Abstract
Osteoporosis (OP) is a chronic metabolic bone disease characterized by the decrease of bone tissue per unit volume under the combined action of genetic and environmental factors, which leads to the decrease of bone strength, makes the bone brittle, and raises the possibility of bone fracture. However, the exact mechanism that determines the progression of OP remains to be underlined. There are hundreds of trillions of symbiotic bacteria living in the human gut, which have a mutually beneficial symbiotic relationship with the human body that helps to maintain human health. With the development of modern high-throughput sequencing (HTS) platforms, there has been growing evidence that the gut microbiome may play an important role in the programming of bone metabolism. In the present review, we discuss the potential mechanisms of the gut microbiome in the development of OP, such as alterations of bone metabolism, bone mineral absorption, and immune regulation. The potential of gut microbiome-targeted strategies in the prevention and treatment of OP was also evaluated.
Cite this article: Bone Joint Res 2020;9(8):524–530.
Article focus
-
The gut microbiome has long been thought to be a potential targeted regulation of bone mass.
-
This article mainly explains the relationship between the gut microbiome and osteoporosis (OP).
Key messages
-
In this manuscript, we review the potential mechanisms of the gut microbiome in the development of OP, such as bone metabolism, bone mineral absorption, and immune regulation.
-
In addition, we evaluate the potential of strategies aimed at the gut microbiome in the prevention and treatment of OP.
Strengths and limitations
-
Starting from the mechanism, we analyzed the relationship between the gut microbiome and OP, and have presented the potential of gut microbiome in the treatment of OP from several treatment strategies.
-
The formation of OP is the result of genetics, environment, nutrition, and other factors. We failed to analyze these factors together, which may lead to a one-sided analysis.
Introduction
Osteoporosis (OP) is defined as a systemic and metabolic bone disease, which is characterized by a decrease in bone mass per unit volume and deterioration of the microstructure of bone tissue, thereby increasing bone fragility and susceptibility to fracture. With the general ageing of the population, OP has become a serious disease affecting society and families. OP can occur in both sexes and at any age, and is most common in postmenopausal women.1 Heredity, hormone levels, nutrition, and lifestyle are closely related to the pathogenesis of OP.2-4 Oestrogen, parathyroid hormone, vitamin D, and inflammatory factors are all important regulators of bone metabolism. Although many aetiologies have been identified, the pathogenesis of OP is still being explored.
There are hundreds of trillions of symbiotic bacteria living in the human gut. Their varieties and numbers are astonishing, more than ten times the total number of human cells. So far, more than 80% of the microbes are still unknown.5 These gut microbiota have a mutually beneficial symbiotic relationship with the human body, which plays an important role in maintaining human health. When the balance of the gut microbiota is disturbed due to certain factors, the body may suffer from diseases such as obesity, diabetes, or even cancers (such as colorectal cancer).6,7 Accumulative research proposes that the gut microbiota is closely related to the regulation of bone metabolism, although its related mechanism has not been fully discovered. The rapid development of modern molecular biotechnology has facilitated the continuous discovery of the human microbiome, and may provide more information on early events about the pathogenesis of OP.8 In this review, the available data on the potential relationship between gut microbiota and OP will be retrospectively analyzed to understand the role of gut microbiota imbalance in the development of OP, and to evaluate the potential of targeting microbiota in novel therapeutic strategies for preventing or treating OP.
Roles of the gut microbiome in osteoporosis
The gut microbiome, often referred to as 'the second largest human genome',9 is a rich and diverse microbial community composed of bacteria, fungi, viruses, and protozoa. The microflora, host, and environment are always in a stable dynamic equilibrium in healthy individuals. Most of them are located in the gastrointestinal tract, but they are responsible for diseases crucial to not only the gut but also the whole body. A large body of evidence10–16 has already demonstrated that the gut microbiome is closely related to bone metabolism and the absorption of bone-related minerals under physiological conditions, while being involved in OP pathologically.
Gut microbiome regulates bone metabolism
Bone remodelling is a dynamic coordination process between bone formation with osteoblasts and bone resorption with osteoclasts.17 More recently, it has been shown that the development of OP is influenced by the gut microbiome.10 Many studies11–14,18–20 have also displayed the role of the gut microbiome in the pathogenesis of OP.
Osteoblasts originate from the primitive mesenchymal cells of bone marrow stroma, which are the main functional cells of bone formation responsible for the synthesis, secretion, and mineralization of the bone matrix.18 Osteocalcin, as a sign of osteoblast maturation, represents the active state of newly formed osteoblasts.19 Researchers often used germ-free (GF) mice or mice treated with antibiotics to make models of the absence or impaired function of gut microbiome, with the help of a control group with normal microbiome to further understand the specific effect of gut microbiome in the development of the disease. Uchida et al11 found that, compared with the primary osteoblasts isolated from alveolar bones and calvarias of the GF mice, the osteoblasts from the specific pathogen-free (SPF) mice expressed substantially more osteocalcin, alkaline phosphatase (ALP), and insulin-like growth factor-I/-II (IGF-I/IGF-II), while the ratio of osteoprotegerin (OPG)/receptor activator of NF-κB ligand (RANKL) was decreased. In the end, the bone density of SPF mice was lower than that of GF mice, indicating that although the gut microbiome has a higher regulation impact on osteoclasts, the regulation effects of osteoblasts on bone density cannot be denied. By studying the mice with sickle cell disease (SCD), Tavakoli and Xiao12 revealed that SCD mice had increased intestinal bacterial load, infection, and intestinal permeability, resulting in the gut microbiome changing. This was followed by bone mass reduction and impairment of osteoblast function. However, after antibiotic treatment the osteoblast function of SCD mice was enhanced and the expression of osteoblast-related transcriptor Runt-related transcription factor 2 (Runx2) and IGF-I was increased to improve bone mass in SCD mice. Unfortunately, these studies did not delve into the pivotal role of specific microbiota. The method by which the gut microbiome regulates bone structure through direct action on osteoblasts remains to be elucidated, and further work should be done to fully address this mechanism.
Osteoclasts, originated from the mononuclear phagocyte system, are special terminally differentiated cells that perform bone absorption.20 Recent studies13,14 have shown that the gut microbiome is more likely to regulate bone metabolism by affecting the activity of osteoclasts. Sjögren et al13 found that lack of gut microbiome can lead to an increase in bone mass. Compared with conventionally raised (Conv.R) mice, the bone mineral density (BMD) of cancellous bone and cortical bone of GF mice increased, and the number of osteoclasts on each bone surface decreased. Meanwhile, the frequency of the CD4+ T cells and CD11b+/ Gr1- osteoclast precursor cells, derived from the bone marrow of GF mice, reduced. However, bone formation was not affected. Thus, the increased bone mass in GF mice is mainly achieved by inhibiting osteoclastogenesis.
Li et al14 further proved that under GF conditions, the production of osteoclast-promoting factors such as tumour necrosis factor-α (TNF-α), RANKL, and interleukin-17 (IL-17) did not increase in the mice model of sex steroid deficiency with the help of leuprolide to induce. On the contrary, after recolonization with different gut microbiomes the gut permeability and osteoclastogenic cytokine production of the sex steroid deficiency mice was increased, resulting in trabecular bone loss. In addition, in order to verify the availability of probiotics the authors used Lactobacillus rhamnosus GG (LGG) and a commercially available complex-probiotic-preparation VSL#3, which contains eight strains of live bacteria (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophilus).
The results showed that the probiotic preparations also prevented increased intestinal permeability caused by sex steroid depletion, thereby limiting the production of osteoclasts to produce cytokines. This serves as a proof-of-concept that gut microbiome and probiotic preparations are involved in trabecular bone loss caused by sexual steroid deficiency. However, limited evidence indicates that the gut microbiome affects osteoblasts or osteoclasts alone. The activity between the osteoblast and the osteoclast is often carried out simultaneously, although the mechanism of the gut microbiome on bone metabolism is still unknown.
Gut microbiome monitors bone mineral absorption
Calcium is the main mineral in human bones, and under normal circumstances food is the only source of calcium for the human body.21 The calcium in food is mainly transported to the upper part of the small intestine and absorbed through the intestinal wall.22 In general, the effect of the gut microbiome on calcium is mainly regulated by short-chain fatty acids (SCFAs).15 SCFAs are the main products of intestinal bacterial fermentation in organisms, including acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid. The colon is the main site of SCFA production, and butyric acid is the main energy source of colonic epithelial cells. Several studies15,23,24 indicate that probiotics can break down dietary fibre into SCFAs, which can promote intestinal secretion of more SCFAs by increasing the number of probiotics and/or prebiotics. On the other hand, SCFAs can increase the absorption of calcium in the intestinal wall by reducing intestinal pH, thus increasing bone mass.23 Further research has confirmed that butyric acid can also regulate antigen presentation by inhibiting dendritic cells, which not only stimulate the differentiation of osteoblasts but also inhibit the formation of osteoclasts in bone marrow cells.16,25
It is well established that vitamin D also pushes forward an immense influence on promoting gut calcium, phosphorus absorption, and bone calcification. Vitamin D deficiency could lead to a decrease in the proportion of Firmicutes and Deferribacteres, and more likely to induce colitis in Cyp knockout mice, whereas inflammation was controlled after vitamin D supplementation or antibiotic treatment. Remarkably, the proportion of Firmicutes and Deferribacteres could be restored after vitamin D intervention. The research sustains the notion that vitamin D can regulate the development of OP by controlling the gut microbiome directly.26 Since a variety of evidence firmly implies that the gut microbiome contributes to the development of OP, the species composition of the gut microbiome associated with OP, and the exact mechanism, need to be clarified.
Gut microbiome orchestrates bone metabolism by cytokines
Both the immune system and the skeletal system are regulated by a pattern of cytokine secretion, which both originates from the bone marrow and shares common transcription factors and signal pathways between the two systems.27 As noted by Zhao et al,28 CD4+ T cells are the key lymphocytes involved in regulating the immune response to OP. These cells not only activate osteoblasts, but also strongly inhibit osteoclasts. Activated CD4+ T cells can produce cytokines such as RANKL, OPG, and TNF-α.29 RANKL can promote osteoclast activation and bone absorption through the OPG-receptor activator of NF-κB (RANK)-RANKL signal transduction pathway.30 TNF-α can directly stimulate osteoclast formation, or increase the expression of RANKL and OPG to regulate osteoclasts indirectly.31
Recent studies13,14,32 have begun to reveal a close relationship between the gut microbiome and OP. Li et al14 reported that the lack of sex steroid did not drive the expansion of T cells and instead increased the production of TNF-α, IL-17, and RANKL in bone marrow and intestine in GF mice. Instead, the expression of these inflammatory cytokines increased in GF mice with reconventional microbiota, which were treated with LGG or VSL#3, and their expression was similar to that in the Conv.R mice. Similarly, these results can also be verified in ovariectomy (OVX). Serotonin (5-hydroxytryptamine (5-HT)), as the most widely studied neurotransmitter, has been reported in regulating bone metabolism through the gut microbiome in recent years.13,32 Both osteocytes and osteoblasts can synthesize and regulate the uptake of 5-HT;33 5-HT knockout mice showed a decrease in bone mass and strength. Interestingly, gut-derived 5-HT has a negative effect on bone formation, while brain-derived 5-HT has the opposite effect.32 Furthermore, Sjögren et al13 not only confirmed that the gut microbiome drove CD4+ T cells and CD11b+/ Gr1- osteoclast precursor cells to regulate bone metabolism, but also determined the inactivation and degradation indexes of intestinal derivative 5-HT in GF mice. Subsequently, they found that the expression of 5-HT rate-limiting enzyme tryptophan hydroxylase-1 (TPH-1) decreased, while the expression of serotonin transporter (SERT) increased. Although there was only a small change in 5-HT after the normalization of bone mass caused by the recolonization microbiota in GF mice, it cannot be ruled out that the gut microbiome had a potential mechanism for regulating bone mass. Thus, further evidence suggests that the gut microbiome may influence the progression of OP with the help of the immune system or neurotransmitter levels.
Gut microbiome as a target for osteoporosis treatment
The current drugs for the treatment of OP are divided into three main categories: bone mineralizing drugs (calcium, etc.); bone forming drugs (parathyroid hormone, fluoride, etc.); and bone absorption inhibitors (oestrogen arthroplasty therapy, bisphosphonate, etc.).34 All these drugs have achieved good outcomes in the treatment of OP, but some potential side effects are also highlighted. Calcium supplementation should be appropriate, and taking excessive amounts could lead to side effects such as kidney stones, hypercalcaemia, and myocardial infarction.35 Treatment with parathyroid hormone may incur higher medical costs and increase the risk of hypercalcaemia, therefore it is not recommended to be used for more than two years.36 A systematic review found that raloxifene is prohibited in patients with a history of venous embolism or thrombotic tendencies, such as long-term bedridden or sedentary patients.37
As disorder of the gut microbiome is one of the important pathogeneses of OP, development of OP treatment strategies targeting the gut microbiome may be promising. In fact, as an alternative therapy to the current complications of OP drugs, treatment for the gut microbiome can safely and effectively alleviate the development of OP. Among these treatments, diet, antibiotics, and probiotics are recommended.
Diet
Bone is considered a nutritionally regulated tissue and diet has a great impact on bone health throughout its life cycle, which is often overlooked. On the other hand, diet is the major factor in determining the type and proportion of microorganisms in host organisms.38 Instead, the gut microbiome contributes to the proteins or enzymes related to digestion and energy metabolism, as it ferments undigested nutrients into SCFAs, resulting in a decrease in gut pH and an increase in intestinal permeability, as well as increased absorption of minerals such as calcium.23 Given this association, diet may be considered as an important confounding factor when assessing the impact of changes in gut microbiome on bone health.
There is a belief that all diets are fermented by the gut microbiome, and that the fibres are decomposed into SCFAs to reduce intestinal pH, which then affects bone minerals. This explanation, however, may be too simplistic, and the underlying mechanisms are varied. There are special foods called prebiotics that cannot be hydrolyzed and absorbed by the digestive system.24 Prebiotics can be classified into two categories: oligosaccharides and polysaccharides. Oligosaccharides mainly include fructooligosaccharides (FOS), galacto-oligosaccharides (GOS), and xylooligosaccharides, while polysaccharides mainly include inulin and microalgae.39 Prebiotics, especially non-digestible oligosaccharides (NDOs), can selectively stimulate, activate, and proliferate beneficial bacteria in the gastrointestinal tract. This effect inhibits the survival and development of harmful bacteria, promotes the metabolism of beneficial bacteria, secretes more SCFAs represented by butyric acid in the gut tract, and then promotes calcium absorption.40,41 Kleessen et al42 found that the effects of dietary FOS on the alterations of intestinal mucosa and the thickness and composition of the colonic epithelial mucus layer were mediated by the gut microbiome rather than changes in the mucosal structure itself. Compared with GF mice, mice fed with oligofructose-inulin mixture have higher villi and deeper crypts, which represent an indicator of the stability of the intestinal mucosal barrier. Meanwhile, the number of probiotics including bifidobacteria and Bacteroides-Prevotella increased. This study further demonstrates that a healthy diet will promote a stable gut microbiome, maintain intestinal health, and improve the gut's absorption function by increasing the number of probiotics. In addition, dietary FOS can increase the bioavailability of isoflavones, which have a similar structure to oestrogen and alleviate the bone mineral content and bone density decreased by OVX. Although there are no further studies on this subject, it has still been proved that FOS has a synergistic effect on the prevention of OP in OVX mice.43 It has been further verified that diet can increase the absorption of bone mineral content in intestines and regulate BMD.
Although these studies did not observe a direct effect of diet on bones, they showed that a healthy diet represented by prebiotics will have beneficial effects on the whole gut microbiota, maintain intestinal homeostasis, improve intestinal absorption, and play a positive role in regulating bone loss.
Antibiotics
Antibiotics are commonly used as anti-inflammatory drugs. Long-term use of antibiotics or in a GF environment can greatly reduce the biodiversity of the gut microbiome, increase intestinal metabolites and serotonin, and then alter the absorption rates of bone-related minerals.44 Scientists have recently discovered that antibiotics can not only change the structure of the microbial community, but also affect biological metabolism.45,46 Cox et al45 analyzed the gut microbiome and bone histomorphometry of mice given low-dose penicillin (LDP) during weaning and at birth. As expected, compared with the mice without LDP, levels of Lactobacillus and segmented filamentous bacteria (SFB) in the female mice with LDP at multiple timepoints after birth were much lower. The female mice with LDP had significantly increased BMD. Interestingly, changes in BMD did not differ significantly in male mice. Similarly, Cho et al46 established an obesity model in which four different formulae of low-dose antibiotics (penicillin, vancomycin, penicillin plus vancomycin, and chlortetracycline) were administered to young mice at weaning. All low-dose antibiotics increased BMD in varying degrees within three weeks, however there was no significant difference in BMD after seven weeks. The 16S sequencing results showed that the proportion of Firmicutes and Lachnospiraceae treated with low-dose antibiotics for mice was significantly increased. Through the in-depth study of intestinal, liver, and adipose tissues, it is clear that the antibiotic activity mainly affected the microflora of the downstream liver, which affected bone development through the metabolism of SCFAs, blood lipids, and cholesterol.
The administration of antibiotics to newbirth or weaning mice (a critical developmental window) can lead to lasting changes in gut microbiome and long-term bone mass changes in mice. At present, only a small number of antibiotic types have been tested, such as penicillin, vancomycin, and chlortetracycline, but more studies are worth analyzing and provide probes for identifying specific antibiotics for the treatment of OP in the future.
Probiotics
Probiotics, mainly referring to the beneficial bacteria in the gut microbiome, are beneficial and harmless bacteria for humans.47 As the second largest human genome, the expression of multiple genes in the gut microbiome is mainly performed by probiotics. These genes encode the regulation of almost all gut activity and the activity in some other organs. Among them, the most prominent function related to the intestinal tract is the regulation of SCFAs, branched-chain fatty acids, and vitamins. Therefore, probiotics are the key components of the gut microbiome for regulating bone metabolism.48
The effects of probiotics on bone mass in animals have been extensively reported.49,50 In a randomized, placebo-controlled, and double-blinded trial, 90 patients with low BMD were randomly divided into two groups. One group orally received Lactobacillus reuteri (L. reuteri 6475) daily, while the other group received a placebo. At the end of the trial, the loss of total BMD and trabecular volume fraction was significantly reduced in patients taking L. reuteri 6475 compared to the placebo group.51 Also, Lambert et al52 discovered a new red clover extract (RCE), which was rich in isoflavone aglycones and probiotic lactic acid bacteria. They found that this could improve bone turnover and promote the production of oestrogen metabolites in 78 patients with postmenopausal OP. The final result showed that the bone loss in the RCE group was two-times lower than that in the control group. These clinical trials51,52 further demonstrated the clinical feasibility of probiotic supplementation in the prevention of bone loss and OP.
Taken together, the gut microbiome can be positively regulated by probiotics such as Bacteroidetes, Firmicutes, Lactobacillus, and SFB, and this method can be an adjuvant therapy for OP in the future.
Other factors
The positive effects of exercise on bone mass have long been confirmed by numerous studies.53,54 As usual, we emphasize the effect of drugs on the gut microbiome but neglect the role of exercise. A new clinical trial55 suggests that exercise can also affect the health of the gut microbiome. The authors recruited 32 volunteers for six weeks of endurance-based exercise training. Sequencing analysis showed that exercise increased the fecal concentration of SCFAs. Once the exercise stops, the gut microbiome will soon be reversed. Beta diversity analysis conclusively showed that exercise can increase the diversity of the gut microbiome, which was positively correlated with host health and other related indicators. Whether exercise actually affects bone mass through gut microbiome is unknown, but exercise is certainly of clinical significance.
Fecal microbiota transplantation (FMT) also has great potential in the treatment of OP.56 FMT was first reported to be effective in the treatment of Clostridioides difficile infection (CDI).57 In recent years, FMT has been widely used in treating various diseases such as Crohn's disease, metabolic syndrome, diabetes, and nervous system diseases.58 Different from single or mixed bacteria, FMT can restore stabilization of the gut microbiome more quickly and efficiently. Due to its abundant species and number, the original active microbiome can be retained to the maximum extent and the gut microbiome can be substantially improved.
In conclusion, the development of OP is the result of the combination of genetics, environment, nutrition, and other factors such as activity loss and bone loss. Many studies have proved that there are highly plausible links between the gut microbiome and OP, so human gut microbiota might be an important regulator in OP aetiology. Several mechanisms have been proposed to explain the role of gut microbiome in the aetiology of OP, such as bone metabolism, immune responses, and changes in microbial composition (Figure 1). However, none of these can fully explain the causes of OP.
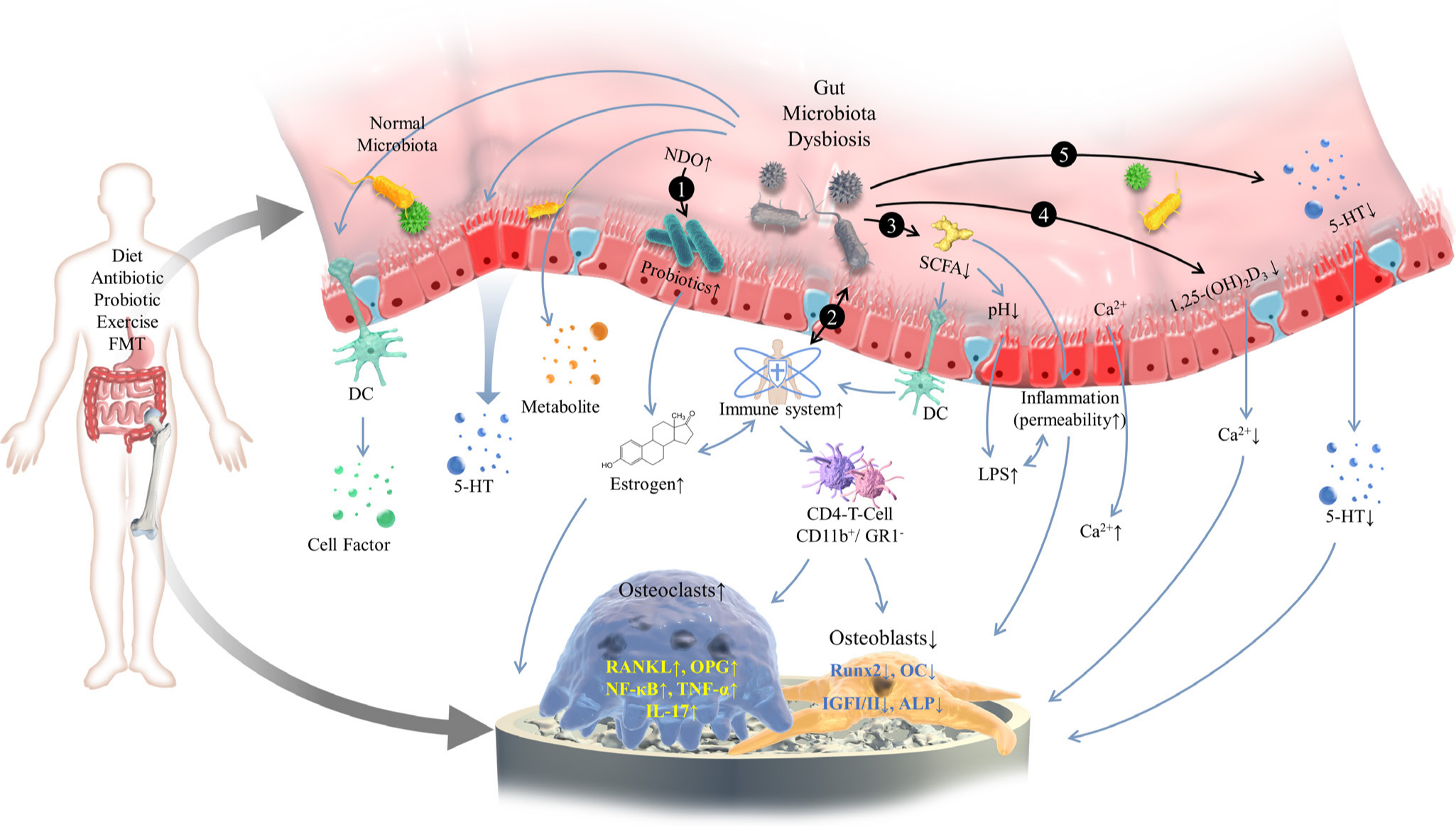
Fig. 1
A schematic diagram for the mechanism of gut microbiome in the development of osteoporosis. The gut microbiome is mainly influenced by diet, antibiotics, and probiotics. The gut microbiome exerts a notable influence on regulating bone mass via a variety of mechanisms including: 1) affecting beneficial bacteria, increasing oestrogen bioavailability, and then regulating bone mass with the aid of prebiotics; 2) increasing the expression of inflammatory cytokine responses by the immune system; 3) producing metabolites of gut microbiome such as short-chain fatty acids; 4) changing intestinal permeability and increasing the promoting effect of vitamin D on bone mineral absorption; and 5) impacting the gut-brain axis and the level of endocrines. 5-HT, 5-hydroxytryptamine; ALP, alkaline phosphatase; DC, dendritic cell; FMT, fecal microbiota transplantation; GR1-, granulocytes 1; IGF-I/II, insulin like growth factor-I/II; IL-17, interleukin-17; LPS, lipopolysaccharide; NDO, non-digestible oligosaccharide; NF-κB, nuclear factor kappa-B; OC, osteocalcin; OPG, osteoprotegerin; RANKL, receptor activator of NF-κB ligand; Runx2, runt-related transcription factor 2; SCFA, short-chain fatty acid; TNF-α, tumour necrosis factor-α.
With the rapid development of modern sequencing technology, we can find out the composition of microbial communities related to environmental factors (diseases, etc.) more comprehensively and accurately. However, many challenges still remain in this area. The main challenge nowadays is to confirm the role of gut microbiota in OP and to investigate how gut microbiota participates in bone metabolism. The revelation of these results may be of great significance for us to analyze the early pathogenesis of OP. Meanwhile, the effects of diet, antibiotics, probiotics, and FMT on OP are to be studied as a potential future treatment strategy. These therapies may help to avoid the adverse effects of existing drugs in the treatment of OP and provide ideas for new clinical treatments in the future.
References
1. Watts NB , Bilezikian JP , Camacho PM , et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis . Endocr Pract . 2010 ; 16 ( Suppl 3 ): 1 – 37 . Crossref PubMed Google Scholar
2. Tang CH . Osteoporosis: from molecular mechanisms to therapies . Int J Mol Sci . 2020 ; 21 ( 3 ): 714 . Crossref PubMed Google Scholar
3. Peng X , Wu X , Zhang J , et al. The role of CKIP-1 in osteoporosis development and treatment . Bone Joint Res . 2018 ; 7 ( 2 ): 173 – 178 . Crossref PubMed Google Scholar
4. Zheng W , Liu C , Lei M , et al. Evaluation of common variants in the CNR2 gene and its interaction with abdominal obesity for osteoporosis susceptibility in Chinese post-menopausal females . Bone Joint Res . 2019 ; 8 ( 11 ): 544 – 549 . Crossref PubMed Google Scholar
5. Qin J , Li R , Raes J , et al. A human gut microbial gene catalogue established by metagenomic sequencing . Nature . 2010 ; 464 ( 7285 ): 59 – 65 . Crossref PubMed Google Scholar
6. Salgaço MK , Oliveira LGS , Costa GN , Bianchi F , Sivieri K . Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus . Appl Microbiol Biotechnol . 2019 ; 103 ( 23-24 ): 9229 – 9238 . Crossref PubMed Google Scholar
7. Xu H , Wang X , Feng W , et al. The gut microbiota and its interactions with cardiovascular disease . Microb Biotechnol . 2020 ; 13 ( 3 ): 637 – 656 . Crossref PubMed Google Scholar
8. Chen MF , Chang CH , Chiang-Ni C , et al. Rapid analysis of bacterial composition in prosthetic joint infection by 16S rRNA metagenomic sequencing . Bone Joint Res . 2019 ; 8 : 367 – 377 . Crossref PubMed Google Scholar
9. Zhu B , Wang X , Li L . Human gut microbiome: the second genome of human body . Protein Cell . 2010 ; 1 ( 8 ): 718 – 725 . Crossref PubMed Google Scholar
10. Charles JF , Ermann J , Aliprantis AO . The intestinal microbiome and skeletal fitness: connecting bugs and bones . Clin Immunol . 2015 ; 159 ( 2 ): 163 – 169 . Crossref PubMed Google Scholar
11. Uchida Y , Irie K , Fukuhara D , et al. Commensal microbiota enhance both osteoclast and osteoblast activities . Molecules . 2018 ; 23 ( 7 ): 1517 . Crossref PubMed Google Scholar
12. Tavakoli S , Xiao L . Depletion of intestinal microbiome partially rescues bone loss in sickle cell disease male mice . Sci Rep . 2019 ; 9 ( 1 ): 8659 . Crossref PubMed Google Scholar
13. Sjögren K , Engdahl C , Henning P , et al. The gut microbiota regulates bone mass in mice . J Bone Miner Res . 2012 ; 27 ( 6 ): 1357 – 1367 . Crossref PubMed Google Scholar
14. Li JY , Chassaing B , Tyagi AM , et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics . J Clin Invest . 2016 ; 126 ( 6 ): 2049 – 2063 . Crossref PubMed Google Scholar
15. Scholz-Ahrens KE , Ade P , Marten B , et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure . J Nutr . 2007 ; 137 ( 3 Suppl 2 ): 838S – 846S . Crossref PubMed Google Scholar
16. D'Amelio P , Sassi F . Gut microbiota, immune system, and bone . Calcif Tissue Int . 2018 ; 102 ( 4 ): 415 – 425 . Crossref PubMed Google Scholar
17. Hadjidakis DJ , Androulakis II . Bone remodeling . Ann N Y Acad Sci . 2006 ; 1092 : 385 – 396 . Crossref PubMed Google Scholar
18. Sanghani-Kerai A , Osagie-Clouard L , Blunn G , Coathup M . The influence of age and osteoporosis on bone marrow stem cells from rats . Bone Joint Res . 2018 ; 7 ( 4 ): 289 – 297 . Crossref PubMed Google Scholar
19. Al-Suhaimi EA , Al-Jafary MA . Endocrine roles of vitamin K-dependent- osteocalcin in the relation between bone metabolism and metabolic disorders . Rev Endocr Metab Disord . 2019 ; 21 ( 1 ): 117 – 125 . Crossref PubMed Google Scholar
20. Indo Y , Takeshita S , Ishii KA , et al. Metabolic regulation of osteoclast differentiation and function . J Bone Miner Res . 2013 ; 28 ( 11 ): 2392 – 2399 . Crossref PubMed Google Scholar
21. Chen Y , Strasser S , Cao Y , Wang KS , Zheng S . Calcium intake and hypertension among obese adults in United States: associations and implications explored . J Hum Hypertens . 2015 ; 29 ( 9 ): 541 – 547 . Crossref PubMed Google Scholar
22. Christakos S , Dhawan P , Porta A , Mady LJ , Seth T . Vitamin D and intestinal calcium absorption . Mol Cell Endocrinol . 2011 ; 347 ( 1-2 ): 25 – 29 . Crossref PubMed Google Scholar
23. Abrams SA , Griffin IJ , Hawthorne KM , et al. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents . Am J Clin Nutr . 2005 ; 82 ( 2 ): 471 – 476 . Crossref PubMed Google Scholar
24. Blaut M . Relationship of prebiotics and food to intestinal microflora . Eur J Nutr . 2002 ; 41 ( Suppl 1 ): 1 – 16 . Crossref PubMed Google Scholar
25. Morozumi A . High concentration of sodium butyrate suppresses osteoblastic differentiation and mineralized nodule formation in ROS17/2.8 cells . J Oral Sci . 2011 ; 53 ( 4 ): 509 – 516 . Crossref PubMed Google Scholar
26. Ooi JH , Li Y , Rogers CJ , Cantorna MT . Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis . J Nutr . 2013 ; 143 ( 10 ): 1679 – 1686 . Crossref PubMed Google Scholar
27. Nance DM , Sanders VM . Autonomic innervation and regulation of the immune system (1987-2007) . Brain Behav Immun . 2007 ; 21 ( 6 ): 736 – 745 . Crossref PubMed Google Scholar
28. Zhao W , Liu Y , Cahill CM , et al. The role of T cells in osteoporosis, an update . Int J Clin Exp Pathol . 2009 ; 2 ( 6 ): 544 – 552 . PubMed Google Scholar
29. Saidenberg-Kermanac'h N , Cohen-Solal M , Bessis N , De Vernejoul MC , Boissier MC . Role for osteoprotegerin in rheumatoid inflammation . Joint Bone Spine . 2004 ; 71 ( 1 ): 9 – 13 . Crossref PubMed Google Scholar
30. Wittrant Y , Théoleyre S , Chipoy C , et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis . Biochim Biophys Acta . 2004 ; 1704 ( 2 ): 49 – 57 . Crossref PubMed Google Scholar
31. Azuma Y , Kaji K , Katogi R , Takeshita S , Kudo A . Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts . J Biol Chem . 2000 ; 275 ( 7 ): 4858 – 4864 . Crossref PubMed Google Scholar
32. Pawlak D , Domaniewski T , Znorko B , et al. The impact of peripheral serotonin on leptin-brain serotonin axis, bone metabolism and strength in growing rats with experimental chronic kidney disease . Bone . 2017 ; 105 : 1 – 10 . Crossref PubMed Google Scholar
33. Bliziotes M , Eshleman A , Burt-Pichat B , et al. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells . Bone . 2006 ; 39 ( 6 ): 1313 – 1321 . Crossref PubMed Google Scholar
34. Compston JE , McClung MR , Leslie WD . Osteoporosis . The Lancet . 2019 ; 393 ( 10169 ): 364 – 376 . Crossref PubMed Google Scholar
35. Cano A , Chedraui P , Goulis DG , et al. Calcium in the prevention of postmenopausal osteoporosis: EMAS clinical guide . Maturitas . 2018 ; 107 : 7 – 12 . Crossref PubMed Google Scholar
36. Curry SJ , Krist AH , et al. US Preventive Services Task Force . Screening for osteoporosis to prevent fractures: US preventive services Task force recommendation statement . JAMA . 2018 ; 319 ( 24 ): 2521 – 2531 . Crossref PubMed Google Scholar
37. Adomaityte J , Farooq M , Qayyum R . Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis . Thromb Haemost . 2008 ; 99 ( 2 ): 338 – 342 . PubMed Google Scholar
38. Weaver CM , Diet WCM . Diet, gut microbiome, and bone health . Curr Osteoporos Rep . 2015 ; 13 ( 2 ): 125 – 130 . Crossref PubMed Google Scholar
39. Valcheva R , Dieleman LA . Prebiotics: definition and protective mechanisms . Best Pract Res Clin Gastroenterol . 2016 ; 30 ( 1 ): 27 – 37 . Crossref PubMed Google Scholar
40. Delcour JA , Aman P , Courtin CM , Hamaker BR , Verbeke K . Prebiotics, fermentable dietary fiber, and health claims . Adv Nutr . 2016 ; 7 ( 1 ): 1 – 4 . Crossref PubMed Google Scholar
41. Swennen K , Courtin CM , Delcour JA . Non-digestible oligosaccharides with prebiotic properties . Crit Rev Food Sci Nutr . 2006 ; 46 ( 6 ): 459 – 471 . Crossref PubMed Google Scholar
42. Kleessen B , Hartmann L , Blaut M . Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa-associated bifidobacteria in gnotobiotic rats . Br J Nutr . 2003 ; 89 ( 5 ): 597 – 606 . Crossref PubMed Google Scholar
43. Ohta A , Uehara M , Sakai K , et al. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice . J Nutr . 2002 ; 132 ( 7 ): 2048 – 2054 . Crossref PubMed Google Scholar
44. Yan J , Charles JF , Microbiome G . Gut microbiome and bone: to build, destroy, or both? Curr Osteoporos Rep . 2017 ; 15 ( 4 ): 376 – 384 . Crossref PubMed Google Scholar
45. Cox LM , Yamanishi S , Sohn J , et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences . Cell . 2014 ; 158 ( 4 ): 705 – 721 . Crossref PubMed Google Scholar
46. Cho I , Yamanishi S , Cox L , et al. Antibiotics in early life alter the murine colonic microbiome and adiposity . Nature . 2012 ; 488 ( 7413 ): 621 – 626 . Crossref PubMed Google Scholar
47. Sánchez B , Delgado S , Blanco-Míguez A , et al. Probiotics, gut microbiota, and their influence on host health and disease . Mol Nutr Food Res . 2017 ; 61 ( 1 ): 1600240 . Crossref PubMed Google Scholar
48. Louis P , Flint HJ , Michel C . How to manipulate the microbiota: prebiotics . Adv Exp Med Biol . 2016 ; 902 : 119 – 142 . Crossref PubMed Google Scholar
49. Chen YC , Greenbaum J , Shen H , Deng HW . Association between gut microbiota and bone health: potential mechanisms and prospective . J Clin Endocrinol Metab . 2017 ; 102 ( 10 ): 3635 – 3646 . Crossref PubMed Google Scholar
50. McCabe LR , Parameswaran N . Advances in probiotic regulation of bone and mineral metabolism . Calcif Tissue Int . 2018 ; 102 ( 4 ): 480 – 488 . Crossref PubMed Google Scholar
51. Nilsson AG , Sundh D , Bäckhed F , Lorentzon M . Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial . J Intern Med . 2018 ; 284 ( 3 ): 307 – 317 . Crossref PubMed Google Scholar
52. Lambert MNT , Thybo CB , Lykkeboe S , et al. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial . Am J Clin Nutr . 2017 ; 106 ( 3 ): 909 – 920 . Crossref PubMed Google Scholar
53. Nordström A , Karlsson C , Nyquist F , et al. Bone loss and fracture risk after reduced physical activity . J Bone Miner Res . 2005 ; 20 ( 2 ): 202 – 207 . Crossref PubMed Google Scholar
54. Xu J , Lombardi G , Jiao W , Banfi G . Effects of Exercise on Bone Status in Female Subjects, from Young Girls to Postmenopausal Women: An Overview of Systematic Reviews and Meta-Analyses . Sports Med . 2016 ; 46 ( 8 ): 1165 – 1182 . Crossref PubMed Google Scholar
55. Allen JM , Mailing LJ , Niemiro GM , et al. Exercise alters gut microbiota composition and function in lean and obese humans . Med Sci Sports Exerc . 2018 ; 50 ( 4 ): 747 – 757 . Crossref PubMed Google Scholar
56. Borody TJ , Khoruts A . Fecal microbiota transplantation and emerging applications . Nat Rev Gastroenterol Hepatol . 2012 ; 9 ( 2 ): 88 – 96 . Crossref PubMed Google Scholar
57. Lawson PA , Citron DM , Tyrrell KL , Finegold SM . Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O'Toole 1935) Prévot 1938 . Anaerobe . 2016 ; 40 : 95 – 99 . Crossref PubMed Google Scholar
58. Borody TJ , Eslick GD , Clancy RL . Fecal microbiota transplantation as a new therapy: from Clostridioides difficile infection to inflammatory bowel disease, irritable bowel syndrome, and colon cancer . Curr Opin Pharmacol . 2019 ; 49 : 43 – 51 . Crossref PubMed Google Scholar
Author contributions
S. Li: Reviewed the literature, Wrote and edited the paper.
Y. Mao: Reviewed the literature, Wrote and edited the paper.
F. Zhou: Oversaw the review and writing process.
H. Yang: Oversaw the review and writing process.
Q. Shi: Supervised the study, Reviewed the literature, Reviewed and edited the paper.
B. Meng: Supervised the study, Reviewed the literature, Reviewed and edited the paper.
S. Li and Y. Mao contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81772312, 891972059), the Natural Science Foundation of Jiangsu Province (BK2019668, BK20151210), and the Suzhou Science and Technology Project (SS2019054).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









