Abstract
Aims
This study aimed to evaluate calprotectin in synovial fluid for diagnosing chronic prosthetic joint infection (PJI) .
Methods
A total of 63 patients who were suspected of PJI were enrolled. The synovial fluid calprotectin was tested by an enzyme-linked immunosorbent assay (ELISA). Laboratory test data, such as ESR, CRP, synovial fluid white blood cells (SF-WBCs), and synovial fluid polymorphonuclear cells (SF-PMNs), were documented. Chi-squared tests were used to compare the sensitivity and specificity of calprotectin and laboratory tests. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was calculated to determine diagnostic efficacy.
Results
The median calprotectin level was 776 μg/ml (interquartile range (IQR) 536.5 to 1132) in the PJI group and 54.5 μg/ml (IQR, 38.75 to 78.25) in the aseptic failure (AF) group (p < 0.05). Using a threshold of 173 ug/ml, the sensitivity was 95.2%, with a 97.6% specificity, and the AUC was 0.993. The sensitivity of calprotectin of the antibiotic-treated PJI group was 100% versus 90.9% of the non-antibiotic-treated PJI group. Although 47.6% (ten cases) of the patients in the PJI group received antibiotics before aspiration, the diagnostic efficacy of calprotectin was not affected. The sensitivity and specificity of ESR, CRP, SF-WBCs, and SF-PMNs ranged from 76.2% to 90.5% and 64.3% to 85.7%, respectively.
Conclusion
Calprotectin in synovial fluid has great diagnostic efficacy for PJI diagnosisand outperformed ESR, CRP, SF-WBCs, and SF-PMNs.
Cite this article: Bone Joint Res 2020;9(8):450–456.
Article focus
The present work focuses on the role of calprotectin in synovial fluid for diagnosing chronic PJI of the hip and knee.
Key messages
The sensitivity and specificity of synovial calprotectin was 95.2% and 97.6%, respectively, at a threshold of 173 µg/ml, and the AUC was 0.993. Calprotectin in synovial fluid has great diagnostic efficacy for PJI of the hip and knee and outperformed ESR, CRP, SF-WBCs, and SF-PMNs.
Strengths and limitations
-
Strengths: This study showed that calprotectin in synovial fluid has great diagnostic efficacy for chronic PJI and outperformed ESR, CRP, SF-WBCs, and SF-PMNs.
-
Limitations: This study involved a single centre, so the sample size was relatively small; only 63 cases were enrolled (with 21 cases in the PJI group and 42 cases in the AF group).
Introduction
Prosthetic joint infection (PJI) is a devastating complication after arthroplasty.1 According to the USA Nationwide Inpatient Sample (NIS) database, PJI was the most common cause of revision after primary total knee arthroplasty (15,233 revisions; 25.2%).2 In clinical practice, diagnosing chronic PJI is difficult due to atypical symptoms, which may delay diagnosis and proper treatment, leading to infection with delayed healing, severe bone defects, and joint dysfunction. The timely and correctly diagnosis of chronic PJI is critical for treatment and goodclinical prognosis.
Although many guidelines, such as the Musculoskeletal Infection Society (MSIS) criteria and the Infectious Diseases Society of America (IDSA) guidelines,3-6 have been published for PJI, the diagnosis of PJI is still challenging. Currently, a diagnosis of PJI is made based on the clinical manifestations of patients and laboratory tests, such as serology,7 joint fluid analysis, intraoperative frozen sections, and tissue bacterial culture. Although microbial culture has been considered the "gold standard" for the diagnosis of PJI, the positivity rate of microbial culture was only 60% to 70%.8,9 The emerging molecular diagnosis showed a promising role in PJI diagnosis, however, the issue of contamination has not yet been addressed.10-12 In addition, the specificity of blood markers is affected by obesity, surgical trauma, and other factors.7,13,14 Synovial fluid white blood cell (SF-WBC) counts and classification are effective tools for diagnosing PJI, but their diagnostic value remains controversial.15,16 The results of intraoperative frozen sections depend on pathologists’ experience, especially when preoperative serology and synovial fluid analysis cannot achieve a clear diagnosis.17 Chronic PJI can be caused by low-virulence pathogens, approximately 4% of which caused normal levels of the ESR and CRP.18,19
Recent efforts that have aimed to improve the accuracy of PJI diagnosis have focused on synovial fluid biomarkers.20 The assessment of inflammatory biomarkers in synovial fluid, such as alpha defensin and leucocyte esterase, could facilitate the diagnosis of focal infections and has demonstrated better diagnostic efficacy than routinely available clinical laboratory tests.21-23 However, due to relative high costs and poor applicability, these biomarkers have not yet been included in routine clinical practice. Calprotectin (also named L1, cystic fibrosis antigen, calgranulin A/calgranulin B, and protein MRP-8/MRP-14) constitutes more than 40% of the soluble cytosolic protein content in neutrophilic granulocytes, is primarily secreted by neutrophilic granulocytes and monocytes at sites of local inflammation, and modulates the migration of leucocytes. The calprotectin levels in plasma, urine, gingival crevicular fluid, synovial fluid, and faeces are markedly elevated in certain inflammatory or infectious conditions, such as acute appendicitis, pyelonephritis, candidal periodontitis, abscesses, and inflammatory bowel disease, indicating its role as a reliable marker for the diagnosis of these diseases.24-28 Recent studies have also shown that calprotectin has a role in PJI diagnosis.29,30 However, its role in diagnosing chronic PJI has not yet been fully elucidated and these results need to be confirmed in additional studies. Therefore, this study used enzyme-linked immunosorbent assay (ELISA) methods to detect the concentration of calprotectin in synovial fluid and to study its diagnostic value in chronic infections in prosthetic hip and knee joints.
Methods
This study was approved by our Institutional Review Board. From January 2014 to December 2016, 63 patients suspected of PJI were enrolled, 42 patients were confirmed to have aseptic failure (AF), and 21 patients were diagnosed with PJI. The characteristics of the 63 patients with AF or PJI are shown in Table I. A diagnosis of PJI was made based on the MSIS criteria: one of the following must have been met to diagnose PJI: 1) a sinus tract communicating with the prosthesis; 2) a pathogen isolated by culture from two separate tissue or fluid samples obtained from the affected prosthetic joint; 3) four of the following six criteria exist: a) elevated ESR and CRP (ESR > 30 mm/hour; CRP > 10 mg/L); b) elevated synovial fluid WBC count ( > 3,000 cells/μL); c) elevated synovial fluid neutrophil percentage ( > 65%); d) presence of purulence in the affected joint; e) isolation of a microorganism in one periprosthetic tissue or fluid culture; f) > five neutrophils per high-powered field in five high-power fields observed from histological analysis of periprosthetic tissue at × 400 magnification. The course of PJI > three months was defined as a chronic PJI.
Table I.
Characteristics of patients with aseptic failure (AF) or prosthetic joint infection (PJI).
| Characteristic | PJI (n = 21) | AF (n = 42) | p-value* |
|---|---|---|---|
| Sex, n,(M/F) | 4/17 | 17/25 | 0.089 |
| Age (yrs) | < 0.001 | ||
| < 60 | 7 | 23 | |
| 60 to 70 | 8 | 9 | |
| > 70 | 6 | 10 | |
| BMI (kg/m2) | 0.04 | ||
| < 24 | 8 | 14 | |
| 24 to 28 | 10 | 20 | |
| > 28 | 3 | 8 | |
| Hypertension | 8 | 22 | 0.29 |
| Diabetes | 7 | 18 | 0.47 |
| Joint involved | 0.00 | ||
| Hip | 15 | 30 | |
| Knee | 6 | 12 | |
| Preoperative antibiotic treatment | 15 | 5 | < 0.001 |
| Administration of immunosuppressive drugs | 12 | 22 | 0.721 |
-
*
Chi-squared test was performed.
-
BMI, Body mass index
The inclusion criteria were as follows: 1) patients with suspected PJI; 2) patients who underwent laboratory tests for PJI diagnosis according to MSIS criteria; and 3) sufficient synovial fluid (without blood contamination) to perform calprotectin analysis. To avoid the interference of other inflammatory processes or pre-existing conditions on elevated inflammatory markers, patients with inflammation at sites other than prosthesis joint, and patients with inflammatory disease like rheumatoid arthritis (RA) were excluded.
Synovial fluid calprotectin results were compared between the PJI and AF groups. A uniform preoperative work-up for PJI, which included the serum ESR and CRP and preoperative or intraoperative aspiration for synovial fluid culture and calprotectin analysis, was performed on all patients.
Synovial fluid was either preoperatively or intraoperatively obtained for culture and calprotectin analysis by direct needle aspiration, to examine preoperative conditions and to avoid blood contamination.31 Synovial fluid was examined for WBCs and PMNs using a haematology analyzer (Symex XE-5000 haematology analyzer, Symex, Japan). Synovial fluid and tissue specimens were processed according to standard laboratory protocols; aerobic cultures were incubated for two days, and anaerobic cultures were incubated for five days. To detect slowly growing and less virulent organisms, the incubation time was extended to 14 days. Intraoperative tissue specimens were also sent for the histological analysis of frozen sections.
For calprotectin testing, synovial fluid samples were placed in a purple EDTA Tubes (Blood Collection Tubes, Becton-Dickinson, Franklin Lakes, New Jersey, USA). The samples were centrifuged at 400 g for ten minutes to obtain synovial fluid devoid of cells. The supernatants were transferred to 2 ml sterile cryotubes and stored at -80℃ within 30 minutes after collection before detection. Immunoassays for synovial fluid calprotectin were generated with reagents from Hycult Biotech (Uden, the Netherlands) and were measured in duplicate by standard ELISAs.
Statistical analysis
Continuous variables of non-normal distributionwere described as median and inter-quartile range (IQR). Mann-Whitney U test was used to analyze the statistical significance, and chi-squared test was used to compare the sensitivity and specificity of laboratory tests (ESR, CRP, SF-WBCs, and SF-PWNs) and calprotectin. The receiver operating characteristic curve (ROC) and area under the curve (AUC) were used to compare the diagnostic efficacy. The optimal threshold was determined according to Youden’s Index (sensitivity + specificity - 1). A p-value ≤ 0.05 was considered statistically significant. Statistical analysis was performed with GraphPad Prism 7.0 and SPSS 21.0 (IBM, Armonk, New York, USA).
Results
The AF group included 16 males and 26 females, with a mean age of 57 years (41 to 86); 30 patients underwent total hip arthroplasty (THA), and 12 patients underwent TKA. The causes of failure included 33 cases of aseptic loosening, four cases of periprosthetic fracture, two cases of prosthetic dislocation, and three cases of failure due to other reasons (e.g. ankylosis,). Two patients in the AF group had a positive result from the bacterial culture (only one positive culture was obtained from each patient), which was considered a false positive. These two cases isolated Staphylococcus epidermidis on solid medium. The bacterial culture results of all other preoperative and intraoperative samples from these two cases were negative. No antibiotics were administered to these patients, as the surgeon (WZ) considered the results to be false positives. There have been no signs of infection, and no further surgeries have been performed on these two patients at the last follow-up visit with their surgeon.
The patients diagnosed with PJI included four males and 17 females, with a mean age of 64 years (54 to 83); 15 patients underwent THA, and six patients underwent TKA. Multiple periprosthetic tissue specimens were also obtained intraoperatively (mean 4.4; 2 to 6). A total of 15 patients had positive culture results (eight cases had a positive synovial fluid culture), while six had negative culture results in PJI group. The isolated organisms included S. epidermidis (six), methicillin-resistant Staphylococcus aureus (MRSA) (two), methicillin-sensitive S. aureus MSSA) (one), Escherichia coli (one), Pseudomonas aeruginosa (one), Streptococcus agalactiae (one), Staphylococcus hominis aureus (one), Staphylococcus saprophyticus (one), and Candida tropicalis (one). Six patients who received preoperative antibiotic treatment had a negative bacterial culture result and were diagnosed with PJI according to the MSIS criteria.
The MSIS-related clinical and laboratory test values for patients with PJI and AF are shown in Table II. The synovial fluid was obtained preoperatively in 23 cases and intraoperatively in 56 cases (some samples were obtained both preoperatively and intraoperatively). The volume of synovial fluid ranged from 0.5 ml to greater than 2 ml. The median of synovial calprotectin in PJI group (776 μg/ml (IQR 536.5 to 1132)) was higher than that of AF group (54.5 μg/ml (IQR 38.75 to 78.25)) (p < 0.001, Mann-Whitney U test) (Figure. 1). There was also a difference in the serum CRP, ESR, synovial WBC count, and neutrophil differential between the groups (p < 0.001 for all, Mann-Whitney U test) (Table III).
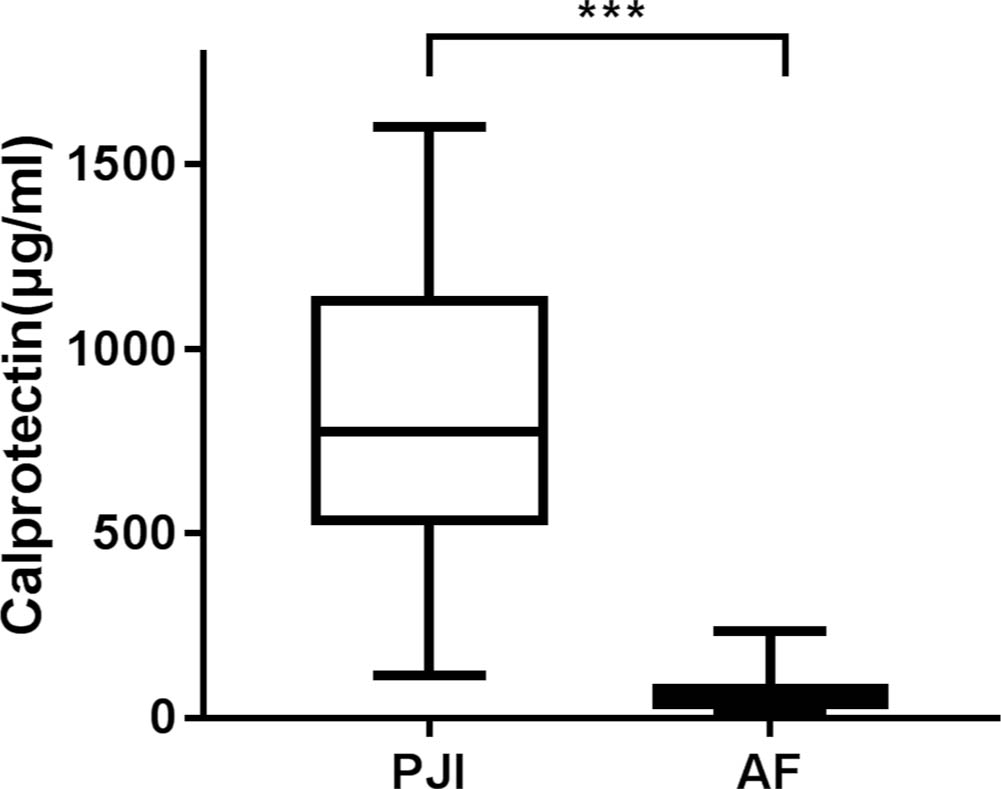
Fig. 1
The median of synovial calprotectin in prosthetic joint infection (PJI) group was higher than that of aseptic failure (AF) group. ***p <0.001, Mann-Whitney U test.
Table II.
Results of Musculoskeletal Infection Society (MSIS)-related clinical and laboratory tests and calprotectin.
| Variable | PJI (n = 21) (median, IQR) | AF (n = 42) (median, range) | p-value* |
|---|---|---|---|
| Sinus | 4 | 0 | < 0.001 |
| ESR (mm/h) | 52 (10 to 120) | 18 (2 to 67) | < 0.001 |
| CRP (mg/ml) | 25.7 (3.3 to 139) | 5 (1.3 to 51) | < 0.001 |
| SF-WBC (cells/μl) | 9,738 (100 to 62,051) | 1,288 (64 to 9,570) | < 0.001 |
| SF-PMN (%) | 86.4 (41 to 97.6) | 45.1 (11 to 91.2) | < 0.001 |
| Calprotectin (μg/ml) | 776 (536.5 to 1,132) | 54.5 (38.75 to 78.25) | < 0.001 |
-
*
Mann-Whitney U test
-
SF-PMN, Synovial fluid polymorphonuclear; SF-WBC, Synovial fluid white blood cell
Table III.
The sensitivity and specificity of synovial calprotectin, ESR, CRP, synovial fluid white blood cells (SF-WBCs), and SF-PMNs (synovial fluid polymorphonuclear cells).
| Variable (cut-off value) | Sensitivity* | p-value† | Specificity* | p-value† | NPV* | PPV* | LR+* | LR-* |
|---|---|---|---|---|---|---|---|---|
| Calprotectin (173 μg/ml) | 95.2% (74.1% to 99.8%) |
N/A | 97.6% (85.9% to 99.9%) |
N/A | 97.6% (85.9% to 99.9%) |
95.2% (74.1% to 99.8%) |
39.6 | 0.049 |
| ESR (30 mm/h) | 76.2% (52.5% to 90.9%) |
0.184 | 64.3% (48.0% to 78.0%) |
0.001 | 84.4% (66.5% to 94.1%) |
51.6% (33.4% to 69.4%) |
2.13 | 0.37 |
| CRP (10 mg/ml) | 85.7% (62.6% to 96.2%) |
0.606 | 81.0% (65.4% to 90.9%) |
0.029 | 91.9% (77.0% to 97.9%) |
69.2% (48.1%84.9%) |
4.51 | 0.177 |
| SF-WBCs (3,000 cells/μl) | 85.7% (62.6% to 96.2%) |
0.606 | 83.3% (68.0% to 92.5%) |
0.109 | 92.1% (77.5% to 97.9%) |
72% (50.4% to 87.2%) |
5.13 | 0.171 |
| SF-PMN (65%) | 90.5% (68.2% to 98.3%) |
1.000 | 85.7% (70.8% to 94.1%) |
0.057 | 94.7% (80.9% to 99.1%) |
76% (54.5% to 89.8%) |
6.32 | 0.111 |
-
*
95% confidence intervals
-
†
Chi-squared test
-
LR+, Positive likelihood ratio; LR-, Negative likelihood ratio; CI, confidence interval; NPV, Negative predictive value; PPV, Positive predictive value; N/A, Not applicable
The ROC curve showed that a CRP threshold of 173 μg/ml had a 95.2% sensitivity and a 97.6% specificity as a diagnostic marker for chronic PJI. The AUC of synovial calprotectin was 0.993, outperforming the routinely available clinical laboratory tests, which included the ESR, CRP, SF-WBC, and SF-PMN; this result indicated that calprotectin was a nearly perfect diagnostic test to identify patients with chronic PJI (Figure. 2). The positive likelihood ratio of synovial calprotectin was estimated to be 39.6, and the negative likelihood ratio was 0.049.
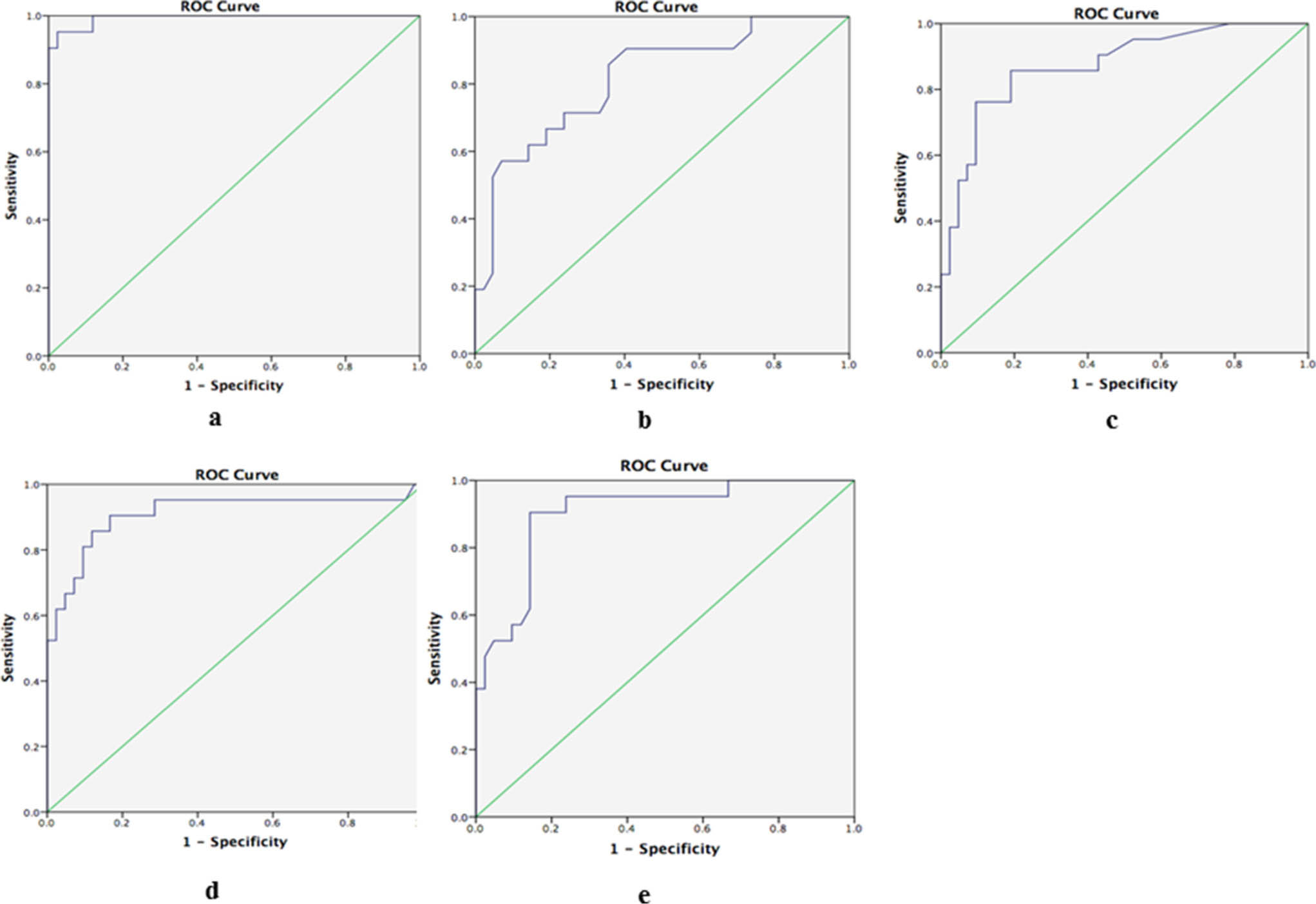
Fig. 2
The area under the curve (AUC) of a) synovial calprotectin, b) ESR, c) CRP, d) synovial fluid white blood cells (SF-WBCs), and e) synovial fluid polymorphonuclear cells (SF-PMNs).
When the SF-WBC cut-off was 3,000 cells/μl, we found a sensitivity of 85.7% and specificity of 83.3%, it is worthwhile to mention that there were seven cases had a SF leucocyte count < 3,000, while the calprotectin was positive, and three cases had a SF leucocyte count > 3,000, while the calprotectin was negative. Additionally, cut-offs of 30 mm/hour for the ESR and 10 mg/ml for CRP resulted in sensitivities of 76.2% and 85.7% and specificities of 64.3% and 81.0%, respectively. When these tests were compared with the calprotectin assay, only the specificity of the ESR and CRP reached statistical significance (p = 0.001 and p = 0.029, respectively, chi-squared test). The remaining tests were not significantly different from the calprotectin assay based on the available numbers (p < 0.05, chi-squared test).
The course of antibiotic treatment ranged from two weeks and three months. Furthermore, we found that the concentration of calprotectin in the PJI group was not affected by antibiotic treatment preoperatively(Figure. 3). The concentration of calprotectin in the antibiotic treatment group was 663 (IQR, 480 to 1,106) μg/ml, while that in the non-antibiotic treatment group was 792 (IQR, 577 to 1,203) μg/ml. The concentration of calprotectin in the antibiotic treatment group was lower than that in non-antibiotic treatment group, but the difference was not statistically significant (p = 0.343, Mann-Whitney U test). However, regardless of the use of antibiotics, the concentration of calprotectin in the PJI group was significantly higher than that in the AF group, and the difference was statistically significant (p < 0.001, Mann-Whitney U test).
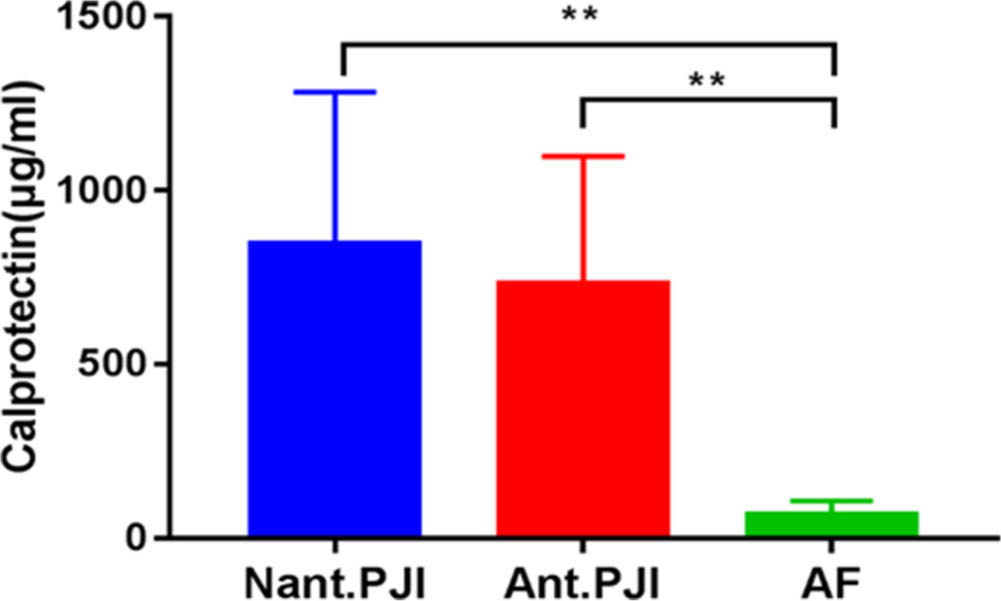
Fig. 3
The concentraction of calprotectin in the prosthetic joint infection (PJI) group was not affected by antibiotics treatment preoperatively, but both of them were higher than that of aseptic failure (AF) group.**p <0.01, Mann-Whitney U test. Ant., antibiotics; Nant., no antibiotics.
Discussion
The preoperative diagnosis of chronic PJI is still a challenge for orthopaedic surgeons, as persistent non-specific pain is often the only manifestation. Although bacterial culture was regarded as “gold standard”, the results were subjected to preoperative antibiotic treatment and the presence of biofilms. For the minor diagnostic criteria of MSIS, the threshold and accuracy of laboratory tests are also controversial. Recently, the assessment of Alpha-defensin in the synovial fluid has been shown to closely match the results of the more complex MSIS definition of PJI.32,33 However, Wouthuyzen-Bakker et al34 found that synovial calprotectin had both high sensitivity and specificity, which was quantitatively detected by the Quantum Blue calprotectin test and was less expensive and faster than the Alpha-defensin test. Wouthuyzen-Bakke et al29 and Salari et al30 also showed that synovial calprotectin have a promising role in chronic PJI diagnosis. Nevertheless, the reliability of calprotectin in PJI diagnosis was not clear enough, and more studies should be performed to further confirmed its ability in diagnosing PJI. The present study detected the concentration of synovial calprotectin with an ELISA and evaluated the role of synovial calprotectin in chronic PJI of the hip and knee.
A significant difference was found in the median synovial calprotectin level between the PJI and AF groups (p < 0.001, Mann-Whitney U test), and the former was 14 times higher than that in the AF group. Wouthuyzen-Bakker et al34 reported a concentration difference between the cohorts of up to 90-fold, which was partly because 40% of the patients who accepted primary total joint replacement were included in the AF cohort. Although 47.6% of the patients in our study received preoperative antibiotics, the ROC curve with a 173 μg/ml cut-off value showed that the level of synovial calprotectin had excellent diagnostic efficacy, with an AUC that reached 0.993, a sensitivity of 95.2%, and a specificity of 97.6%. However, whether this was due to an insufficient number of cases was still not explicit. The cut-off value in this study was determined by the ELISA for synovial Calprotectin and was higher than the cut-off that Wouthuyzen-Bakker et al34 (50 μg/ml) determined by lateral flow immunoassay. This may be because the AF cohort in our study only included revision patients, while Wouthuyzen-Bakker et al34 included patients with primary joint arthroplasty, and more than 50% of the patients were diagnosed with osteoarthritis. However, the AUC of synovial Calprotectin was greater than 0.9, both by using ELISA or the lateral flow immunoassay of Wouthuyzen-Bakker et al34, showing excellent diagnostic accuracy. As joint aspiration was included in the standardized diagnostic procedure for PJI, synovial calprotectin should be taken into consideration, as it could be useful for preoperatively or intraoperatively diagnosing PJI, which may provide an important reference for surgical decisions. In most hospitals, calprotectin is already routinely measured using a faecal calprotectin ELISA-based test, which has great potential to translate into the development of a clinically feasible method for diagnosing PJI.
The sensitivities and specificities of the ESR, CRP, SF-WBCs, and SF-PMNs ranged from 76.2% to 90.5% and 64.3% to 85.7%, respectively, which were consistent with literature reports.35,36 The calprotectin assay outperformed all of these laboratory tests for diagnosing PJI, with a higher sensitivity and specificity, but these differences did not reach statistical significance, except for the specificity of the ESR and CRP. The slightly superior specificity of the calprotectin assay compared with synovial fluid PMNs and synovial WBCs is intriguing because the calprotectin assay can be used on dead or lysed cells. Furthermore, the calprotectin assay requires a lower volume of synovial fluid (only 500 μl) than SF-WBCs and SF-PWNs, which would be useful for PJI diagnosis for patients with insufficient synovial fluid.
Although calprotectin shows excellent diagnostic accuracy, two cases were still misdiagnosed, including a false negative case and a false-positive case. In the false negative case, the diagnosis of PJI was made based on bacterial culture results; namely, Staphylococcus hominis was isolated from four separate tissue samples. This patient was not preoperatively administered antibiotics and had normal CRP, ESR, SF-PMNs, and SF-WBCs and negative pathological results. This patient underwent revision surgery due to persistent pain after primary THA and received corticosteroids (prednisone 20 mg/day) for at least six years because of chronic adrenocortical hypofunction. It has been reported that immunosuppressive treatment may result in false negative results. Therefore, corticosteroid treatment may be a leading cause of low levels of synovial calprotectin. The false positive case underwent a revision for prosthesis dislocation 13 months after primary THA and had an elevated CRP level of 12.3 mg/l; SF-WBCs at 4452 cells/μl; positive pathological results; normal PMN% and ESR; and negative bacterial culture results. Synovial calprotectin was slightly elevated in this patient (234 µg/ml). The pathological results and certain inflammatory indexes of synovial fluid or serum suggested that there was acute inflammation around the hip due to prosthesis dislocation. Based on our limited data, caution should be taken in the interpretation of a positive result in patients who have acute inflammation, as illustrated by the false positive test result in one of the patients who underwent revision for prosthesis dislocation in our study. In addition, the influence of immunosuppressive treatment on the levels of synovial calprotectin needs to be determined in the future.
There were some limitations in the present study. This study involved a single centre, so the sample size was relatively small, with only 21 cases in the PJI group and 42 cases in the AF group. Furthermore, patients with inflammatory disease were excluded from the study, as inflammatory disease has been documented in the literature to trigger the pathogen response and to cause elevations in synovial fluid calprotectin, which may lead to false positive results. Therefore, a larger cohort study that includes patients with inflammatory arthropathy and evaluates the diagnostic efficacy of synovial calprotectin in the diagnosis of PJI is warranted. Moreover, in this study, the synovial fluid calprotectin was detected by ELISA-based approach. Compared to a more rapid point-of-care test / lateral flow method, ELISA is more expensive, less rapid, and not a point-of-care test. However, a rapid point-of-care test / lateral flow method is unavailable now in many countries, so an ELISA-based approach is easier to perform and therefore more suitable for hospitals.
In conclusion, the present work detected synovial fluid calprotectin via an ELISA-based approach, and showed that synovial fluid calprotectin had high sensitivity and specificity for diagnosing PJI.
Reference
1. Lima ALL , Oliveira PR , Carvalho VC , et al. Periprosthetic joint infections . Interdiscip Perspect Infect Dis . 2013 ; 2013 : 542796 . Crossref PubMed Google Scholar
2. Bozic KJ , Kurtz SM , Lau E , et al. The epidemiology of revision total knee arthroplasty in the United States . Clin Orthop Relat Res . 2010 ; 468 ( 1 ): 45 – 51 . Crossref PubMed Google Scholar
3. Parvizi J , Della Valle CJ . AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee . J Am Acad Orthop Surg . 2010 ; 18 ( 12 ): 771 – 772 . Crossref PubMed Google Scholar
4. Osmon DR , Berbari EF , Berendt AR , et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America . Clin Infect Dis . 2013 ; 56 ( 1 ): e1 – e25 . Crossref PubMed Google Scholar
5. Parvizi J , Zmistowski B , Berbari EF , et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society . Clin Orthop Relat Res . 2011 ; 469 ( 11 ): 2992 – 2994 . Crossref PubMed Google Scholar
6. Parvizi J , Gehrke T , Chen AF . Proceedings of the International Consensus on Periprosthetic Joint Infection . Bone Joint J . 2013 ; 95-B ( 11 ): 1450 – 1452 . Crossref PubMed Google Scholar
7. Saleh A , George J , Faour M , et al. Serum biomarkers in periprosthetic joint infections . Bone Joint Res . 2018 ; 7 ( 1 ): 85 – 93 . Crossref PubMed Google Scholar
8. Schäfer P , Fink B , Sandow D , et al. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy . Clin Infect Dis . 2008 ; 47 ( 11 ): 1403 – 1409 . Crossref PubMed Google Scholar
9. Tsang STJ , McHugh MP , Guerendiain D , et al. Underestimation of Staphylococcus aureus (MRSA and MSSA) carriage associated with standard culturing techniques: One third of carriers missed . Bone Joint Res . 2018 ; 7 ( 1 ): 79 – 84 . Crossref PubMed Google Scholar
10. Janz V , Schoon J , Morgenstern C , et al. Rapid detection of periprosthetic joint infection using a combination of 16s rDNA polymerase chain reaction and lateral flow immunoassay: A Pilot Study . Bone Joint Res . 2018 ; 7 ( 1 ): 12 – 19 . Crossref PubMed Google Scholar
11. Kuo F-C , Lu Y-D , Wu C-T , et al. Comparison of molecular diagnosis with serum markers and synovial fluid analysis in patients with prosthetic joint infection . Bone Joint J . 2018 ; 100-B ( 10 ): 1345 – 1351 . Crossref PubMed Google Scholar
12. Chen M-F , Chang C-H , Chiang-Ni C , et al. Rapid analysis of bacterial composition in prosthetic joint infection by 16S rRNA metagenomic sequencing . Bone Joint Res . 2019 ; 8 ( 8 ): 367 – 377 . Crossref PubMed Google Scholar
13. Wang C , Wang Q , Li R , et al. Synovial Fluid C-reactive Protein as a Diagnostic Marker for Periprosthetic Joint Infection: A Systematic Review and Meta-analysis . Chin Med J (Engl) . 2016 ; 129 ( 16 ): 1987 – 1993 . Crossref PubMed Google Scholar
14. Matsen Ko L , Parvizi J . Diagnosis of Periprosthetic Infection: Novel Developments . Orthop Clin North Am . 2016 ; 47 ( 1 ): 1 – 9 . Crossref PubMed Google Scholar
15. Qu X , Zhai Z , Liu X , et al. Evaluation of white cell count and differential in synovial fluid for diagnosing infections after total hip or knee arthroplasty . PLoS One . 2014 ; 9 ( 1 ): e84751 . Google Scholar
16. Yi PH , Cross MB , Moric M , et al. Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clin Orthop Relat Res . 2015 ; 473 ( 2 ): 498 – 505 . Google Scholar
17. Springer BD . The Diagnosis of Periprosthetic Joint Infection . J Arthroplasty . 2015 ; 30 ( 6 ): 908 – 911 . Google Scholar
18. Akgün D , Müller M , Perka C , Winkler T . The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence . Bone Joint J . 2018 ; 100-B ( 11 ): 1482 – 1486 . Crossref PubMed Google Scholar
19. Pérez-Prieto D , Portillo ME , Puig-Verdié L , et al. C-Reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections . Int Orthop . 2017 ; 41 ( 7 ): 1315 – 1319 . Crossref PubMed Google Scholar
20. Chen M-F , Chang C-H , Yang L-Y , et al. Synovial fluid interleukin-16, interleukin-18, and CRELD2 as novel biomarkers of prosthetic joint infections . Bone Joint Res . 2019 ; 8 ( 4 ): 179 – 188 . Crossref PubMed Google Scholar
21. Gomez-Urena EO , Tande AJ , Osmon DR , Berbari EF . Diagnosis of Prosthetic Joint Infection: Cultures, Biomarker and Criteria . Infect Dis Clin North Am . 2017 ; 31 ( 2 ): 219 – 235 . Crossref PubMed Google Scholar
22. Li B , Chen F , Liu Y , Xu G . Synovial fluid α-defensin as a biomarker for Peri-Prosthetic joint infection: a systematic review and meta-analysis . Surg Infect . 2017 ; 18 ( 6 ): 702 – 710 . Crossref PubMed Google Scholar
23. Deirmengian C , Kardos K , Kilmartin P , et al. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res . 2014 ; 472 ( 11 ): 3254 – 3262 . Crossref PubMed Google Scholar
24. Pagano RL , Dias MAA , Dale CS , Giorgi R . Neutrophils and the calcium-binding protein MRP-14 mediate carrageenan-induced antinociception in mice . Mediators Inflamm . 2002 ; 11 ( 4 ): 203 – 210 . Crossref PubMed Google Scholar
25. Azramezani Kopi T , Shahrokh S , Mirzaei S , et al. The role of serum calprotectin as a novel biomarker in inflammatory bowel diseases: a review study . Gastroenterol Hepatol Bed Bench . 2019 ; 12 ( 3 ): 183 – 189 . PubMed Google Scholar
26. Marie I , Leroi A-M , Menard J-F , et al. Fecal calprotectin in systemic sclerosis and review of the literature . Autoimmun Rev . 2015 ; 14 ( 6 ): 547 – 554 . Crossref PubMed Google Scholar
27. Ingram JR , Cawley S , Coulman E , et al. Levels of wound calprotectin and other inflammatory biomarkers aid in deciding which patients with a diabetic foot ulcer need antibiotic therapy (INDUCE study) . Diabet Med . 2018 ; 35 ( 2 ): 255 – 261 . Crossref PubMed Google Scholar
28. Fernandes SR , Santos P , Fatela N , et al. Ascitic Calprotectin is a Novel and Accurate Marker for Spontaneous Bacterial Peritonitis . J Clin Lab Anal . 2016 ; 30 ( 6 ): 1139 – 1145 . Crossref PubMed Google Scholar
29. Wouthuyzen-Bakker M , Ploegmakers JJW , Ottink K , et al. Synovial Calprotectin: An Inexpensive Biomarker to Exclude a Chronic Prosthetic Joint Infection . J Arthroplasty . 2018 ; 33 ( 4 ): 1149 – 1153 . Crossref PubMed Google Scholar
30. Salari P , Grassi M , Cinti B , et al. Synovial Fluid Calprotectin for the Preoperative Diagnosis of Chronic Periprosthetic Joint Infection . J Arthroplasty . 2020 ; 35 ( 2 ): 534 – 537 . Crossref PubMed Google Scholar
31. Morgenstern C , Cabric S , Perka C , et al. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection . Diagn Microbiol Infect Dis . 2018 ; 90 ( 2 ): 115 – 119 . Crossref PubMed Google Scholar
32. Stone WZ , Gray CF , Parvataneni HK , Prieto HA . Clinical Evaluation of Alpha Defensin Test Following Staged Treatment of Prosthetic Joint Infections . J Arthroplasty . 2019 ; 34 ( 7 ): 1446 – 1451 . Crossref PubMed Google Scholar
33. Kleiss S , Jandl NM , Novo de Oliveira A , et al. Diagnostic accuracy of alpha-defensin enzyme-linked immunosorbent assay in the clinical evaluation of painful hip and knee arthroplasty with possible prosthetic joint infection: a prospective study of 202 cases . Bone Joint J . 2019 ; 101-B ( 8 ): 970 – 977 . Crossref PubMed Google Scholar
34. Wouthuyzen-Bakker M , Ploegmakers JJW , Kampinga GA , et al. Synovial calprotectin: a potential biomarker to exclude a prosthetic joint infection . Bone Joint J . 2017 ; 99-B ( 5 ): 660 – 665 . Crossref PubMed Google Scholar
35. Bajada S , Yoong AWH , Hourigan P , et al. Plasma Viscosity Has a Role in the Diagnosis of Prosthetic Joint Infection After Total Knee Arthroplasty . Journal of Arthroplasty . 2019 ; 34 ( 12 ): 3035 – 3039 Crossref PubMed Google Scholar
36. Paziuk T , Rondon AJ , Goswami K , et al. A Novel Adjunct Indicator of Periprosthetic Joint Infection: Platelet Count and Mean Platelet Volume . Journal of Arthroplasty . 2020 ; 35 ( 3 ): 836 – 839 . . Crossref PubMed Google Scholar
Author contributions
Z. Zhang: Performed the study
Y: Cai: Wrote the manuscript
G. Bai: Revised the manuscript
C. Zhang: Translated the manuscript into English
W. Li: Conducted the statistical analysis
B. Yang:Guided the experiment
W. Zhang:Designed the study
Z. Zhang, Y. Cai and G. Bai contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
No competing financial interests exist.
Acknowledgements
This paper was supported by Fujian Education and Scientific Research Projects for Young Teachers (grant number JAT170241), the Natural Science Foundation of Fujian Province (grant numbers 2018I0006 and 2018Y4003) and the Key clinical speciality of Fujian Province (grant number 2012[149]).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









