Abstract
Aims
Limb salvage in bone tumour patients replaces the bone with massive segmental prostheses where achieving bone integration at the shoulder of the implant through extracortical bone growth has been shown to prevent loosening. This study investigates the effect of multidrug chemotherapy on extracortical bone growth and early radiological signs of aseptic loosening in patients with massive distal femoral prostheses.
Methods
A retrospective radiological analysis was performed on adult patients with distal femoral arthroplasties. In all, 16 patients were included in the chemotherapy group with 18 patients in the non-chemotherapy control group. Annual radiographs were analyzed for three years postoperatively. Dimensions of the bony pedicle, osseointegration of the hydroxyapatite (HA) collar surface, bone resorption at the implant shoulder, and radiolucent line (RLL) formation around the cemented component were analyzed.
Results
A greater RLL score (p = 0.041) was observed at three years postoperatively, with those receiving chemotherapy showing greater radiological loosening compared with those not receiving chemotherapy. Chemotherapy patients experience osteolysis at the shoulder of the ingrowth collar over time (p < 0.001) compared with non-chemotherapy patients where osteolysis was not observed. A greater median percentage integration of the collar surface was observed in the non-chemotherapy group (8.6%, interquartile range (IQR) 0.0% to 37.9%; p = 0.021) at three years. Bone growth around the collar was observed in both groups, and no statistical difference in amount of extracortical bony bridging was seen.
Conclusion
Multidrug chemotherapy affects the osseointegration of ingrowth collars and accelerates signs of radiological loosening. This may increase the risk of aseptic loosening in patients with massive segmental implants used to treat bone cancer.
Cite this article: Bone Joint Res 2020;9(7):333–340.
Article focus
-
Investigating the effect of multidrug chemotherapy on early radiological signs of aseptic loosening in patients with massive distal femoral endoprostheses.
Key messages
-
Multidrug chemotherapy significantly affects osseointegration of ingrowth collars and accelerates aseptic loosening of distal femoral endoprostheses appearing as early as three years postoperatively.
-
The authors of this paper recommend that bone tumour units provide stringent follow-up to those patients receiving chemotherapy, particularly in the initial years postoperatively.
Strengths and limitations
-
This is the first adequately powered study to investigate the effect of multidrug chemotherapy on osseointegration.
-
Surgery was carried out by a number of orthopaedic surgeons specializing in treating bone cancers. Preservation of the periosteum at the shoulder of the implant was not recorded.
-
A limitation of the study is that this was a retrospective study design with small numbers.
Introduction
Studies have shown that the introduction of an adjuvant chemotherapy regime improves the survivorship of malignant bone tumour patients.1,2 Prior to this amputation was the standard treatment, however improving patient mortality rates shifted focus to limb salvage involving segmental resection of the tumour with reconstruction using endoprostheses. Although custom endoprostheses require time for manufacture that may delay chemotherapy and worsen prognosis, Rosen et al3 demonstrated that the use of neoadjuvant chemotherapy where patients were treated preoperatively while fabricating the custom endoprosthesis was beneficial. Currently, approximately 80% of osteosarcoma patients are treated with neoadjuvant chemotherapy and limb salvage.4
Although chemotherapy has significantly lowered the risk of metastatic disease, complications from reconstructive techniques continue to result in late morbidity. According to Jeys et al,5 aseptic loosening is the most common reason for failure of endoprostheses and accounts for 28.6% of failures. Implant fixation requires the bone to be healthy and viable. Literature has shown there to be substantial effects of doxorubicin, cisplatin, and ifosfamide on osteotomy healing and incorporation of cortical autografts.6 For bone tumour implants, extracortical bony-bridging (ECBB) where bone forms over the implant shaft and stabilizes the fixation has been shown to reduce loosening.7 Coathup et al8 showed that 98% of distal femoral arthroplasties (DFRs) survive to ten years if their hydroxyapatite (HA) collar had osseointegrated compared to 75% if they had not. Radiologically, aseptic loosening around segmental implants initially manifests itself with the development of a ‘gap’ of localized cortical bone loss at the bone-shoulder implant junction. Over time, as osteolysis increases, there is progression of periprosthetic bone-cement radiolucent lines that advance along the interface eventually leading to aseptic loosening.9
This study investigates the effect of multidrug chemotherapy on extracortical bone growth and early radiological signs of loosening around massive distal femoral bone tumour prostheses. We hypothesized that patients undergoing chemotherapy would show less osseointegration of their endoprosthesis with greater loosening compared to patients who did not receive chemotherapy.
Methods
Study design and setting
A retrospective case-control study was conducted. No patient or animal contact was involved in this study and therefore ethical approval was not required. A database of all DFRs (Stanmore Implants, Elstree, UK) performed in our unit between 2004 and 2013 was collated. Annual radiographs for the first three postoperative years were analyzed using the local Picture Archiving and Communication System (PACS; Centricity; GE Healthcare, Amersham, UK). Demographic data as well as diagnosis and resection level were recorded for each patient with the extracortical bone growth, osseointegration, gap at transection site, and radiolucent line score (RLL) measured on each radiograph.
Patients
Adult patients who had undergone reconstruction using DFRs with HA ingrowth collars were included in our study. The custom DFRs were all based on the Stanmore Modular Individualized Lower Limb System (SMILES) rotating hinge knee arthroplasty (Stanmore Implants). The most significant difference between the implants used for individual patients is the length of proximal implant shaft as this is dictated by the resection level. They all used the HA collar on the shaft and initial fixation was achieved using a cemented intramedullary stem proximally and distally. The chemotherapy group consisted of patients who were diagnosed with primary bone tumours (Table I). The non-chemotherapy control group consisted of patients who were diagnosed with giant cell tumours. All the procedures were performed in one institution and no patients were recalled for the purposes of this study.
Table I.
Patients included in chemotherapy group with chemotherapy regimen.
| Case no. | Diagnosis | Chemotherapy | Agents |
|---|---|---|---|
| 1 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate |
| 2 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate |
| 3 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate with maintenance pegylated interferon* |
| 4 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate |
| 5 | High grade pleomorphic sarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin |
| 6 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate |
| 7 | High grade spindle cell sarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin |
| 8 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate |
| 9 | Ewing’s sarcoma | Neoadjuvant and adjuvant | VIDE (neoadjuvant), VAI (adjuvant)† |
| 10 | High grade pleomorphic sarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin |
| 11 | High grade spindle cell sarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin |
| 12 | Ewing’s sarcoma | Neoadjuvant and adjuvant | VIDE (neoadjuvant), VAC (adjuvant)‡ |
| 13 | High grade spindle cell sarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin |
| 14 | Osteosarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate |
| 15 | High grade spindle cell sarcoma | Neoadjuvant and adjuvant | Cisplatin, doxorubicin, methotrexate§ |
| 16 | Osteosarcoma | Adjuvant | Cisplatin, doxorubicin |
-
*
Five of 12 cycles of methotrexate omitted due to complications.
-
†
Randomized to vincristine, actinomycin D, ifosfamide as part of the EURO-EWING 99 study.10
-
‡
Randomized to vincristine, actinomycin D, cyclophosphamide as part of the EURO-EWING 99 study.
-
§
Methotrexate and cisplatin stopped at later cycles due to complications.
-
VAC, vincristine, actinomycin D, cyclophosphamide; VAI, vincristine, actinomycin D, ifosfamide; VIDE, vincristine, ifosfamide, doxorubicin, etoposide.
The selection criteria were chosen in particular to exclude cases where the potential for bone growth and osseointegration was not biased between the groups. Patients aged less than 16 years at the time of surgery, patients with an underlying diagnosis of rheumatoid arthritis, patients who had received radiotherapy directly to or near the transection site,11 patients who received DFRs used in reconstruction for metastatic lesions,12 and patients who received DFRs used in revision procedures were all excluded. Patients who developed a prosthetic infection were excluded even if this was diagnosed beyond the first three postoperative years, as it was assumed the implant was infected beforehand.
A total of 302 patients were identified using a local database. In all, 216 of these patients were excluded according to our criteria, leaving 86 potentially eligible patients. Overall, 24 patients were allocated to the chemotherapy group with 62 in the non-chemotherapy group. Further exclusions were made based on the quality of the radiographs (Figure 1). This left 16 eligible patients in the chemotherapy group and 18 in the non-chemotherapy group who had radiographs at one, two, and three years. Demographic data as well as resection level (%) and RLL scores are summarized in Table II.
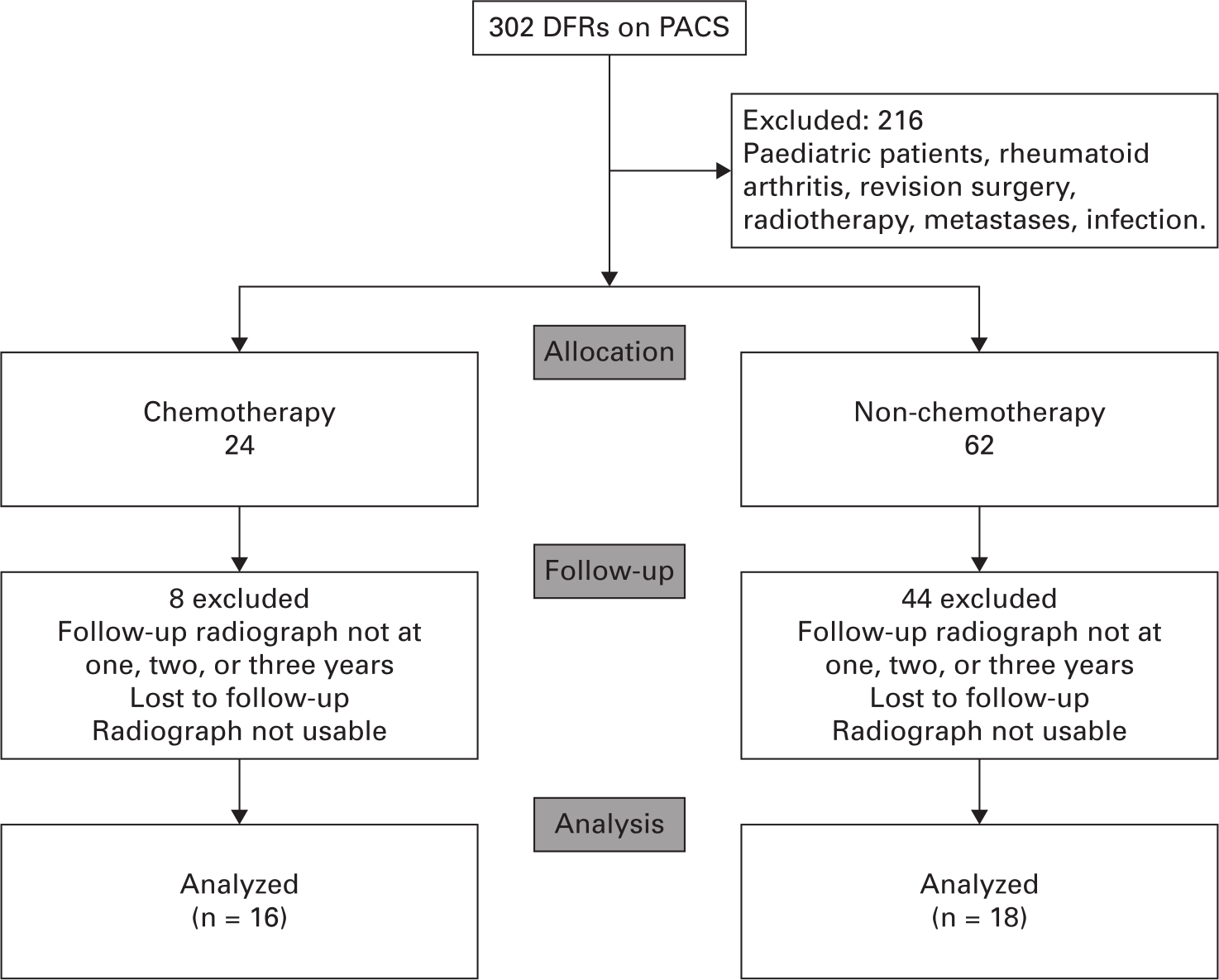
Fig. 1
CONsolidated Standards Of Reporting Trials (CONSORT) flow diagram. DFR, distal femoral arthroplasty; PACS, Picture Archiving and Communication System.
Table II.
Comparisons of demographic data across chemotherapy and non-chemotherapy groups.
| Variable | Chemotherapy | Non-chemotherapy | p-value |
|---|---|---|---|
| Median age, yrs (IQR) | 31 (19 to 50) | 35 (29 to 44) | 0.395* |
| Sex, male:female, n | 9:7 | 9:9 | 0.566† |
| Median resection level, % (IQR) | 39.5 (33.0 to 48.5) | 26.2 (24.8 to 29.7) | < 0.001* |
-
*
Mann-Whitney U test.
-
†
Chi-squared test.
-
IQR, interquartile range.
Variables, outcome measures, data sources, and bias
Radiological analysis was performed by one observer (TCE). Anteroposterior (AP) and lateral (L) radiographs were used to quantify all measurements (Figure 2). Resection level was defined by the proportion of the femur that had been resected, taken from the joint line to the transection site, expressed as a percentage of the total length of the femur. The amount of extracortical bone growth was quantified by measuring the maximal length and thickness (millimetres) of the extracortical bone pedicle growing from the level of the shoulder of the HA collar in orthogonal regions (anterior (A), posterior (P), medial (M), and lateral (L)) surrounding the HA collar and implant shaft. On the radiographs, osseointegration was quantified by expressing the length (millimetres) of the extracortical bony pedicle in direct contact with the HA collar for each region (A, P, M, L) as a percentage of the full length (millimetres) of the collar. The overall osseointegration of the collar was calculated by taking the mean of the integration occurring in each plane. The presence of a gap between the shoulder of the HA collar and the transection site was observed and quantified in each region (A, P, M, L), with no gap being denoted as 0 mm. To evaluate loosening of the cemented intramedullary component the RLL score was calculated. The cement mantle surrounding the intramedullary component is split into 12 equal zones, with six on either side of the component midline. A score of one is given if a zone contains a radiolucent line at the cement-bone interface suggesting loosening. This was done for both AP and lateral radiographs, therefore the maximum RLL score that can be given for any DFR is 24.8 The assessor was blinded as to whether they were scoring the radiograph of a patient who had received or not received chemotherapy.
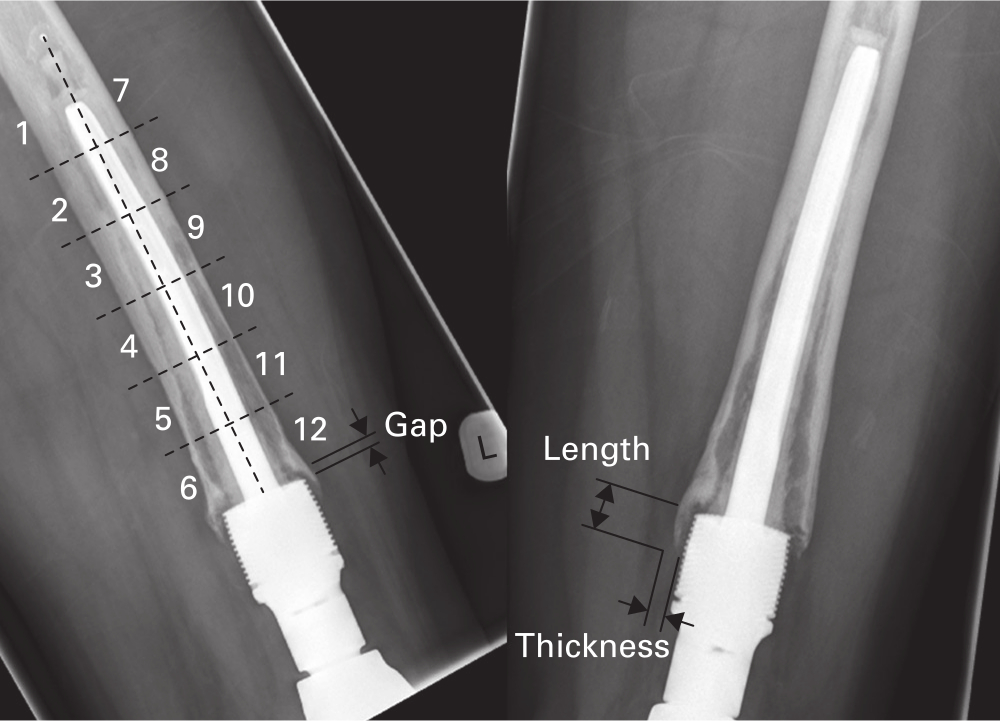
Fig. 2
Anteroposterior (right) and lateral (left) radiographs of distal femoral arthroplasty intramedullary component showing the zones used for the radiolucent line score together with length, thickness, and gap distances measured around the hydroxyapatite (HA) collar. Radiolucent lines are evident in all zones in the lateral radiograph, giving a maximum score of 12.
Statistical analysis
Statistical analysis was performed using SPSS Statistics V22 (IBM, Armonk, New York, USA). Data were tested for normality using the Shapiro-Wilk test and non-parametric data were compared using a Mann-Whitney U test. Multiple data comparisons for non-parametric data were compared using a Kruskal-Wallis test. Categorical data were compared using the chi-squared test. A p-value < 0.05 was considered to be significant. Correlation analysis was conducted using Pearson’s correlation coefficient with p < 0.05 being seen as significant. Regression analysis was performed using RLL as the dependent variable and age, sex, resection length, and whether or not they received chemotherapy as independent variables; those variables with a p-value < 0.05 were seen as significant. The post hoc power analysis was completed using the asymptotic relative efficiency method on G*Power (v3.1.9.2; University of Kiel, Kiel, Germany) using an α-value of 0.05 for RLL only. A medium effect size (d = 0.5) was used as defined by Cohen et al.13
Results
Lucent lines around DFRs, as measured in the RLL score, in chemotherapy patients significantly increased over the three-year period (p = 0.009, Kruskal-Wallis test). This suggests worsening radiological loosening of the intramedullary cemented component over the first three years. In contrast, no significant change in RLL score was observed in the non-chemotherapy group (p = 0.061, Kruskal-Wallis test). The disparity in RLL score between the two groups becomes greater over time, reaching statistical significance at year 3 (p = 0.044, Mann-Whitney U test) (Figure 3).
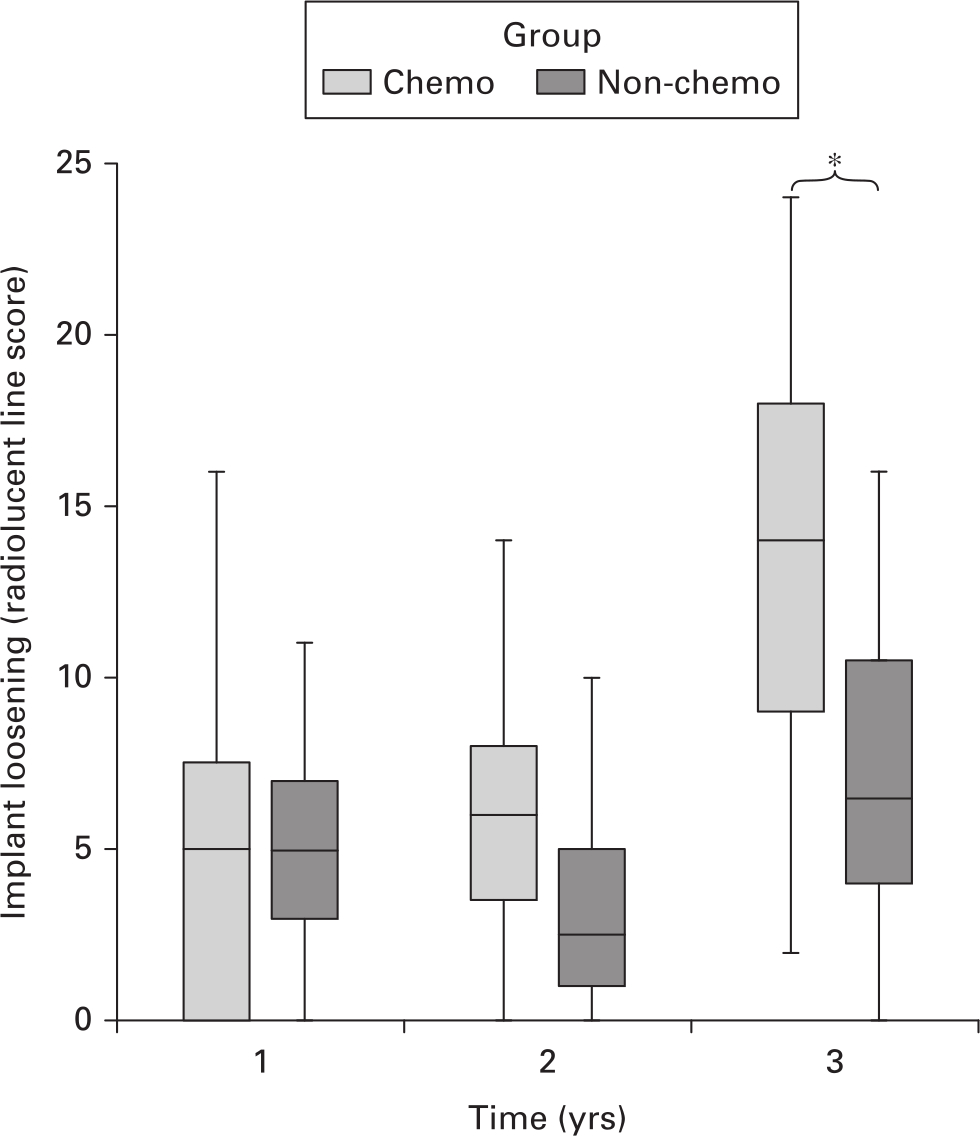
Fig. 3
Boxplot of radiolucent line scores for non-chemotherapy and chemotherapy groups over time. p = 0.044, Mann-Whitney U test.
The median overall size of the gap seen in year 3 (3.2 mm; interquartile range (IQR) 2.6 to 4.1; p < 0.001, Mann-Whitney U test) in the chemotherapy group was significantly greater than that found at year 1 (0.0 mm; IQR 0.0 to 7.0) and 2 (0.2 mm; IQR 0.0 to 1.0). There was no significant difference observed in the median overall gap size between year 1 (0.7 mm; IQR 0.4 to 1.7), year 2 (0.6 mm; IQR 0.0 to 2.0), and year 3 (1.8 mm; IQR 0.1 to 2.7) in the non-chemotherapy group. Subgroup analysis reveals a significant (p = 0.024, Kruskal-Wallis test) difference in the size of the gap at the medial aspect of the HA collar shoulder in the third year. Those undergoing chemotherapy have a median 3.2 mm (IQR 1.8 to 4.7) gap compared to a much smaller median 1.3 mm (IQR 0.0 to 2.3) in the non-chemotherapy group. No statistical differences were detected in the anterior, posterior, and lateral gaps otherwise.
Significantly lower median osseointegration of the HA collar was observed posteriorly in those receiving chemotherapy (0.0%; IQR 0.0% to 0.0%) compared with those in the non-chemotherapy group (8.6%; IQR 0.0% to 37.9%; p = 0.021, Mann-Whitney U test) at three years, however no significant differences were observed anteriorly, medially, or laterally. A median overall increase in osseointegration was observed in the non-chemotherapy group from year 1 (2.6%; IQR 0.0% to 15.2%), year 2 (11.4%; IQR 0.0% to 33.0%), and year 3 (12.8%; IQR 0.4% to 30.1%), however this did not translate into a statistically significant result (p = 0.232, Kruskal-Wallis test).
Greater osseointegration of the HA collar was found to significantly negatively correlate with the RLL score. In contrast, gap formation suggesting osteolysis and RLL score were found to be significantly positively correlated. Resection length was found to not correlate with RLL score, gap formation, or osseointegration (Table III). Chemotherapy, sex, age, or resection length were not significant in predicting RLL score according to the regression analysis. Using our data, a post hoc power analysis reveals that our study was powered adequately.
Table III.
Correlation analysis between variables with Pearson correlation coefficients and p-values.
| Variable | Measure | Gap | RLL | Osseointegration |
|---|---|---|---|---|
| Resection length | Correlation coefficient | -0.078 | 0.193 | 0.086 |
| p-value | 0.493 | 0.086 | 0.450 | |
| Osseointegration | Correlation coefficient | -0.177 | -0.265 | N/A |
| p-value | 0.097 | 0.012* | N/A | |
| RLL | Correlation coefficient | 0.304 | N/A | N/A |
| p-value | 0.004* | N/A | N/A |
-
*
Statistically significant.
-
N/A, not applicable; RLL, radiolucent line.
Extracortical bone pedicles were found to develop around the HA collar in both groups. The length of the pedicles increased through from years 1 to 3 for the chemotherapy group, however this reached a peak in year 2 and was found to have resorbed slightly in year 3 in the non-chemotherapy group. For both groups, the thickness of the bone pedicles peaked in year 2 before again showing resorption in year 3 (Table IV). These observed differences did not translate into statistical differences between or within the groups.
Table IV.
A comparison of bone pedicle size growing around the hydroxyapatite collar in the chemotherapy and non-chemotherapy groups over the first three postoperative years.
| Group | Variable | Year 1 | Year 2 | Year 3 |
|---|---|---|---|---|
| Non-chemo | Median pedicle length, mm (IQR) | 1.9 (0.0 to 10.7) | 7.0 (0.9 to 14.5) | 6.5 (1.1 to 15.8) |
| Median pedicle thickness, mm (IQR) | 1.0 (0.0 to 2.9) | 2.0 (0.2 to 3.8) | 1.9 (1.0 to 3.8) | |
| Chemo | Median pedicle length, mm (IQR) | 1.9 (0.0 to 12.5) | 6.7 (0.0 to 12.6) | 8.3 (0.0 to 18.0) |
| Median pedicle thickness, mm (IQR) | 0.5 (0.0 to 4.4) | 1.4 (0.0 to 3.6) | 0.8 (0.0 to 4.5) |
-
IQR, interquartile range.
Discussion
Limb salvage surgery remains the current gold standard for treatment of bone and soft tissue tumours, however endoprosthetic reconstruction remains fraught with complications with higher rates of aseptic loosening than standard primary arthroplasty procedures.5 A large proportion of these patients have chemotherapy and therefore it is possible that this may contribute to the higher rate of loosening.
Literature has shown the substantial effects of doxorubicin, cisplatin, and ifosfamide on osteotomy healing and incorporation of cortical autografts, with histological evidence of poor new bone formation and decreased strength of healed osteotomies.6 A study evaluating cortical hypertrophy around the Compress distal femoral endoprosthesis (Biomet, Warsaw, Indiana, USA) showed greater hypertrophy around the implant in patients not receiving chemotherapy in the first postoperative year.14 In contrast, Muscolo et al15 found that the use of chemotherapy did not have a significant effect on the overall allograft survival rates in patients undergoing reconstruction using osteoarticular allografts for musculoskeletal sarcomas. This is the first study to show that multidrug chemotherapy worsens radiological loosening of DFRs and reduces osseointegration.
A large gap as a result of osteolysis and the inhibition of bone formation at the shoulder of the implant will cause increased strain on the component leading to loosening.16 The amount of strain and therefore loosening is dictated by the osseointegration of extracortical bone and the quality of the intramedullary component fixation. It is important to consider both the gap size and RLL score when assessing implant loosening radiologically. DFRs in chemotherapy patients show a greater gap formation and higher RLL score than subjects in the control group. These two indicators of radiological loosening are furthermore positively correlated. Finite element analysis has shown osseointegration of extracortical bone reduces stresses passing through the component of the implant;7,17 our study supports this as radiological loosening of the intramedullary component is negatively correlated with osseointegration of the extracortical bone. This indicates that the stresses along the component due to poor integration at the shoulder may result in the formation of radiolucent lines.
Osseointegration of extracortical bone in DFRs has been shown to be effective in reducing loosening.18 Our study has shown that patients undergoing multidrug chemotherapy show reduced rates of osseointegration, together with greater radiological loosening in the initial postoperative years following reconstruction.
Bone integration can be described on a cellular level by the attachment of osteoblasts onto the implant surface and subsequent extracellular mineralization. Osteoblasts attach to substrates using integrins.19 One of the most important extracellular matrix proteins that integrins attach to is fibronectin, of which human blood plasma levels reduce significantly when patients receive chemotherapy. These reduced levels can be seen as early as the next day following chemotherapy,20 with up to a 50% reduction in levels seen over three weeks from the initiation of chemotherapy.21 Treatment with cisplatin has been shown to directly reduce gene expression of fibronectin by almost 2.5 times.22 Reduced osseointegration of HA collars in patients undergoing chemotherapy could therefore be due to the negative effect of chemotherapy on cell attachment.
There was no difference in the amount of ECBB from the transection site over the HA collar between the chemotherapy and non-chemotherapy groups, indicating that bone formation was not impaired by chemotherapy. Evidence also exists which has conversely shown bone turnover to be clearly affected by the use of chemotherapy.6 Avedian et al14 found chemotherapy to temporarily adversely affect bone growth at the bone-implant interface, however after one year they observed no differences between the two groups. Either chemotherapy has no true effect on extracortical bone growth, or the three-year follow-up period in our study is not long enough to detect the difference in ECBB between the two groups. Increased strain at the HA collar region due to early loosening can itself stimulate bone growth without the ability to osseointegrate, much like callus formation in the hypertrophic nonunion scenario where there is failed continuity of callus between the fragments. This may counteract the inhibition of bone growth due to the chemotherapy.
There was a significant difference in resection length between the two groups. In our study, resection length did not correlate with RLL score, osseointegration, and gap formation, nor was it a significant variable in our regression analysis. This suggested that it was not a potential confounding factor according to our data. The sample size is small with a large number of exclusions. This was to keep the groups as homogenous as possible and to exclude other factors that may affect bone growth at the HA collar. Osseointegration was quantified as radiological contact between pedicle and collar. True osseointegration can only be confirmed histologically, which has already been proven for this collar design in a previous study.8 Finally, the control group is very homogenous with all the cases being of patients with giant cell tumours; the same cannot be said for the chemotherapy group. However, the chemotherapeutic agents received by these patients were very similar and therefore we do not believe this limited our results greatly.
In conclusion, this is the first study to evaluate the effect of multidrug chemotherapy on radiological signs of loosening around endoprostheses. We cannot guarantee the findings in our study are due to the chemotherapy alone and not the underlying sarcoma, however it is likely that chemotherapy does affect implant fixation given the in vivo evidence from animal models, which have shown that such treatment disrupts bone formation.23–25 An understanding of the involvement of multidrug chemotherapy on the early osseointegration and loosening of endoprostheses is imperative to allow for continued development of limb salvage procedures. This is crucial, particularly with emerging technologies used to improve osseointegration of endoprostheses.26 We demonstrated that there are radiological signs of loosening as early as three years postoperatively, and differences in osseointegration of the HA collar were observed in the first postoperative year. We recommend that bone tumour units provide stringent follow-up to those patients receiving chemotherapy, particularly in the initial years postoperatively. Studies with longer follow-up of this cohort of patients must be performed to conclude whether these radiological findings lead to clinical symptomatic loosening or implant failure.
References
1. Eilber F , Giuliano A , Eckardt J , et al. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial . J Clin Oncol . 1987 ; 5 ( 1 ): 21 – 26 . Crossref PubMed Google Scholar
2. Link MP , Goorin AM , Miser AW , et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity . N Engl J Med . 1986 ; 314 ( 25 ): 1600 – 1606 . Crossref PubMed Google Scholar
3. Rosen G , Marcove RC , Caparros B , et al. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery . Cancer . 1979 ; 43 ( 6 ): 2163 – 2177 . Crossref PubMed Google Scholar
4. Allison DC , Carney SC , Ahlmann ER , et al. A meta-analysis of osteosarcoma outcomes in the modern medical era . Sarcoma . 2012 ; 2012 : 704872. https://doi.org/10.1155/2012/704872 Crossref PubMed Google Scholar
5. Jeys LM , Kulkarni A , Grimer RJ , et al. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis . J Bone Joint Surg Am . 2008 ; 90-A ( 6 ): 1265 – 1271 . Crossref PubMed Google Scholar
6. Virolainen P , Inoue N , Nagao M , Frassica FJ , Chao EY . The effect of multidrug chemotherapy on bone graft augmented prosthesis fixation . J Orthop Res . 2005 ; 23 ( 4 ): 795 – 801 . Crossref PubMed Google Scholar
7. Mumith A , Coathup M , Fromme P , et al. Utilising additive layer manufactured components to improve osteointegration of endoprostheses: a FEA and histological study . Front Bioeng Biotechnol . 2016 ; 4 . Google Scholar
8. Coathup MJ , Batta V , Pollock RC , et al. Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up . J Bone Joint Surg Am . 2013 ; 95-A ( 17 ): 1569 – 1575 . Crossref PubMed Google Scholar
9. Coathup MJ , Sanghrajka A , Aston WJ , et al. Hydroxyapatite-coated collars reduce radiolucent line progression in cemented distal femoral bone tumor implants . Clin Orthop Relat Res . 2015 ; 473 ( 4 ): 1505 – 1514 . Crossref PubMed Google Scholar
10. Dwek JR . The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol . 2010 ; 39 ( 4 ): 319 – 323 . Crossref PubMed Google Scholar
11. No authors listed . ISRCTN Registry. EURO-EWING 99: European ewing tumour working initiative of national groups . http://www.isrctn.com/ISRCTN61438620?q=61438620&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10&searchType=basic-search (date last accessed 26 June 2020 ). Google Scholar
12. Boden SD , Sumner DR . Biologic factors affecting spinal fusion and bone regeneration . Spine . 1995 ; 20 ( 24 Suppl ): 102S – 112 . PubMed Google Scholar
13. Cohen J . Statistical Power Analysis for the Behavioral Sciences. Mahwah , ed . Second . New Jersey : Lawrence Erlbaum Associates , 1988 . Google Scholar
14. Avedian RS , Goldsby RE , Kramer MJ , O’Donnell RJ . Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses . Clin Orthop Relat Res . 2007 ; 459 ( 459 ): 48 – 53 . Crossref PubMed Google Scholar
15. Muscolo DL , Ayerza MA , Aponte-Tinao LA , Ranalletta M . Use of distal femoral osteoarticular allografts in limb salvage surgery . J Bone Joint Surg Am . 2005 ; 87-A ( 11 ): 2449 – 2455 . Crossref PubMed Google Scholar
16. Perren SM . Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology . J Bone Joint Surg Br . 2002 ; 84-B ( 8 ): 1093 – 1110 . Crossref PubMed Google Scholar
17. Chao EY , Sim FH . Composite fixation of segmental bone/joint defect replacement (SDR) prostheses. biological and biomechanical justifications . Chir Organi Mov . 1990 ; 75 ( 1 Suppl ): 171 – 173 . PubMed Google Scholar
18. Cannon SR . The use of megaprosthesis in the treatment of periprosthetic knee fractures . Int Orthop . 2015 ; 39 ( 10 ): 1945 – 1950 . Crossref PubMed Google Scholar
19. Barrère F , van Blitterswijk CA , de Groot K . Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics . Int J Nanomedicine . 2006 ; 1 ( 3 ): 317 – 332 . PubMed Google Scholar
20. Choate JJ , Mosher DF . Fibronectin concentration in plasma of patients with breast cancer, colon cancer, and acute leukemia . Cancer . 1983 ; 51 ( 6 ): 1142 – 1147 . Crossref PubMed Google Scholar
21. Brodin B , Liedén G , Malm C , Vikrot O . Plasma fibronectin deficiency during chemotherapy of acute myeloid leukaemia . Scand J Haematol . 1983 ; 30 ( 3 ): 247 – 249 . Crossref PubMed Google Scholar
22. Carminati PO , Mello SS , Fachin AL , et al. Alterations in gene expression profiles correlated with cisplatin cytotoxicity in the glioma U343 cell line . Genet Mol Biol . 2010 ; 33 ( 1 ): 159 – 168 . Crossref PubMed Google Scholar
23. Burchardt H , Glowczewskie FP , Enneking WF . The effect of Adriamycin and methotrexate on the repair of segmental cortical autografts in dogs . J Bone Joint Surg Am . 1983 ; 65-A ( 1 ): 103 – 108 . PubMed Google Scholar
24. Friedlaender GE , Tross RB , Doganis AC , Kirkwood JM , Baron R . Effects of chemotherapeutic agents on bone. I. short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model . The Journal of Bone & Joint Surgery . 1984 ; 66 ( 4 ): 602 – 607 . PubMed Google Scholar
25. Pelker RR , Friedlaender GE , Panjabi MM , et al. Chemotherapy-induced alterations in the biomechanics of rat bone . J Orthop Res . 1985 ; 3 ( 1 ): 91 – 95 . Crossref PubMed Google Scholar
26. Mumith A , Coathup M , Chimutengwende-Gordon M , et al. Augmenting the osseointegration of endoprostheses using laser-sintered porous collars: an in vivo study . Bone Joint J . 2017 ; 99-B ( 2 ): 276 – 282 . Crossref PubMed Google Scholar
Author contributions
A. Mumith: Designed the study, Analyzed and interpreted the data, Wrote and edited the manuscript.
M. Coathup: Designed the study, Interpreted the data, Edited the manuscript.
T. C. Edwards: Analyzed the radiographs, Interpreted the data, Edited the manuscript.
P. Gikas: Performed the operations, Interpreted the data, Edited the manuscript.
W. Aston: Performed the operations, Interpreted the data, Edited the manuscript.
G. Blunn: Designed the study, Interpreted the data, Edited the manuscript, Supervised the project.
Funding statement
This work was supported by Royal College of Surgeons of England Enid Linder Research Fellowship, Orthopaedic Research UK, and Skeletal Cancer Action Trust.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
W. Aston reports personal fees from Stanmore Implants Worldwide, unrelated to the study.
Acknowledgements
We thank the Royal College of Surgeons of England, Orthopaedic Research UK, and Skeletal Cancer Action Trust for funding part of this study.
Ethical review statement
This study did not require ethical approval.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









