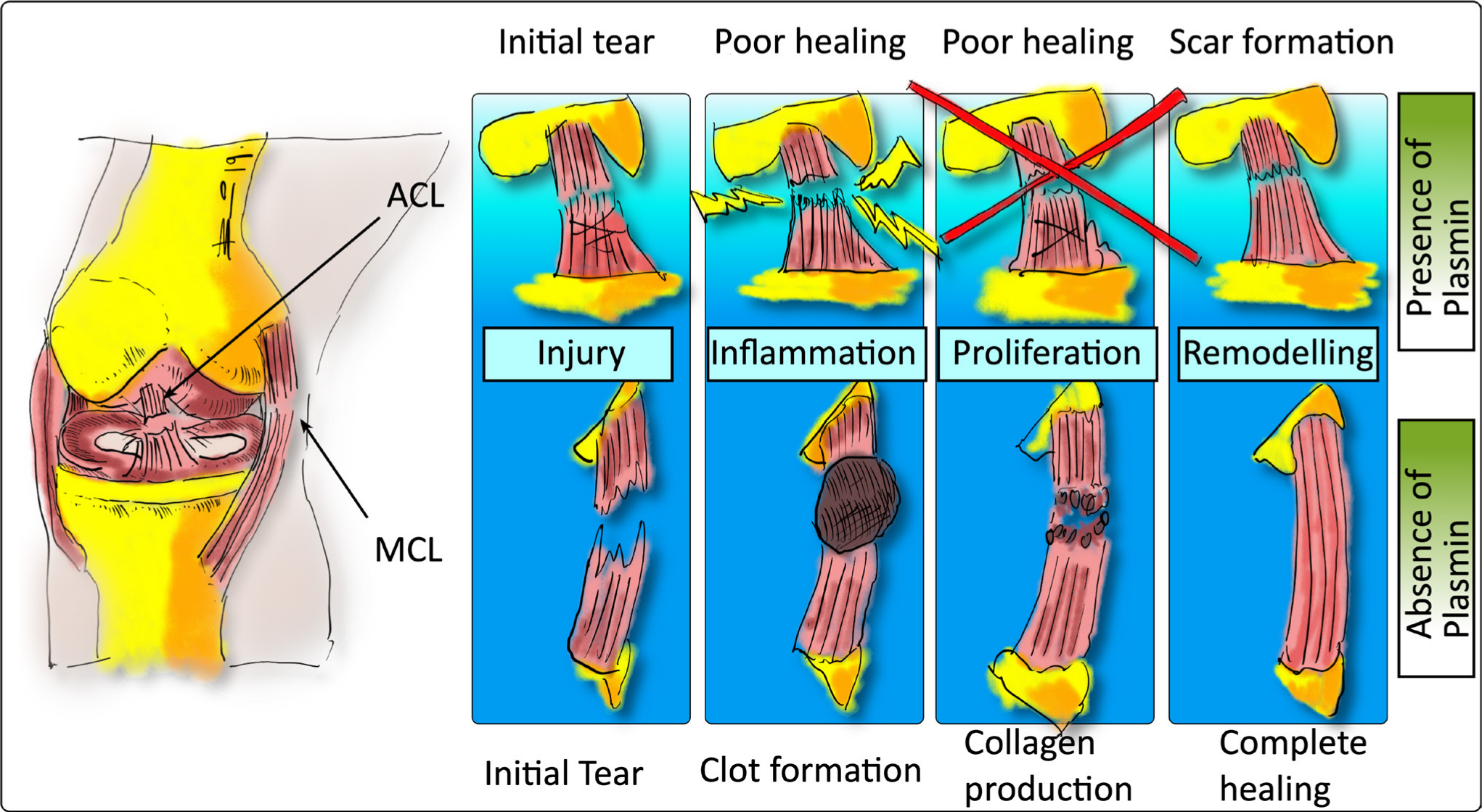
Fig. 1
Diagram of the difference in the healing process of the anterior cruciate ligament (ACL) inside the joint capsule filled with synovial fluid compared to the medial collateral ligament (MCL), which is located outside of the joint capsule, thus lacking contact with synovial fluid, which contains plasmin to some extent. The original hypothesis was formulated by Kiapour and Murray3 and has been redrawn here to illustrate the principle.
In this study, it was the aim to determine the effect of different plasmin concentrations on the specific response of primary human-derived ACL-LCs using standardized wound scratch assays. Furthermore, we investigated for the first time the changes in gene expression using selected ACL-LC specific genes, i.e. collagenase 1A2 (col 1) and collagenase 3A1 (col3A1), tenascin C (TNC), tenomodulin (TNMD), and catabolic genes such as MMP3, MMP13, and ADAMTS4 with respect to increasing doses of plasmin.
Methods
Primary ACL-LCs isolation and culture conditions
The ACL tissue was obtained from surgeries of the knee team at the Insel Hospital, University of Bern, Switzerland, with ethical approval and written consent of the patients. The tissue sample was placed in a tissue culture dish containing phosphate buffered saline (PBS) and cut into small pieces of approximately 0.3 cm3. Cells were then harvested by mild overnight digestion with 275 U/mg collagenase type II (Worthington Biochemical Corporation, Lakewood, New Jersey, USA) that was added to 25 ml low glucose Dulbeccos’s Modified Eagle Medium (LG-DMEM; Gibco, ThermoFisher Scientific, Basel, Switzerland) at a final concentration of 0.5 mg/ml. The cells were then seeded into T75 culture flask (Techno Plastic Products [TPP], Alsardingen, Switzerland) and placed for 16 to 20 hours on a shaker in the incubator (37°C, 5% CO2) for digestion. The digested material was filtered through a 70 μm cell strainer (BD Falcon; Becton-Dickinson, Brussels, Belgium). The cells were transferred into a T150 culture flask and cultured in growth media which contained LG-DMEM + 10% FCS (Gibco, ThermoFisher Scientific) and 1% antibiotics (100 μg/ml penicillin, 100 IU/ml streptomycin; Gibco, ThermoFisher Scientific). For the first week Primocin (InvivoGen, Toulouse, France; distributed by Lucerna-Biochem, Lucerne, Switzerland) with a concentration of 100 μg/ml was applied. Table I provides an overview of the hACL donors, which were used for this study.
Table I.
List of anterior cruciate ligament donors used with the IncuCyte S3 System (Essen Bioscience, Royston, UK).
| Donor ID and passage number | Age, yrs | Sex | Significance of WCS* |
|---|---|---|---|
| ACL42 P2 | 47 | F | n.s. |
| ACL48 P3 | 27 | F | p ≤ 0.01 |
| ACL49 P2 | 41 | M | p ≤ 0.001 |
| ACL72 P2 | 67 | F | p ≤ 0.001 |
| ACL75 P3 | 25 | F | p ≤ 0.001 |
| ACL77 P2 | 53 | F | n.s. |
-
*
One-way analysis of variance (ANOVA).
-
WCS, wound closing speed; n.s., not significant
Real-time life cell imaging of wound scratch assays using ACL-LCs
The in vitro scratch wound assay was performed using the WoundMaker 96-pin tool, a mechanical device to create homogenous, 700 to 800 µm wide scratches in cell monolayers (Essen Bioscience, Royston, UK) (Figure 2). Cells were seeded in LG-DMEM + 10% FCS into specialized multi-well plates (Essen Bioscience) with an optimized cell density of 15,000 cells per well. At about 90% confluency, a wound was induced. The cells were washed twice with PBS to remove cell debris and the desired plasmin concentrations were added. The plasmin was derived from human plasma (Sigma-Aldrich, St. Louis, Missouri, USA) and was diluted in PBS to a stock solution of 0.5 µg/µl. To ensure that wound closure does not occur mainly due to cell proliferation, the serum concentration was reduced in all experimental groups to 5% right after scratch induction. The rate of wound closure was monitored with time-lapse microscopy and images were acquired at 15-minute intervals from the time the cells filed the ‘induced wound’. The procedure was followed the guidelines of wound scratch assays as described in Grada et al.15 Human-derived plasmin was added to LG-DMEM with different concentrations (0, 0.1, 1, 10, and 50 µg/ml), and two controls were included to test the influence of plasmin on wound healing. Serum-free LG-DMEM was used as a negative control and media containing PDGF (50 µg/ml; Peprotech, London, UK) and 10% FCS (growth medium) were run as two positive controls. The experimental groups and the media concentrations are outlined in Table II.
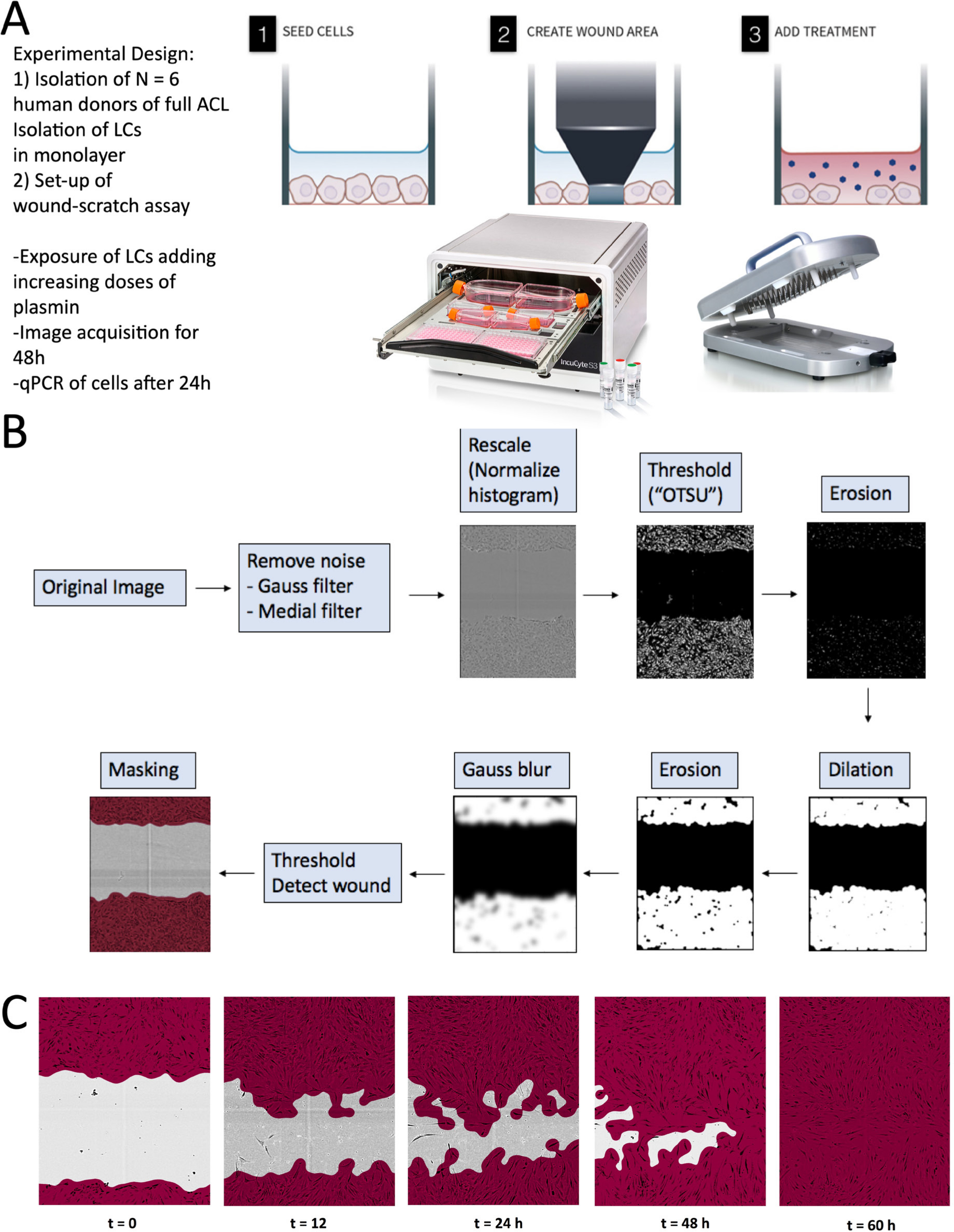
Fig. 2
Experiment design of the performed study to test the effect of plasmin, a component of the synovial fluid, on the wound healing behaviour of primary anterior cruciate ligament (ACL)-derived ligamentocytes (ACL-LCs). a) Experiment set-up. b) Customized main image processing chain using open source CellProfiler software to segment the ACL-LCs and wound closure in the wound scratch assays. "OTSU" refers to the image thresholding algorithm named after Nobuyuki Otsu.16 c) Example of phase-contrast images taken at a 10× magnification with the IncuCyte S3 system demonstrating customized segmentation of ACL-LCs. LC, ligamentocyte; qPCR, quantitative PCR.
Table II.
Overview of media used for the experiment groups.
| Experiment group | Medium | Supplements |
|---|---|---|
| Positive control 1 | LG-DMEM + 100 μg/ml penicillin + 100 IU/ml streptomycin | 10% FCS |
| Positive control 2 | LG-DMEM + 100 μg/ml penicillin + 100 IU/ml streptomycin | 5% FCS + 50 μg/ml PDGF |
| Negative control | LG-DMEM + 100 μg/ml penicillin + 100 IU/ml streptomycin | Serum-free medium |
| Experiment groups | LG-DMEM + 100 μg/ml penicillin + 100 IU/ml streptomycin | 5% FCS + different plasmin concentration (0, 0.1, 1, 10, and 50 µg/ml) |
-
DMEM, Eagle’s minimum essential medium; FCS, fetal calf serum; LG, low glucose medium; PDGF, platelet-derived growth factor.
Relative gene expression analysis
All cells were lysed in 1 ml TRI reagent (Molecular Research Center, Cincinnati, Ohio, USA) and frozen at -80°C prior extraction on day zero and after 24 hours, cells and 5 µl of poly-acryl carrier were added (Molecular Research Center, Cincinnati, Ohio, USA). For RNA extraction 100 ml of 1-bromo-3-chloropropane (BCP; Sigma Aldrich, Buchs, Switzerland) was added and a phase separation was performed followed by silicon membrane bound purification technique using GenElute total RNA extraction kit (Sigma-Aldrich). The remaining genomic DNA was digested using AMP-D1 DNase I Kit (Sigma-Aldrich), as described earlier.17
The relative gene expression for selected tendon-relevant extracellular matrix (ECM) homeostasis genes was investigated as previously described.18 Tendon and ACL-LC markers including collagen I and III, and tenomodulin, were tested. In addition, gene expression of two matrix metalloproteinases, enzymes, which regulate matrix turnover, were investigated (Table III). MMP3 (stromelysin 1) and MMP13 (collagenase 3) both break down collagen and proteoglycans quickly and are known to play a role in ligament remodelling.19 RNA integrity was controlled on selected samples by standard sensitivity RNA-chip using Experion Automated Electrophoresis System (Bio-Rad, Reinach, Switzerland). Complementary DNA (cDNA) was synthesized with the all-in-one cDNA Synthesis SuperMix from bimake (Houston, Texas, USA). The 18S ribosomal RNA gene was chosen as the reference gene, which is usually highly expressed in cells and independent from experimental conditions.20 Customized primers designed with Beacon Designer (v.7.7; Premier Biosoft, Palo Alto, California, USA) were tested for efficiency and synthesized by Microsynth (Balgach, Switzerland) as described previously.17,21 The qPCR was run in a two-step protocol with an annealing temperature of 61°C (Table III) and the 2× SYBR green supermix from bimake. Melting curve analysis was run as a standard procedure for control for the specificity of the amplicons.
Table III.
Polymerase chain reaction primers used for quantitative polymerase chain reaction. Primers were designed in Beacon designer v.7.7 (Premier Biosoft, Palo Alto, California, USA) and evaluated for efficiency.
| mRNA (Gene ID) | Primer pairs |
|---|---|
| Reference gene | |
| 18S (100008588) | F: CGA TGC GGC GGC GTT ATT C, R: GTG GCA GTG ATG GAA |
| Anabolic gene | |
| COL1A2 (1278) | F: TCA CCT ACA GCA CGC TTG, R: GGT CTG TTT CCA GGG TTG |
| COL2A1 (1280) | F: AGC AAG AGC AAG GAG AAG, R: GGG AGC CAG ATT GTC ATC |
| COL3A1 (1281) | F: ATA TCA AAC ACG CAA GGC, R: GAT TAA AGC AAG AGG AAC AC |
| TNC (3371) | F: TCT CTG CAC ATA GTG AAA AAC AAT ACC, R: TCA AGG CAG TGG TGT CTG TGA |
| TNMD (64102) | F: ACA AGC AAG TGA GGA AGA A, R: GAC GGC AGT AAA TAC AAC AAT |
| Catabolic genes | |
| ADAMTS4 (9507) | F: TTCCTGGACAATGGCTATGG, R: GTGGACAATGGCGTGAGT |
| MMP3 (4314) | F: GAC AAA GGA TAC AAC AGG GAC, R: TGA GTG AGT GAT AGA GTG GG |
| MMP13 (4322) | F: GTT CAA GGA ATC CAG TCT CTC TAT GG, R: TGG GTC ACA CTT CTC TGG TGT TT |
-
F, Forward primer; R, Reverse primer; mRNA, messenger RNA
To calculate relative gene expression, the Livak & Schmidt method (2-ΔΔCt)22 was used using the CFX-96 cycler software v3.1 (Bio-Rad); 18S (ribosomal RNA) was applied as the reference gene for all samples.23 Relative gene expression levels for samples were calculated relative to the no plasmin reference sample (i.e. with LG-DMEM + 5% FCS) of each ACL donor after 24 hours of exposure.
Image processing and segmentation
The data were analyzed with CellProfiler version 3.1.9, which is open source software for quantitative analysis of biological images.24 The main image processing steps that were established are depicted in Figure 2. Firstly, the cell shapes were smoothed by implementing a Gaussian and median filter, which removed particles that were disturbing the segmentation of the wound area. The smoothened image was then rescaled by histogram normalization. The OTSU’s thresholding method16 was then applied to search the threshold value for which the sum of foreground and background spreads was at its maximum (see Figure 2). In an erosion step single cells, which were detected in the wound site, were removed. Dilation caused the identified objects to dilate and to form a confluent layer. A Gaussian ‘blur’ was applied and the cell monolayers were detected finally as objects. The images were then masked and the cell areas were calculated.
Statistical analysis
The statistical analyses were performed with GraphPad Prism 7.0d (GraphPad Software, La Jolla, California, USA). To quantify WCS of real-time microscopy images a least-square linear regression line was fitted to the wound confluence data within the first 24 hours. The slopes were then compared with one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test. Gene expression analyses were tested identically and additionally, if significantly different from the control (no plasmin control) using a hypothetical mean of 1.0. A p-value of < 0.05 was considered significant.
Results
Wound scratch assays of primary ACL-LCs
Real-time microscopy revealed tiff images and videos, which visualized the WCR of the different experimental groups (Supplementary Videos 1 and 2). The analysis of the slopes from linear regression of each individual scratch assay is depicted in Figure 3. Overall statistical significance of WCR of single experiments using one-way ANOVA was found in four out of six donors (Table I). In two out of the six donors (donors 72 and 75) there were significant decreases in WCR observed between the 50 µg plasmin and the no plasmin group (p = 0.001 in both donors, Tukey’s multiple test); two donors reacted in the opposite way (Figure 3). However, comparing the WCR slopes of all six donors resulted in highly significant changes (p < 0.001, one-way ANOVA; Figure 4). From the pairwise comparisons, however, only the PDGF positive control was highly significant (p < 0.001, Tukey’s multiple test) from all others and the serum-free control versus the 10% FCS control was found to be significant (p < 0.05, Tukey’s multiple test) (Figure 4). If the slopes were compared pairwise across all six donors there were no significant differences between the means of the 50 µg, or among any of the weaker plasmin concentrations to the no plasmin control (Figure 4). These results demonstrated the sensitivity of the chosen wound scratch assay.
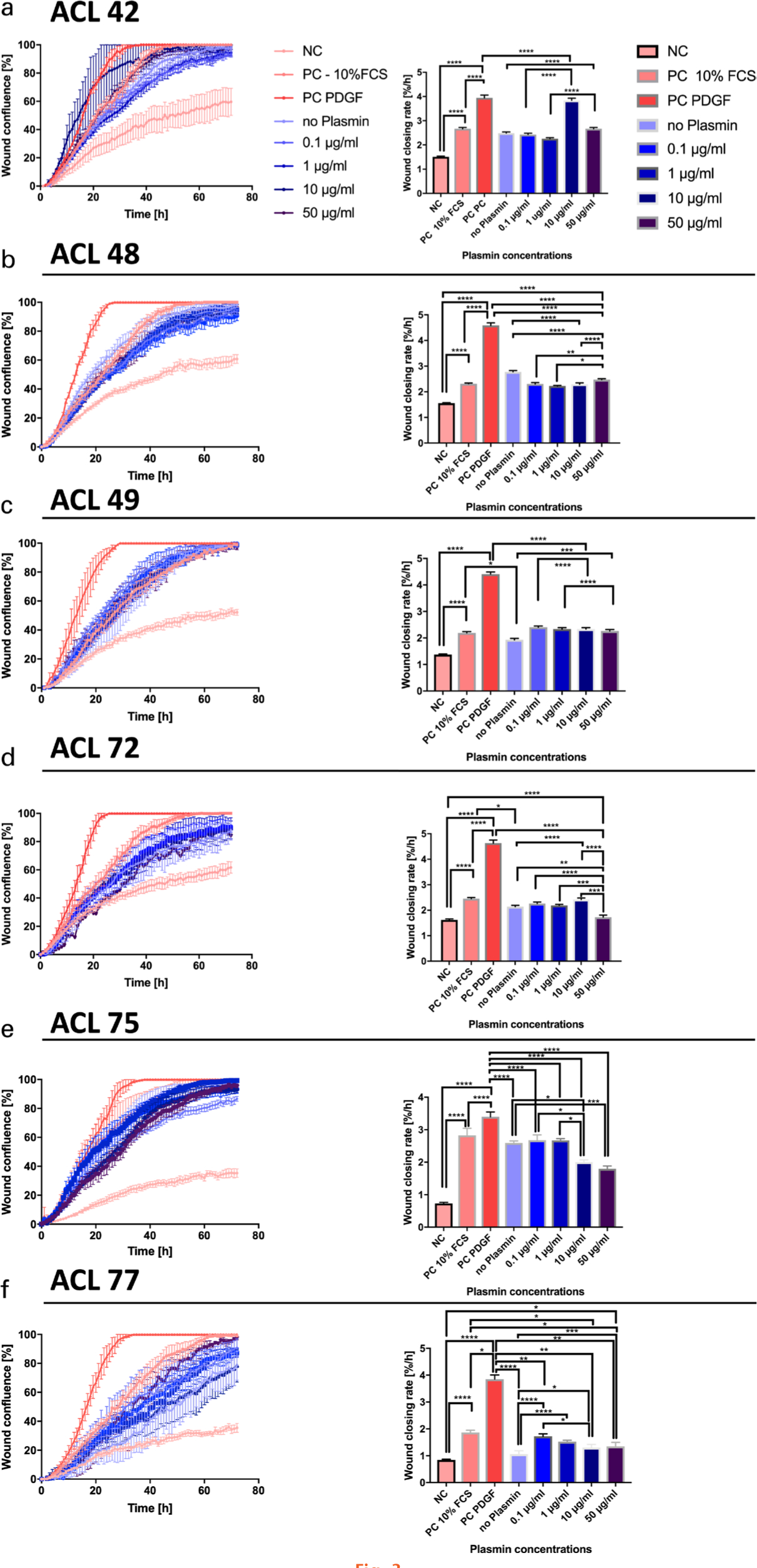
Fig. 3
Statistical comparisons of slopes of the least-square fitted linear regressions, defined as wound closing rate (WCR) of the wound scratch assays. a) to f) Wound confluence over time for anterior cruciate ligament (ACL)-derived ligamentocytes (ACL-LCs) of each of the six different donors: on the left side wound confluency is presented for the 72-hour period; on the right side the estimated slopes after 24 hours are shown for the nine technical replicates for each assay. The significance from pairwise testing is stated as follows: non-significant (ns) p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. FCS, fetal calf serum; NC, negative control; PC, positive control; PDGF, platelet-derived growth factor.
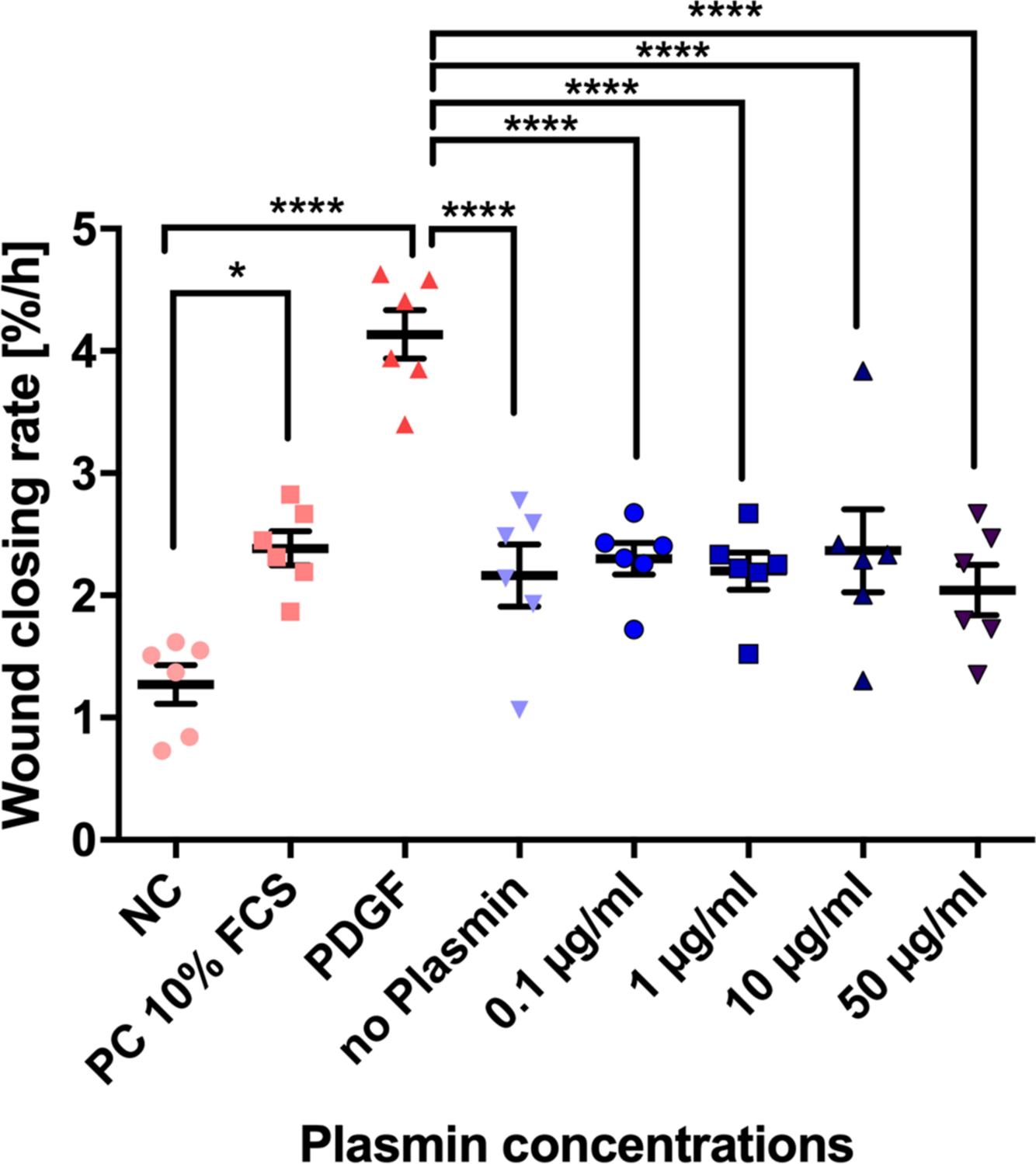
Fig. 4
Overall means of slopes across six donors of wound scratch assays to investigate the effects of plasmin on primary anterior cruciate ligament (ACL)-derived ligamentocytes (ACL-LCs). The means and SDs of the wound closing rate, given as area %/hour, were inferred from the slopes by fitting a linear regression within the first 24 hours after induction of the wound using the WoundMaker 96-pin tool (six biological replicates = human ACL donors). FCS, fetal calf serum; NC, negative control; PC, positive control; PDGF, platelet-derived growth factor. The significance from pairwise testing is stated as follows: non-significant p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001.
Gene expression analyses
Relative gene expression after the scratching of monolayer and the exposure to plasmin was quantified after 24 hours. The gene expression did not significantly change among groups (Figure 5). Exceptions were obtained for ADAMTS4 between FCS and the negative control group. There were trends visible of up-regulation of ADAMTS4, MMP3, and MMP13 with increasing concentrations of plasmin in the medium (Figure 5).
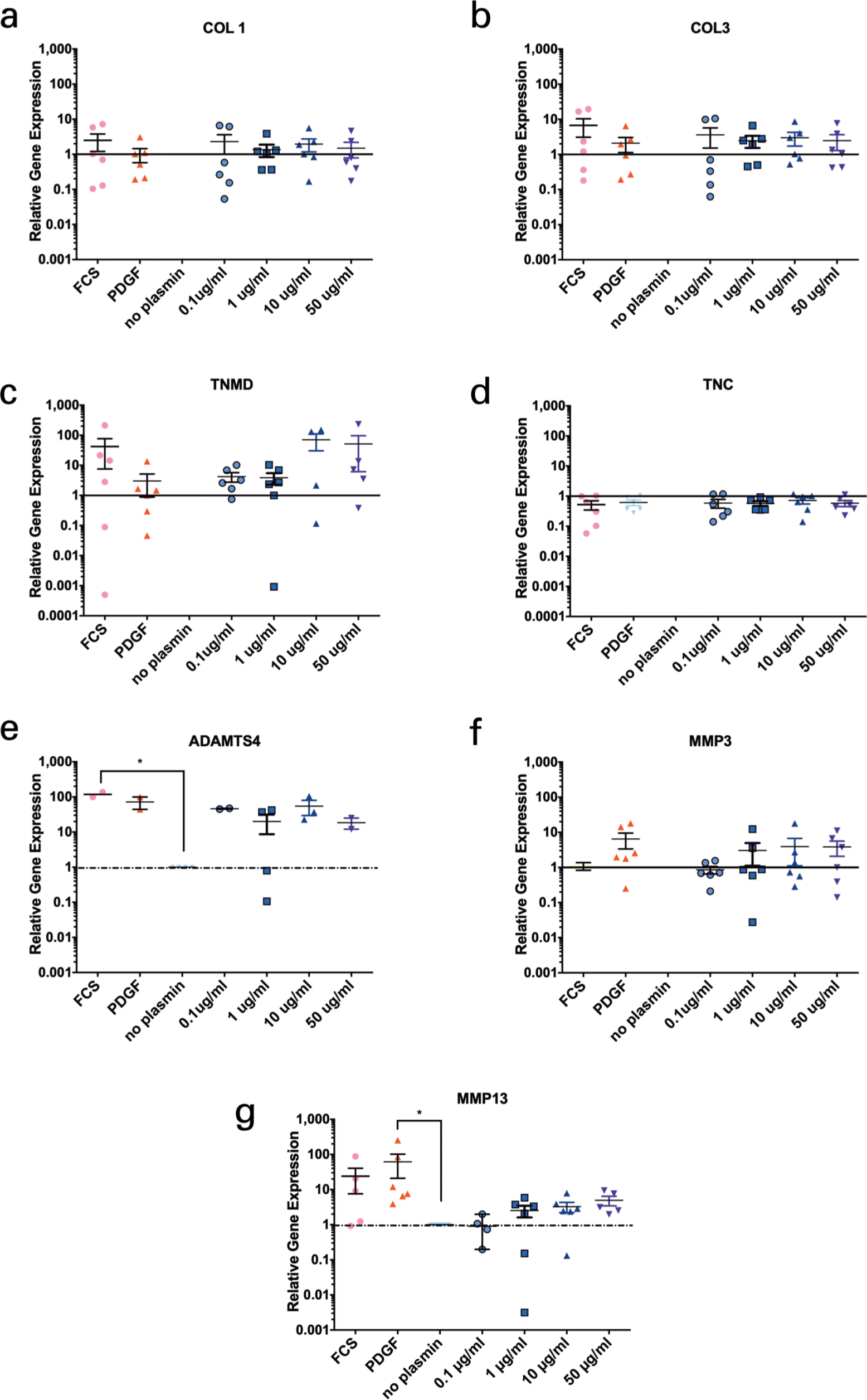
Fig. 5
Relative gene expression in the anterior cruciate ligament (ACL)-derived ligamentocytes (ACL-LCs) of six human ACL donors of induced wound scratch assays and exposure to different concentrations of plasmin in medium. a) Collagen type 1 (COL1), b) Collagen type 3 (COL3), c) Tenomodulin (TNMD), d) Tenascin (TNC), e) ADAMTS-4, f) Stromelysin 1 (MMP3), g) Collagenase 3 (MMP13). The significance is stated as follows: non-significant p > 0.05, *p ≤ 0.05. FCS, fetal calf serum; PDGF, platelet-derived growth factor.
Discussion
The current experimental data showed evidence of reduction of WCS with an increased plasmin concentration in four out of six donors (Table I and Figure 3). However, averaging across all six donors the data did not confirm that plasmin concentration seemed to have an effect on the wound-closing behaviour of primary ACL-LCs using slope comparisons among groups of each donor.
The finding that PDGF clearly accelerated WCR was evident in wound scratch assays but also supported by qPCR (Figures 3 to 5). Thus, PDGF should be discussed for improved wound healing in the clinics for ACL therapeutic purposes. Platelet-rich plasma (PRP) isolated from peripheral blood has been demonstrated to contain higher amounts of PDGF, and thus remains a feasible clinically approved method to ‘boost’ healing.21,25 These results suggested that either plasmin is not important for the observed poor wound healing response or other factors in combination with plasmin should be tested. Plasmin, however, seemed to modulate gene expression of TNMD but also ADAMTS4, MMP3, and MMP13. It is possible that these gene expression changes were induced by the addition of plasmin, thus switching on the enzymatic cascade after exposure to plasmin. This study describes for the first time the effect of plasmin presence on the MMP gene expression of primary ACL-LCs.
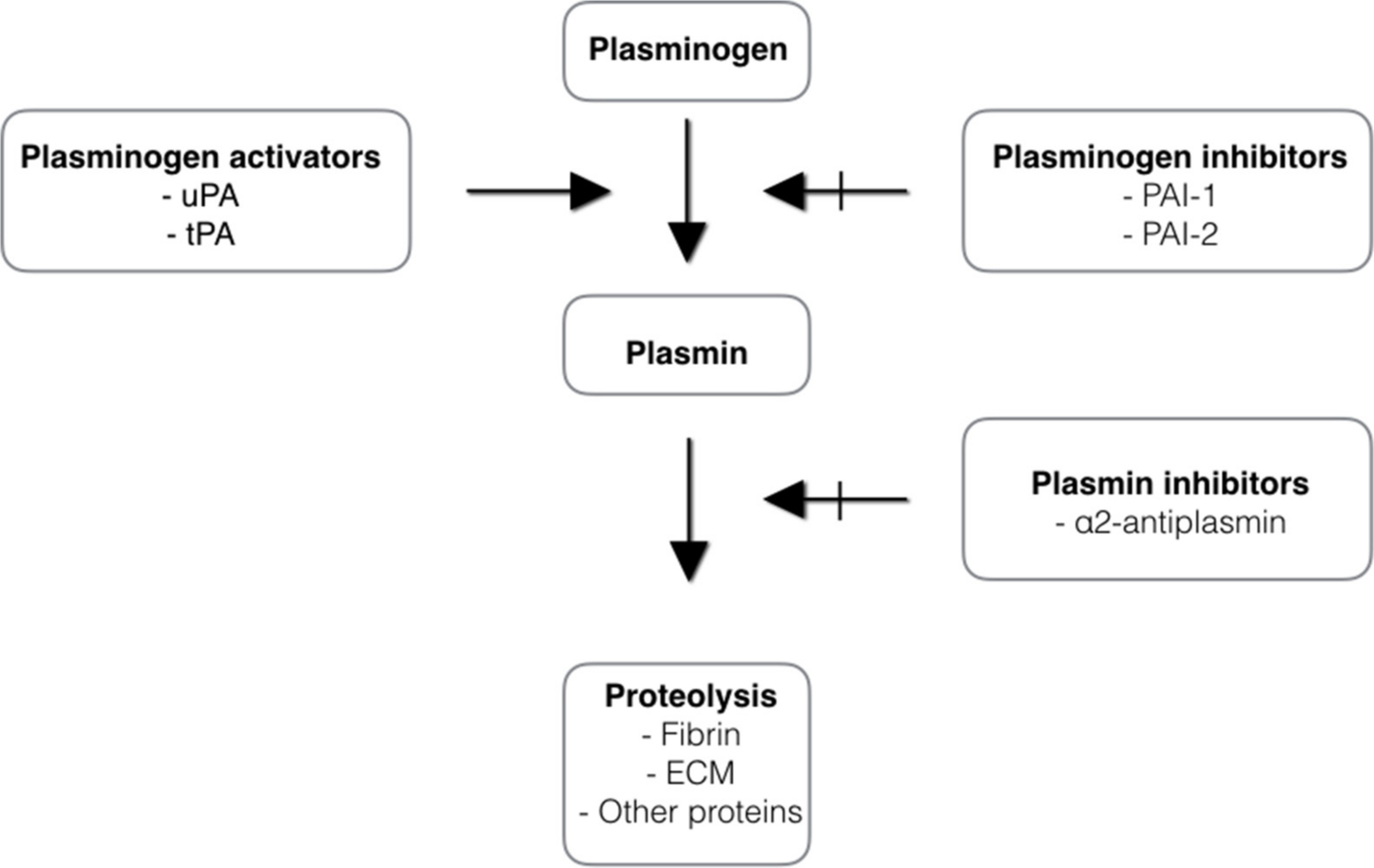
The fibrinolytic system is involved in the dissolution of fibrin clots during wound healing. It degrades fibrin and fibrinogen to products that act to inhibit the enzyme thrombin and therefore reduce transformation of fibrinogen to fibrin. The activation of the fibrinolytic system depends on the conversion of the plasma zymogen plasminogen to the serine protease plasmin. Plasminogen is mainly synthesized in the liver and circulates in the blood. The plasma concentration of plasminogen is approximately 200 µg/ml.26 Considerable amounts are also found in extravascular fluids. The main physiological function of plasmin is blood clot fibrinolysis and restoration of normal blood flow. When a blood clot forms, the blood cells and proteins become cross-linked with fibrin fibres. Fibrin formation induces activation of plasminogen. There are two types of plasminogen activators: tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) (see Figure 6). Tissue plasminogen activator is synthesized by endothelium cells and oocytes. As plasminogen, tPA possesses a high affinity for fibrin. Its activity increases sharply in the presence of fibrin. This makes tPA a useful therapeutic agent, since its activity is largely confined to the sites of recent thrombosis.27 uPA is synthesized by leucocytes, tumour cells, macrophages, and fibroblasts. The uPA molecule has no affinity to fibrin. It activates plasminogen preferably in plasma and ECM and it is thought to play a role in rheumatoid arthritis. Urokinase plasminigen activator has been identified as the predominant synovial fluid plasminogen activator in joint diseases.14 The activities of tPA and uPA are regulated by the plasminogen activator inhibitor (PAI)-1 and PAI-2 (see Figure 6). The importance of the plasminogen/plasmin system in wound healing has been demonstrated in one study.28 Plasminogen-deficient mice have a delay in the epithelialization of skin wounds. The same was observed for uPA/tPA double-deficient mice.29 In contrast, mice deficient in PAI-1 experienced accelerated wound healing. Several in vitro studies showed that plasmin induces signalling pathways that are important for cell activation and expression of many pro-inflammatory mediators during wound healing.30 The fibrinolytic system is also involved in degradation of ECM, embryogenesis, cell migration, tissue remodelling, angiogenesis, inflammation, oncogenesis, and metastasis.12
To advance ACL research future designs are needed to combine influences of cytokine and/or fibrin clots combined with automated wound healing models.31 This article is a first step towards the development of a high-throughput screening platform to test chemicals or specific substances from the synovial fluid of the knee joint in a 2D wound scratch model. Here, apart from customized imaging processing software using artificial intelligence, customized bioreactors will also be needed that allow for a combination of mechanics and biology.32,33 Current commercial solutions for real-time imaging are often limited to 2D models but would need to be optimized for 3D applications, such as screening scaffolds and tissues under mechanical loading.
Factors other than plasmin content in synovial fluid, such as mechanical loading, are likely to play an additional key role for the healing of the ACL.34,35 The ACL undergoes daily cyclic loading during natural motion of the knee.36,37 Therefore, if surgical sutures were considered as rigid fixation materials, the likelihood of failure of the repair-construct will be due to the cyclic loads that act on the ACL during the healing phase, resulting from natural knee motion.38 This concept was proven in biomechanical research, which involved a variety of ACL fixation techniques.39 Therefore, it was in the interest of some clinical research groups to introduce a dynamic form of fixation to address the biomechanical barriers of healing.39,40 The first clinical results of such an approach were recently published and demonstrated beneficial outcomes for patients using a dynamic intraligamentary stabilization technique.41 It was also recently found that in case of ruptures the anterolateral ligament may act as a secondary stabilizer in the knee joint kinematics.35
To summarize these results, it was demonstrated that increasing plasmin concentration had a significant effect on the WCR of ACL-LCs in vitro for four out of the six donors, especially increasing the plasmin concentration to 50 µg/ml which is equivalent value of plasmin concentration in the synovial fluids (Table I and Figure 3).13 However, comparing the means of the slopes of all six donors no statistical significance was found. There were visible trends of up-regulation of ADAMTS4, MMP3, and MMP13 with increasing concentrations of plasmin in the medium.
References
1. Markatos K , Kaseta MK , Lallos SN , et al. The anatomy of the ACL and its importance in ACL reconstruction . Eur J Orthop Surg Traumatol . 2013 ; 23 ( 7 ): 747 – 752 . Crossref PubMed Google Scholar
2. Siegel L , Vandenakker-Albanese C , Siegel D . Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management . Clin J Sport Med . 2012 ; 22 ( 4 ): 349 – 355 . Crossref PubMed Google Scholar
3. Kiapour AM , Murray MM . Basic science of anterior cruciate ligament injury and repair . Bone Joint Res . 2014 ; 3 ( 2 ): 20 – 31 . Crossref PubMed Google Scholar
4. Murray MM , Vavken P , Fleming BC . The ACL Handbook . NY : Springer , 2013 . Google Scholar
5. Schmalzl J , Plumhoff P , Gilbert F , et al. Tendon-derived stem cells from the long head of the biceps tendon: Inflammation does not affect the regenerative potential . Bone Joint Res . 2019 ; 8 ( 9 ): 414 – 424 . Google Scholar
6. Costa-Almeida R , Gershovich P , Rodrigues MT , et al. Tendon Stem Cell Niche Turksen K . Tissue-Specific Stem Cell Niche . Cham : Springer International Publishing , 2015 : 221 – 244 . Google Scholar
7. Andrish J , Holmes R . Effects of synovial fluid on fibroblasts in tissue culture . Clin Orthop Relat Res . 1979 ; 138 : 279 – 283 . PubMed Google Scholar
8. Kleesiek K , Reinards R , Brackertz D , Neumann S , Lang H , Greilin H . Granulocyte elastase as a new biochemical marker in the diagnosis of chronic joint diseases . Rheumatol Int . 1986 ; 6 ( 4 ): 161 – 169 . Crossref PubMed Google Scholar
9. Gysen P , Malaise M , Gaspar S , Franchimont P , et al. Measurement of proteoglycans, elastase, collagenase and protein in synovial fluid in inflammatory and degenerative arthropathies . Clin Rheumatol . 1985 ; 4 ( 1 ): 39 – 50 . Crossref PubMed Google Scholar
10. RoŚć D , Powierza W , Zastawna E , et al. Post-traumatic plasminogenesis in intraarticular exudate in the knee joint . Med Sci Monit . 2002 ; 8 ( 5 ): CR371 – 378 . PubMed Google Scholar
11. Murray MM , Fleming BC . Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery . Am J Sports Med . 2013 ; 41 ( 8 ): 1762 – 1770 . Crossref PubMed Google Scholar
12. Aisina RB , Mukhametova LI . Structure and functions of plasminogen/plasmin system . Bioorg Khim . 2014 ; 40 ( 6 ): 642 – 657 . Google Scholar
13. Palmer M , Stanford E , Murray MM . The Effect of Synovial Fluid Enzymes on the Biodegradability of Collagen and Fibrin Clots . Materials (Basel) . 2011 ; 4 ( 8 ): 1469 – 1482 . Crossref PubMed Google Scholar
14. Kummer JA , Abbink JJ , de Boer JP , et al. Analysis of intraarticular fibrinolytic pathways in patients with inflammatory and noninflammatory joint diseases . Arthritis Rheum . 1992 ; 35 ( 8 ): 884 – 893 . Crossref PubMed Google Scholar
15. Grada A , Otero-Vinas M , Prieto-Castrillo F , et al. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay . J Invest Dermatol . 2017 ; 137 ( 2 ): e11 – e16 . Crossref PubMed Google Scholar
16. Otsu N . A threshold selection method from gray-level histograms . IEEE Trans Syst Man Cybern . 1979 ; 9 ( 1 ): 62 – 66 . Google Scholar
17. May RD , Tekari A , Frauchiger DA , et al. Efficient non-viral transfection of primary intervertebral disc cells by electroporation for tissue engineering application . Tissue Eng Part C Methods . 2017 ; 23 ( 1 ): 30 – 37 . Google Scholar
18. Gantenbein B , Gadhari N , Chan SC , Kohl S , Ahmad SS . Mesenchymal stem cells and collagen patches for anterior cruciate ligament repair . World J Stem Cells . 2015 ; 7 ( 2 ): 521 – 534 . Crossref PubMed Google Scholar
19. Attia E , Bohnert K , Brown H , et al. Characterization of total and active matrix metalloproteinases-1, -3, and -13 synthesized and secreted by anterior cruciate ligament fibroblasts in three-dimensional collagen gels . Tissue Eng Part A . 2014 ; 20 ( 1-2 ): 171 – 177 . Crossref PubMed Google Scholar
20. Lee CR , Grad S , Maclean JJ , et al. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk . Anal Biochem . 2005 ; 341 ( 2 ): 372 – 375 . Crossref PubMed Google Scholar
21. Krismer AM , Cabra RS , May RD , et al. The biologic response of human anterior cruciate ligamentocytes on collagen-patches to platelet-rich plasma formulations with and without leucocytes . Journal of Orthopaedic Research . 2017 ; 35 ( 12 ): 2733 – 2739 . Google Scholar
22. Livak KJ , Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method . Methods . 2001 ; 25 ( 4 ): 402 – 408 . Crossref PubMed Google Scholar
23. Marino JH , Cook P , Miller KS . Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and real-time RT-PCR . J Immunol Methods . 2003 ; 283 ( 1-2 ): 291 – 306 . Crossref PubMed Google Scholar
24. Carpenter AE , Jones TR , Lamprecht MR , et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes . Genome Biology . 2006 ; 7 ( 10 ): 1 – 11 . Crossref PubMed Google Scholar
25. Murray IR , LaPrade RF . Platelet-rich plasma: Renewed scientific understanding must guide appropriate use . Bone Joint Res . 2016 ; 5 ( 3 ): 92 – 94 . Crossref PubMed Google Scholar
26. Ogston D . Biochemistry of the plasmin system . J Clin Pathol Suppl (R Coll Pathol) . 1980 ; 14 : 5 – 9 . PubMed Google Scholar
27. Bhattacharjee P , Bhattacharyya D . An insight into the abnormal fibrin clots - its pathophysiological roles. Krasimir K . Fibrinolysis and thrombolysis . Croatia : InTechOpen, Croatia University Press , 2014 : 3 – 29 . Google Scholar
28. Judex MO , Mueller BM . Plasminogen activation/plasmin in rheumatoid arthritis: matrix degradation and more . Am J Pathol . 2005 ; 166 ( 3 ): 645 – 647 . Crossref PubMed Google Scholar
29. Shen W , Chen J , Yin Z , et al. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair . Cell Transplant . 2012 ; 21 ( 5 ): 943 – 958 . Crossref PubMed Google Scholar
30. Syrovets T , Simmet T . Novel aspects and new roles for the serine protease plasmin . Cell Mol Life Sci . 2004 ; 61 ( 7-8 ): 873 – 885 . Crossref PubMed Google Scholar
31. Baar K . Minimizing Injury and Maximizing Return to Play: Lessons from Engineered Ligaments . Sports Med . 2017 ; 47 ( Suppl 1 ): 5 – 11 . Crossref PubMed Google Scholar
32. Gantenbein B , Frauchiger DA , May RD , et al. Developing Bioreactors to host Joint-derived Tissues that require Mechanical Stimulation In: Reis RL, Gomes ME, ed . Encyclopedia of Tissue Engineering and Regenerative Medicine . NY : Elsevier , 2019 . Google Scholar
33. Hohlrieder M , Teuschl AH , Cicha K , et al. Bioreactor and scaffold design for the mechanical stimulation of anterior cruciate ligament grafts . Biomed Mater Eng . 2013 ; 23 ( 3 ): 225 – 237 . Crossref PubMed Google Scholar
34. Whitaker S , Edwards JH , Guy S , et al. Stratifying the mechanical performance of a decellularized xenogeneic tendon graft for anterior cruciate ligament reconstruction as a function of graft diameter: An animal study . Bone Joint Res . 2019 ; 8 ( 11 ): 518 – 525 . Crossref PubMed Google Scholar
35. Kang KT , Koh YG , Park KM , et al. The anterolateral ligament is a secondary stabilizer in the knee joint: A validated computational model of the biomechanical effects of a deficient anterior cruciate ligament and anterolateral ligament on knee joint kinematics . Bone Joint Res . 2019 ; 8 ( 11 ): 509 – 517 . Crossref PubMed Google Scholar
36. Buschmann J , Bürgisser GM . Biomechanics of Tendons and Ligaments: Tissue Reconstruction and Regeneration . Woodhead Publishing : Elsevier , 2017 : 1 – 340 . Google Scholar
37. Snedeker JG , Foolen J . Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy . Acta Biomater . 2017 ; 63 : 18 – 36 . Crossref PubMed Google Scholar
38. Ateschrang A , Salewski C , Ahrend MD , et al. The Elastic Capacity of a Tendon-Repair Construct Influences the Force Necessary to Induce Gapping . Knee Surg Sports Traumatol Arthrosc. Crossref PubMed Google Scholar
39. Ahmad SS , Schürholz K , Liechti EF , et al. Seventy percent long-term survival of the repaired ACL after dynamic intraligamentary stabilization . Knee Surg Sports Traumatol Arthrosc . 2020 ; 28 ( 2 ): 594 – 598 . Crossref PubMed Google Scholar
40. Kohl S , Stock A , Ahmad SS , et al. Dynamic intraligamentary stabilization and primary repair: A new concept for the treatment of knee dislocation . Injury . 2014 ; 46 ( 4 ): 724 – 728 . Crossref PubMed Google Scholar
41. Eggli S , Kohlhof H , Zumstein M , et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL . Knee Surg Sports Traumatol Arthrosc . 2014 ; 23 ( 4 ): 1215 – 1221 . Crossref PubMed Google Scholar
Author contributions
E. Bakirci: Collected the qPCR data, Wrote and edited the manuscript.
K. Tschan: Collected the data on live cell imaging, Wrote the manuscript, Performed the wound scratch assays.
R. D. May: Assisted during the cell cultures, Edited and proofread the manuscript.
S. S. Ahmad: Provided the clinical input, Drafted and edited the manuscript, Provided the funding.
B. Kleer: Provided the clinical samples with ethical approval, Edited and approved the final manuscript.
B. Gantenbein: Designed the study, Assisted in the statistical analysis, Wrote and edited the manuscript, Provided the funding for the study.
E. Bakirci and K. Tschan contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. This study was funded by an independent peer-reviewed start-up grant from the Centre for Applied Biotechnology and Molecular Medicine (CABMM) platform of the University of Zurich, granted to B. Gantenbein and S. S. Ahmad.
Acknowledgements
We are thankful to Joel Werren and Selina Steiner for assistance and data analysis for qPCR. Sandro Kohl provided clinical samples. We thank Nicolas Barsuglia from Essen Bioscience, Switzerland/UK for providing the demo equipment of the Incucyte S3 device and the MIC Centre of the University of Bern (www.mic.unibe.ch) for the support.
Ethical review statement
The research was approved by the local ethical committee of the Cantone of Bern, Switzerland.
Supplementary material
Two supplementary videos that demonstrate a representative wound scratch assay of a responsive donor (Supplementary Video 1, ACL72) and a non-responsive donor (Supplementary Video 2, ACL48).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.