Cite this article: Bone Joint Res 2020;9(6):311–313.
Debridement is a central tenet in the management of prosthetic joint infections (PJIs). Modern debridement has three distinct stages: 1) surgical; 2) mechanical/physical; and 3) chemical.1 Given more recent understanding in the pathogenesis of PJI,2 the microscopic targeting of the bacterial biofilm and intracellular pathogens should also be included in the art of debridement.2,3 The adequacy of debridement is critical to the success of single-stage procedures, which have been shown to result in lower patient morbidity and healthcare costs.4 There is currently an unmet clinical need to optimize debridement in the management of PJI.
Surgical debridement
Novel ultrasonic cutting devices may be beneficial as they selectively apply high strain to hard tissues, while soft tissues such as ligaments and nerves can be deflected without damage.5
Mechanical/physical debridement
Non-contact induction heating of metal prostheses delivers localized thermal damage to the biofilm, resulting in bacterial eradication and antibiotic synergism.6-8 Pulsed electromagnetic fields are used to induce eddy currents within metallic prostheses to generate heat.7 Low-intensity pulsed ultrasound potentiates antimicrobials when used against biofilms,3 as well as increasing the in vitro elution of antimicrobials from polymethylmethacrylate cement.9,10
Chemical debridement
Topical antiseptic agents, such as Betadine, are only partially effective in the eradication of bacterial biofilms.11 Ethylenediaminetetraacetic acid (EDTA) is a safe and effective debridement adjunct against multidrug-resistant pathogens in implant-related infections.12 EDTA, similar to acetic acid,11,13 already has regulatory approval for its use in the management of superficial wound infections.14
Biological debridement
There has been a renewed interest in bacteriophage therapy due to the pending global crisis in antimicrobial resistance.15 Bacteriophages are viruses that infect and inactivate bacteria. Unlike traditional antibiotics, bacteriophage activity is not limited by bacterial cell dormancy, furthermore the biological cost of acquired resistance to bacteriophages permits phage-susceptible clones to persist within wild-type populations.15 Host cell internalization allows pathogens to avoid antimicrobial exposure and immune system interaction. One strategy to overcome this problem is the addition of cell-penetrating peptides to both established antimicrobials and novel therapeutics.16 A further approach is the development of liposome nanocarriers, which are phospholipid vesicles that are able to penetrate both biofilms and mammalian cells.17 Modified delivery systems using cell-penetrating peptides or liposome nanocarriers would allow co-localization of antimicrobial agents with intracellular pathogens. Monoclonal antibodies that target matrix components of biofilms such as extracellular DNA, virulence factors, and adhesion factors can disperse established biofilms,18 act synergistically with established antimicrobials to eradicate pathogens,18 and even inhibit biofilm formation.19
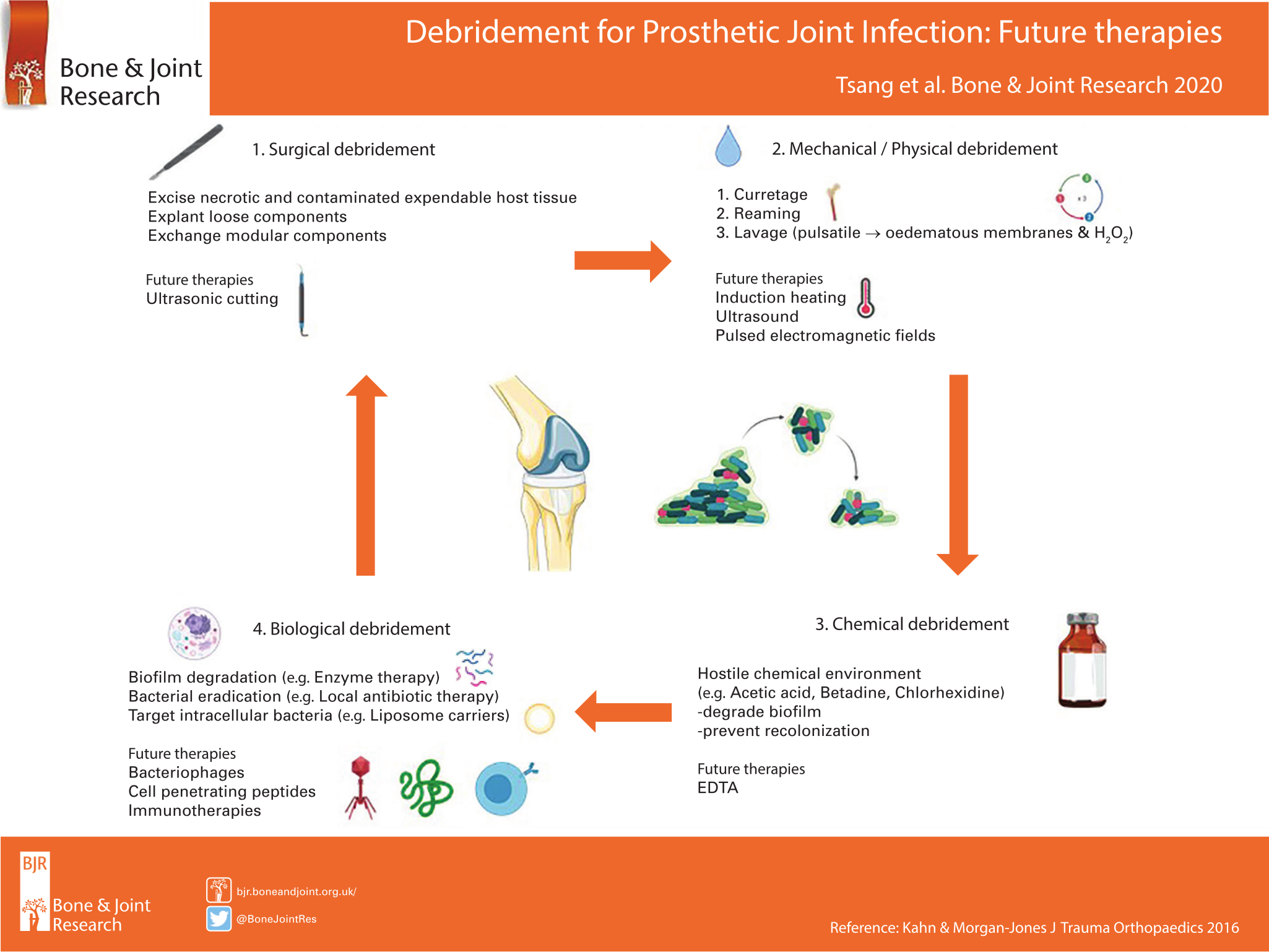
Fig. 1
References
1. Khan W , Morgan-Jones R . Debridement: defining something we all do . J Trauma Orthop . 2016 ; 4 ( 1 ): 48 – 50 . Google Scholar
2. Yang D , Wijenayaka AR , Solomon LB , et al. Novel Insights into Staphylococcus aureus Deep Bone Infections: the Involvement of Osteocytes . mBio . 2018 ; 9 ( 2 ): e00415 – e00418 . Crossref PubMed Google Scholar
3. Tsang STJ , Simpson A . Pathogenesis of biomaterial-associated infection. In: Li B, Moriarty TF, Webster T, Xing M, eds . Racing for the surface: pathogenesis of implant infection and advanced antimicrobial strategies . Cham : Springer International Publishing , 2020 : 109 – 169 . Google Scholar
4. Grammatopoulos G , Bolduc ME , Atkins BL , et al. Functional outcome of debridement, antibiotics and implant retention in periprosthetic joint infection involving the hip: a case-control study . Bone Joint J . 2017 ; 99-B ( 5 ): 614 – 622 . Crossref PubMed Google Scholar
5. Bejarano F , Feeney A , Wallace R , Simpson H , Lucas M . An ultrasonic orthopaedic surgical device based on a cymbal transducer . Ultrasonics . 2016 ; 72 : 24 – 33 . Crossref PubMed Google Scholar
6. Pijls BG , Sanders I , Kuijper EJ , Nelissen R . Non-contact Electromagnetic Induction Heating for Eradicating Bacteria and Yeasts on Biomaterials and Possible Relevance to Orthopaedic Implant Infections: In vitro Findings . Bone Joint Res . 2017 ; 6 ( 5 ): 323 – 330 . Crossref PubMed Google Scholar
7. Pijls BG , Sanders I , Kuijper EJ , Nelissen R . Segmental induction heating of orthopaedic metal implants . Bone Joint Res . 2018 ; 7 ( 11 ): 609 – 619 . Crossref PubMed Google Scholar
8. Pijls BG , Sanders I , Kujiper EJ , Nelissen R . Induction heating for eradicating Staphylococcus epidermidis from biofilm . Bone Joint Res . 2020 ; 9 ( 4 ): 192 – 199 . Crossref PubMed Google Scholar
9. Nicholson JA , Tsang STJ , MacGillivray TJ , Perks F , Simpson A . What is the role of ultrasound in fracture management?: diagnosis and therapeutic potential for fractures, delayed unions, and fracture-related infection . Bone Joint Res . 2019 ; 8 ( 7 ): 304 – 312 . Crossref PubMed Google Scholar
10. Wendling A , Mar D , Wischmeier N , Anderson D , McIff T . Combination of modified mixing technique and low frequency ultrasound to control the elution profile of vancomycin-loaded acrylic bone cement . Bone Joint Res . 2016 ; 5 ( 2 ): 26 – 32 . Crossref PubMed Google Scholar
11. Tsang STJ , Gwynne PJ , Gallagher MP , Simpson A . The biofilm eradication activity of acetic acid in the management of periprosthetic joint infection . Bone Joint Res . 2018 ; 7 ( 8 ): 517 – 523 . Crossref PubMed Google Scholar
12. Deng Z , Liu F , Li C . Therapeutic effect of ethylenediaminetetraacetic acid irrigation solution against wound infection with drug-resistant bacteria in a rat model: an animal study . Bone Joint Res . 2019 ; 8 ( 5 ): 189 – 198 . Crossref PubMed Google Scholar
13. Williams RL , Ayre WN , Khan WS , Mehta A , Morgan-Jones R . Acetic Acid as Part of a Debridement Protocol During Revision Total Knee Arthroplasty . J Arthroplasty . 2017 ; 32 ( 3 ): 953 – 957 . Crossref PubMed Google Scholar
14. Finnegan S , Percival SL . EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care . Adv Wound Care (New Rochelle) . 2015 ; 4 ( 7 ): 415 – 421 . Crossref PubMed Google Scholar
15. Akanda ZZ , Taha M , Abdelbary H . Current review-The Rise of Bacteriophage as a Unique Therapeutic Platform in Treating Peri-Prosthetic Joint Infections . J Orthop Res . 2018 ; 36 ( 4 ): 1051 – 1060 . Crossref PubMed Google Scholar
16. Zahid M , Robbins PD . Cell-type specific penetrating peptides: therapeutic promises and challenges . Molecules . 2015 ; 20 ( 7 ): 13055 – 13070 . Crossref PubMed Google Scholar
17. Rukavina Z , Vanić Ž . Current trends in development of liposomes for targeting bacterial biofilms . Pharmaceutics . 2016 ; 8 ( 2 ): 8 . Crossref PubMed Google Scholar
18. Estellés A , Woischnig AK , Liu K , et al. A high-affinity native human antibody disrupts biofilm from Staphylococcus aureus bacteria and potentiates antibiotic efficacy in a mouse implant infection model . Antimicrob Agents Chemother . 2016 ; 60 ( 4 ): 2292 – 2301 . Crossref PubMed Google Scholar
19. Wang Y , Cheng LI , Helfer DR , et al. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets . Proc Natl Acad Sci U S A . 2017 ; 114 ( 26 ): E5094 – E5102 . Crossref PubMed Google Scholar
Author contributions
S-T. J. Tsang: Conceptualized, created, and edited the infographic.
R. Morgan-Jones: Conceptualized, created, and edited the infographic.
A. H. R. W. Simpson: Conceptualized, created, and edited the infographic.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
None declared.
Acknowledgements
None declared.
Ethical review statement
This study did not require ethical approval.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









