Abstract
Aims
The aim of this study was to examine whether tourniquet use can improve perioperative blood loss, early function recovery, and pain after primary total knee arthroplasty (TKA) in the setting of multiple-dose intravenous tranexamic acid.
Methods
This was a prospective, randomized clinical trial including 180 patients undergoing TKA with multiple doses of intravenous tranexamic acid. One group was treated with a tourniquet during the entire procedure, the second group received a tourniquet during cementing, and the third group did not receive a tourniquet. All patients received the same protocol of intravenous tranexamic acid (20 mg/kg) before skin incision, and three and six hours later (10 mg/kg). The primary outcome measure was perioperative blood loss. Secondary outcome measures were creatine kinase (CK), CRP, interleukin-6 (IL-6), visual analogue scale (VAS) pain score, limb swelling ratio, quadriceps strength, straight leg raising, range of motion (ROM), American Knee Society Score (KSS), and adverse events.
Results
The mean total blood loss was lowest in the no-tourniquet group at 867.32 ml (SD 201.11), increased in the limited-tourniquet group at 1024.35 ml (SD 176.35), and was highest in the tourniquet group at 1,213.00 ml (SD 211.48). The hidden blood loss was lowest in the no-tourniquet group (both p < 0.001). There was less mean intraoperative blood loss in the tourniquet group (77.48 ml (SD 24.82)) than in the limited-tourniquet group (137.04 ml (SD 26.96)) and the no-tourniquet group (212.99 ml (SD 56.35); both p < 0.001). Patients in the tourniquet group showed significantly higher levels of muscle damage and inflammation biomarkers such as CK, CRP, and IL-6 than the other two groups (p < 0.05). Outcomes for VAS pain scores, limb swelling ratio, quadriceps strength, straight leg raising, ROM, and KSS were significantly better in the no-tourniquet group at three weeks postoperatively (p < 0.05), but there were no significant differences at three months. No significant differences were observed among the three groups with respect to transfusion rate, thrombotic events, or the length of hospital stay.
Conclusion
Patients who underwent TKA with multiple doses of intravenous tranexamic acid but without a tourniquet presented lower total blood loss and hidden blood loss, and they showed less postoperative inflammation reaction, less muscle damage, lower VAS pain score, and better early knee function. Our results argue for not using a tourniquet during TKA.
Cite this article: Bone Joint Res 2020;9(6):322–332.
Article focus
-
The purpose of this study was to examine whether the tourniquet can improve perioperative blood loss, early recovery, and pain from total knee arthroplasty (TKA) beyond the benefits of multiple-dose intravenous tranexamic acid.
Key messages
-
Total blood loss and hidden blood loss were lowest in the no-tourniquet group compared with the other two groups.
-
Patients in the tourniquet group showed significantly higher levels of muscle damage and inflammation biomarkers such as CK, CRP, and IL-6 than the other two groups.
-
Early clinical outcomes were significantly better in the no-tourniquet group at three weeks postoperatively, but there were no significant differences at three months.
Strengths and limitations
-
This is the first study to evaluate whether the tourniquet can improve clinical outcomes in TKA beyond the benefits of multiple-dose intravenous tranexamic acid.
-
The study was to determine if the use of multiple doses of tranexamic acid without tourniquet can reduce muscle damage and inflammatory reaction.
-
Our study did not investigate prosthetic fixation or survival outcomes in the long term.
Introduction
The application of a tourniquet during total knee arthroplasty (TKA) has been proposed to improve the visibility of the operative field and reduce blood loss.1 Although widely used by orthopaedic surgeons, the tourniquet remains controversial.2-4 Some reports have shown that the tourniquet can reduce blood loss, postoperative inflammation, and muscle damage, as well as slight postoperative pain, but without improving early recovery.5,6 Previous studies found that avoidance of the tourniquet has several benefits, including reduction in total blood loss, knee pain, limb swelling, and inflammatory biomarkers.3,7 Previous work also showed that patients undergoing primary TKA without a tourniquet presented earlier recovery and higher patient satisfaction.7-9 Tourniquet use has been associated with numerous disadvantages, including perioperative pain, reperfusion injury, limb swelling, delayed rehabilitation, wound complication, venous thrombosis, and peripheral nerve injury.10-12 One study showed that the tourniquet can reduce intraoperative blood loss but increase the total and postoperative blood losses, such that the rate of transfusion during TKA is similar to that with a tourniquet.13 The reason for this may be activation of fibrinolysis following tourniquet release.14,15 The higher volume of postoperative blood loss may be due to unintended coagulopathy because tourniquet deflation can alter the normal coagulation balance.7
Several strategies have been developed to use the tourniquet while mitigating its adverse effects, including the inflation of the tourniquet at the start of the procedure and deflation after cement hardening, inflation of the tourniquet only during cementation, or use of the tourniquet throughout the entire surgery.16 However, one study did not find differences in operating time, pain score, pain medication requirements, rehabilitation, haemoglobin (Hb) change, or total blood loss between limited tourniquet use or tourniquet use from the start of the procedure until cement hardening.17
At our hospital, tranexamic acid was recently incorporated as a routine procedure in TKA. Several studies have shown that the use of tranexamic acid reduces postoperative bleeding and transfusion rate without increasing thrombotic events.18-23 Administration of multiple doses of tranexamic acid can decrease blood loss and reduce postoperative inflammatory response, pain, limb swelling, and length of hospital stay. Multiple doses of intravenous tranexamic acid can also lead to significantly lower blood loss than a single dose of intravenous tranexamic acid.24
In view of the controversial results, no consensus has been reached on the use of a tourniquet during TKA. To our knowledge, few studies have asked whether the tourniquet can improve outcomes in TKA beyond the benefits of multiple-dose intravenous tranexamic acid. The hypothesis was that the primary TKA without tourniquet use would reduce blood loss, muscle damage, and improve early function recovery and pain in an enhanced recovery protocol. The main purpose of this study was to identify the risks and benefits of tourniquet use with multiple doses of intravenous TXA in primary TKA.
Methods
Study population
This study was approved by the Institutional Review Board of the First Hospital of Lanzhou University, Lanzhou, China, and registered in the Chinese Clinical Trial Registry (ChiCTR-INR-17013110). Patients provided written informed consent prior to being enrolled. Patients over 55 years of age undergoing unilateral primary TKA for end-stage osteoarthritis from January 2017 to December 2018 were included in the trial. The exclusion criteria included rheumatoid arthritis, coagulopathy, anaemia (Hb level < 12 g/dl for women and < 13 g/dl for men), contraindications for tranexamic acid, uncontrolled hypertension, previous knee surgery, extreme limb deformity (varus or valgus angle ≥ 20°), renal dysfunction, peripheral vascular disease, and body mass index (BMI) > 35 kg/m2. In total, 232 patients were screened for inclusion in the study, of whom 28 patients refused to participate. In total 15 patients receiving anticoagulant therapy were excluded, as were nine patients with peripheral vascular disease. The remaining 180 patients were enrolled in the study and randomized to each treatment group (n = 60 per group; Figure 1). The three groups presented similar baseline characteristics. There were no significant differences in terms of preoperative laboratory values, including Hb, HCT, PT, APTT, INR, FIB, or platelet count (Table I). All patients had been diagnosed with knee osteoarthritis. Patients were randomized into three groups in a 1:1:1 ratio using a computer-generated random number table. The randomization numbers were contained in sealed, opaque envelopes and kept in the office by staff who were not involved in the research and did not measure outcomes. Surgeons were informed of the assignment for each patient 24 hours before the operative procedure. The researcher assistant opened a sealed envelope to reveal the allocation. Surgeons, patients, nurses, data collectors, and research assistants were blinded during the entire study. Preoperative and postoperative data were collected by two physician assistants, not by the operating surgeon. Intraoperative data were recorded by a research fellow. Postoperative recovery during the hospital stay was evaluated by an experienced physical therapist, who was blinded to patient allocation.
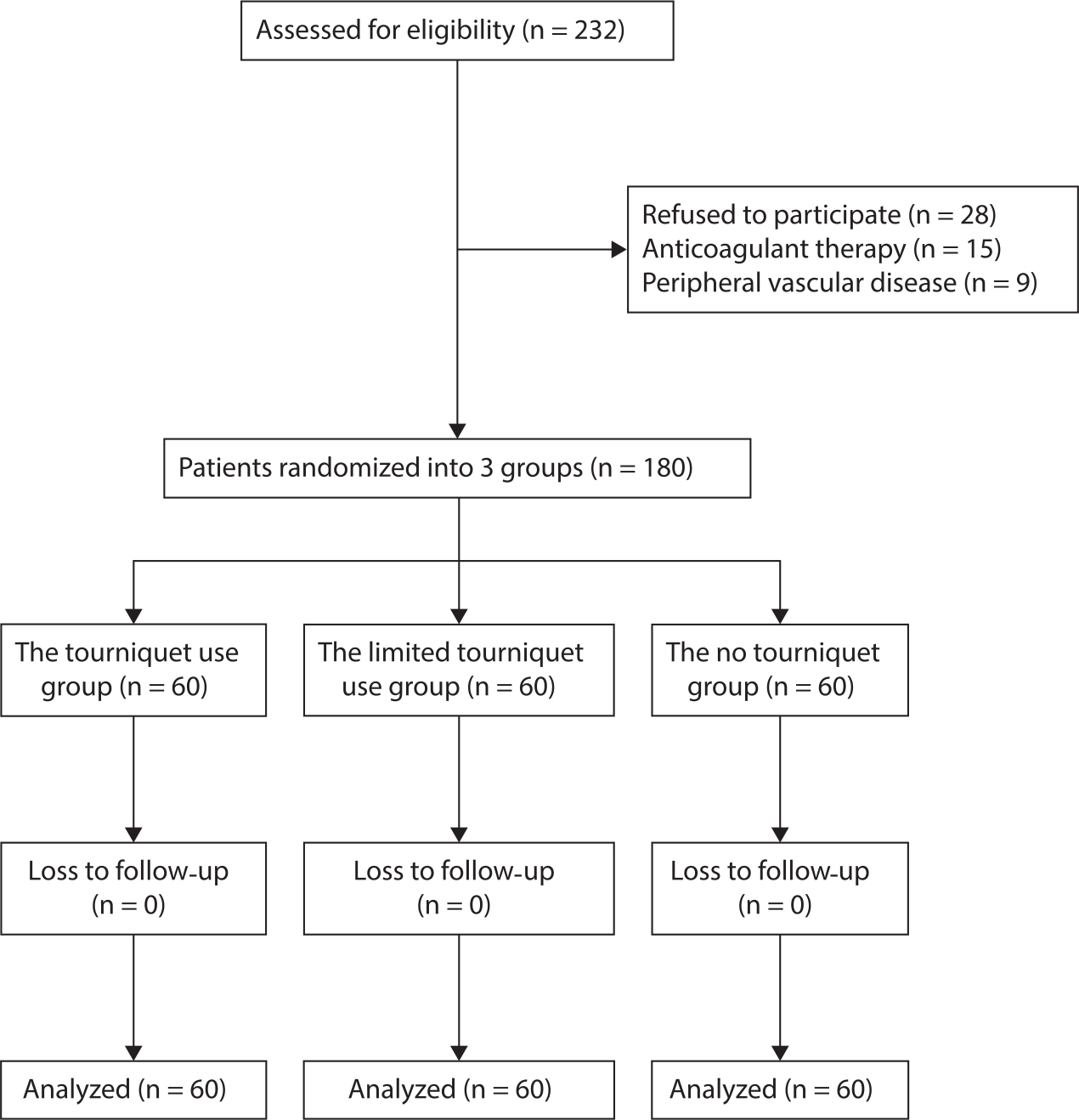
Fig. 1
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the present trial showing patient selection and exclusion.
Table I.
Clinical and demographic characteristics of patients before surgery.
| Characteristic | Tourniquet (n = 60) | Limited tourniquet (n = 60) | No tourniquet (n = 60) | p-value |
|---|---|---|---|---|
| Mean age, yrs (SD) | 65.01 (9.59) | 65.55 (7.87) | 64.53 (8.54) | 0.132* |
| Sex, n (%) | ||||
| Female | 23 (38) | 18 (30) | 20 (33) | 0.216 |
| Male | 37 (62) | 42 (70) | 40 (67) | 0.341 |
| Mean BMI, kg/m2 (SD) | 26.35 (3.52) | 25.72 (4.78) | 25.74 (5.41) | 0.692* |
| ASA classification, n (%) | ||||
| I | 11 (19) | 12 (20) | 13 (21) | 0.704† |
| II | 32 (53) | 32 (53) | 31 (52) | 0.693† |
| III | 17 (28) | 16 (27) | 16 (27) | 0.562† |
| Mean haemoglobin, g/dl (SD) | 137.85 (5.58) | 135.25 (15.96) | 138.71 (14.64) | 0.972* |
| Mean haematocrit, % (SD) | 40.98 (4.32) | 40.50 (4.62) | 40.82 (3.76) | 0.915* |
| Mean prothrombin time, s (SD) | 11.67 (2.25) | 11.28 (1.57) | 11.42 (0.58) | 0.312* |
| Mean APTT, s (SD) | 26.08 (3.27) | 27.30 (3.30) | 26.75 (3.67) | 0.143* |
| Mean INR (SD) | 0.94 (0.08) | 0.93 (0.05) | 0.95 (0.06) | 0.462* |
| Mean fibrinogen, g/l (SD) | 210.66 (42.15) | 198.81 (38.09) | 205.45 (39.30) | 0.316* |
| Mean platelet count, × 109/l (SD) | 213.66 (82.15) | 194.81 (58.09) | 205.45 (60.20) | 0.315* |
-
*
One-way analysis of variance.†Chi-squared test.
-
APTT, activated partial thromboplastin time; ASA, American Society of Anesthesiologists; BMI, body mass index; INR, international normalized ratio.
The tourniquet group was treated with a tourniquet during the entire TKA procedure. The tourniquet was inflated before the incision and was deflated after closure of the incision. In the limited-tourniquet group, the tourniquet was inflated only during component cementing and was deflated after the cement had hardened prior to closure of the joint capsule. In the no-tourniquet group, the tourniquet was wrapped around the thigh but was not inflated during the surgery.
All patients received the same protocol of intravenous tranexamic acid (20 mg/kg) before skin incision, and three and six hours later (10 mg/kg). In the tourniquet and limited-tourniquet groups, the tourniquet was inflated to a systolic blood pressure over 100 mmHg.
Operative procedures
All TKAs were performed by the same medical team composed of three senior surgeons (WJW and HYZ), and surgical procedures were performed according to routine practice. A standard midline skin incision and the medial parapatellar approach were used in the operative procedure, as well as a measured resection technique. All patients received spinal anaesthesia and the same type of cemented prosthesis (Genesis II Total Knee System; Smith & Nephew, Memphis, Tennessee, USA). An intramedullary guide was used for femoral cuts, and an extramedullary guide was used for tibial preparation. Electrocauterization was used to ensure haemostasis throughout the procedure, and no topical chemical haemostatic agents were used. An 80 ml cocktail with 200 mg ropivacaine and 1% epinephrine was injected into the joint space to enhance postoperative pain control prior to capsule closing. We did not use drains postoperatively. All knees were packed with ice immediately after surgery in order to decrease blood loss and alleviate pain. Operating time, tourniquet time, and intraoperative blood loss were recorded by research assistants.
Postoperative care protocol
All patients were treated with a comprehensive, multidisciplinary approach of postoperative care, including multiple pain management, anticoagulation regimens, and other systemically physical therapies. Patients without contraindications were administered a standard pain control protocol that involved preoperative celecoxib (200 mg two times per day), hydrocodone (10 mg two times per day), and parecoxib (40 mg two times per day) for postoperative pain control. Celecoxib (200 mg two times per day for two weeks) was routinely prescribed for pain control after discharge if there was no contraindication. All patients received a half dose of enoxaparin (0.2 ml, 2000 IU, Clexane; Sanofi-Aventis, Paris, France) at six hours postoperatively, followed by a full dose (0.4 ml, 4000 IU) once daily for three days. After discharge, rivaroxaban was administered orally at 10 mg once a day for ten days in all cases if no bleeding events occurred. A Doppler ultrasound examination of both lower limbs was performed in all patients to assess potential deep venous thrombosis at the time of discharge and at three weeks and three months after operation. CT, in combination with clinical symptom assessment, was used to evaluate potential pulmonary embolism. All patients were managed through an early rehabilitation programme that included muscle power training, passive and active range of motion (ROM) training, and walking training on the day of the surgery.
Outcome measurements
Patient demographic data were recorded, including age, sex, BMI, and the American Society of Anesthesiologists (ASA) class. Before surgery, Hb, haematocrit (HCT), prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrinogen (FIB), and platelet counts were collected. Recorded intraoperative characteristics included the use of a tourniquet, the time of tourniquet use, and the operative time (from incision to end of closure). The primary outcome measure was total blood loss. In addition, intraoperative blood loss, hidden blood loss, and blood transfusion rate were measured. Total blood loss was calculated according to the modification of the Gross formula, which includes sex, height, weight, and preoperative and postoperative day 2 HCT values.25 If blood transfusion was performed before the lowest Hb level was obtained, the total blood loss was calculated as the change in Hb plus the volume of blood transfused.26 Intraoperative blood loss was defined as the amount of blood collected in the suction canister and in saturated surgical sponges. There is one patient who was transfused prior to HB being taken in the tourniquet use group. A perioperative blood transfusion was performed if Hb levels were < 7 g/dl, or patients showed clinical symptoms and signs of anaemia (light-headedness, fatigue, palpitation, tachycardia > 100 beats per minute, urine output < 30 ml per hour, drop in systolic blood pressure < 90 mmHg).
Secondary outcomes were measured, including creatine kinase (CK), CRP, and interleukin-6 (IL-6) at each timepoint (before surgery and at postoperative days 1, 2, 3, and 4). The perioperative visual analogue scale (VAS) pain score, the length of hospital stay, venous thromboembolism events, and other complications were also recorded. The swelling ratio was determined during the hospital stay as well as at three weeks and three months after TKA based on the mean knee circumference of the upper pole of the patella and the lower pole of the patella, and was presented as a percentage of the value for the contralateral limb.24 Other measures that were assessed for postoperative knee function included quadriceps strength, time interval of straight leg raising, knee active ROM, and Harris Hip Score (HHS) at discharge as well as at three weeks and three months after the operation.
Statistical analysis
Sample size was based on the power analysis from a pilot study involving 60 patients, in which a 15% reduction in total blood loss was detected in the no-tourniquet group compared with the limited-tourniquet and tourniquet groups, and the pooled SD was 283 ml. Thus, we calculated that 53 individuals per group would be required to detect a statistically significant difference between groups with a two-sided alpha level of 0.05 and a power of 90%. Considering the risk of dropouts, 60 patients were included in each of the three groups. Data were analyzed using SPSS v22.0 (IBM, Chicago, Illinois, USA). Continuous variables are presented as means with SDs if the data followed a normal distribution, whereas categorical data are shown as percentages and frequencies. Differences in continuous variables between the groups were assessed using one-way analysis of variance (ANOVA). Differences in categorical variables were assessed using a chi-squared test or Fisher's exact test. Differences associated with p < 0.05 were considered significant.
Results
The mean operating time in the tourniquet group (72.70 minutes (SD 10.84)) was significantly shorter than in the limited-tourniquet group (83.11 minutes (SD 11.27); p < 0.001) and the no-tourniquet group (90.57 minutes (SD 13.20); p = 0.021, all by one-way ANOVA). The mean tourniquet time was significantly longer in the tourniquet group (72.70 minutes (SD 10.84)) than in the limited-tourniquet group (19.50 minutes (SD 4.43); p < 0.001). Mean intraoperative blood loss was significantly lower in the tourniquet group than in the other two groups (both p < 0.001, one-way ANOVA). The mean length of hospital stay was similar across the three groups: 5.65 days (SD 1.14) in the tourniquet group, 5.51 days (SD 1.18) in the limited-tourniquet group, and 5.48 days (SD 1.54) in the no-tourniquet group.
Blood loss
Mean total blood loss was significantly smaller in the no-tourniquet group (867.32 ml (SD 201.11); p < 0.001) than in the tourniquet group (1,213.00 ml (SD 211.48); p < 0.001) and limited-tourniquet group (1,024.35 ml (SD 176.35); p < 0.001, all by one-way ANOVA) (Figure 2a). Similarly, mean hidden blood loss was significantly lower in the no-tourniquet group (654.33 ml (SD 124.82)) than in the tourniquet group (1,135.53 ml (SD 189.17); p = 0.001) and limited-tourniquet group (887.31 ml (SD 171.51); p < 0.001, all by one-way ANOVA) (Table II). Mean Hb and HCT levels fell significantly in all groups during surgery (Figures 2b and 2c), but the decreases were significantly smaller in the no-tourniquet group (28.35 g/dl (SD 8.79) and 8.53% (SD 3.48%), respectively; both p < 0.05, all by one-way ANOVA) (Figures 2d and 2e). Transfusion rates were similar across the three groups: three patients in the tourniquet group; two in the limited-tourniquet group; and two in the no-tourniquet group (Table II).
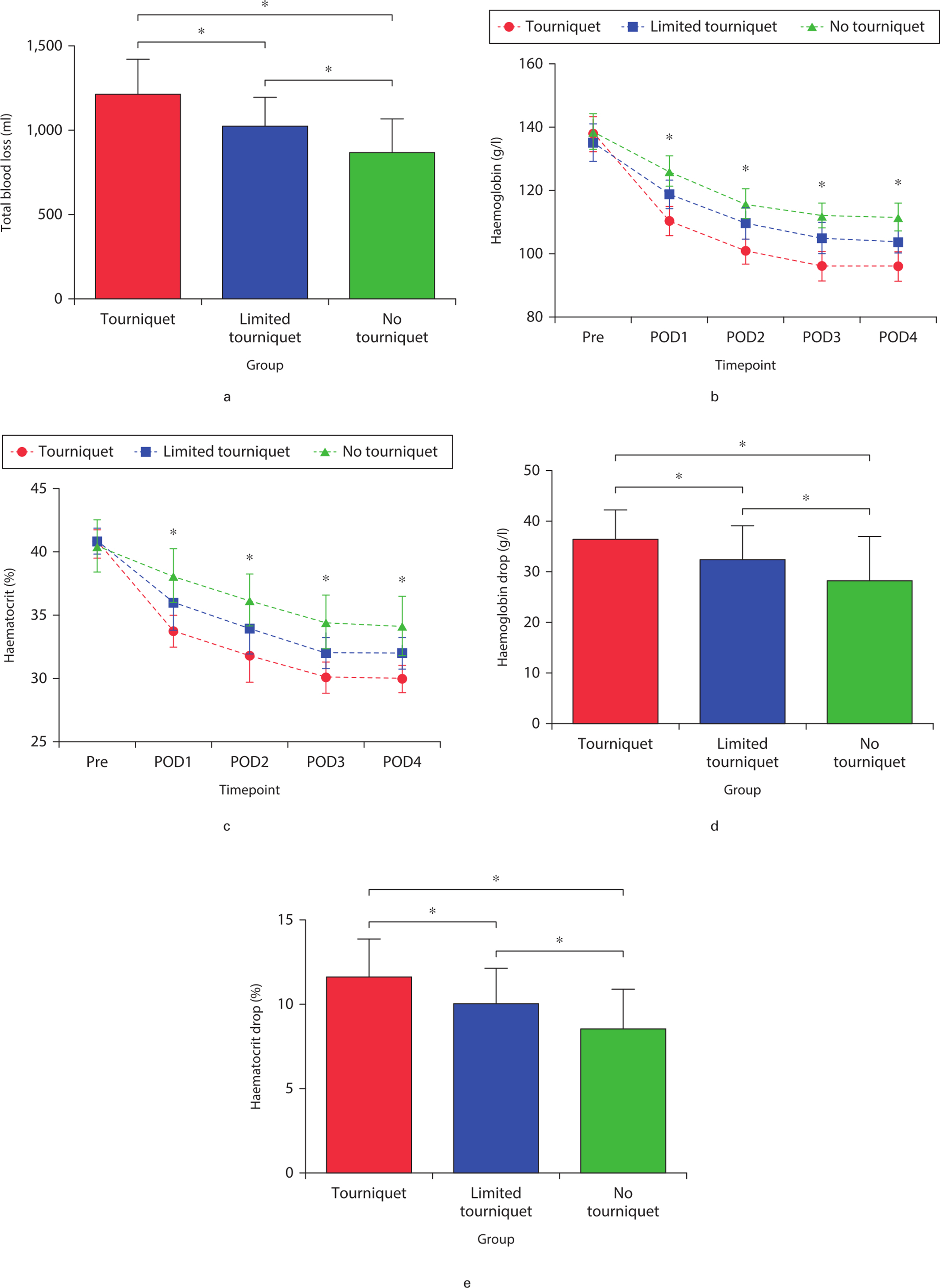
Fig. 2
a) Total blood loss, b) haemoglobin levels, and c) haematocrit levels preoperatively and at postoperative days 1 to 4. d) Comparison of the haemoglobin drop between the preoperative value and the postoperative lowest value, and e) haematocrit drop between the preoperative value and the postoperative lowest value among the groups. *Significant differences (p < 0.05) between the indicated groups (Figures 2a, 2d, and 2e) or among all three groups (Figures 2b and 2c), measured using one-way analysis of variance. Pre, preoperative; POD, postoperative day.
Table II.
Comparison of differences in blood loss among the patient groups.
| Parameter | Tourniquet (n = 60) | Limited tourniquet (n = 60) | No tourniquet (n = 60) | P1 value | P2 value | P3 value |
|---|---|---|---|---|---|---|
| Mean calculated blood loss, ml (SD) | 1,213.00 (211.48) | 1,024.35 (176.35) | 867.32 (201.11) | < 0.001* | < 0.001* | < 0.001* |
| Mean intraoperative blood loss, ml (SD) | 77.48 (24.82) | 137.04 (26.96) | 212.99 (56.35) | < 0.001* | < 0.001* | 0.012* |
| Mean hidden blood loss, ml (SD) | 1,135.53 (189.17) | 887.31 (171.51) | 654.33 (124.82) | < 0.001* | 0.001* | < 0.001* |
| Mean haemoglobin drop, g/dl (SD) | 36.40 (5.86) | 32.39 (6.62) | 28.35 (8.79) | < 0.001* | 0.005* | 0.006* |
| Mean haematocrit drop, % (SD) | 12.60 (4.32) | 10.05 (3.08) | 8.53 (3.48) | 0.004* | < 0.001* | 0.004* |
| Transfusion rate, n (%) | 3 (5.0) | 2 (3.3) | 2 (3.3) | 0.351 | 0.256 | 0.283 |
| Mean operating time, mins (SD) | 72.70 (10.84) | 83.11 (11.27) | 90.57 (13.20) | < 0.001* | < 0.001* | 0.021* |
| Mean tourniquet duration, mins (SD) | 72.70 (10.84) | 19.50 (4.43) | N/A | < 0.001* | N/A | N/A |
-
*
One-way analysis of variance.
-
N/A, not applicable; P1, comparison between the tourniquet and limited-tourniquet groups; P2, comparison between the tourniquet and no-tourniquet groups; P3, comparison between the limited-tourniquet and no-tourniquet groups.
Soft tissue and inflammation markers
Levels of soft tissue and inflammation markers increased in all groups as a result of surgery. Significant differences were observed among the three groups at postoperative days 1 to 4. CK, a marker of muscle damage, showed an overall increase in all groups postoperatively, reaching a peak on postoperative day 2. CK increase was significantly smaller in the no-tourniquet and limited-tourniquet groups than in the tourniquet group at postoperative days 1 to 4 (p = 0.015, one-way ANOVA; Figure 3a). CRP levels reached a maximum on postoperative day 2. The CRP level was significantly lower in the no-tourniquet group than in the other two groups at postoperative days 1 to 4 (p = 0.003, one-way ANOVA; Figure 3b). The IL-6 level peaked on postoperative day 1 in all groups, and the level was significantly lower in the no-tourniquet group than in the other two groups on postoperative days 1 to 4 (p = 0.021, one-way ANOVA; Figure 3c). CK, CRP, and IL-6 levels differed significantly between the tourniquet and limited-tourniquet groups at all postoperative timepoints (Figures 3a to 3c).
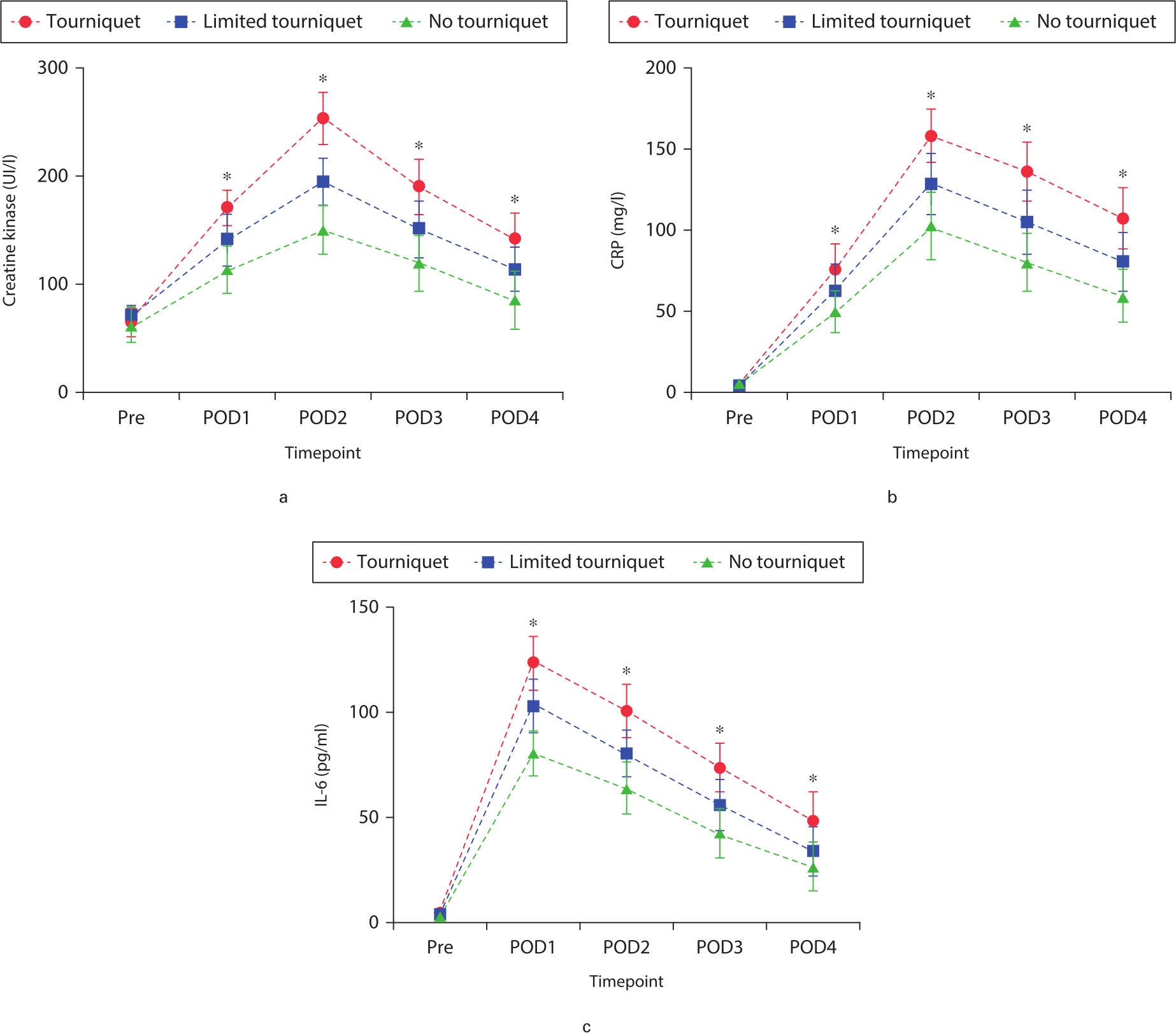
Fig. 3
Mean pre- and postoperative levels of the muscle damage and inflammatory biomarkers: a) creatine kinase (CK), b) CRP, and c) interleukin-6 (IL-6). *Significant differences (p < 0.05) among the three groups, measured by one-way analysis of variance. Pre, preoperative; POD, postoperative day.
Postoperative assessment and rehabilitation
Postoperative knee pain decreased gradually. The no-tourniquet group reported a significantly lower postoperative knee VAS pain score than the other two groups on postoperative days 1 to 4 (both p < 0.031, one-way ANOVA; Figure 4). Preoperative limb swelling was not significantly different among the three groups (Figure 5). The postoperative swelling ratio was significantly lower in the no-tourniquet group than in the other two groups on postoperative days 1 to 4 (both p < 0.021, one-way ANOVA; Figure 5).
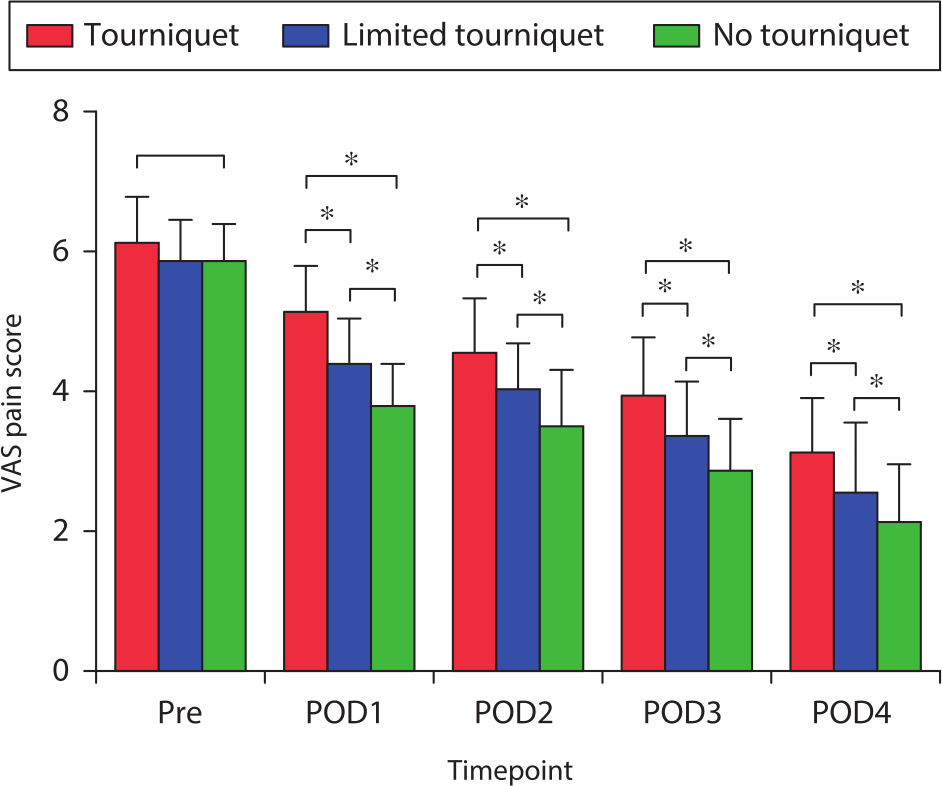
Fig. 4
Mean pre- and postoperative visual analogue scale (VAS) pain scores. *Significant differences among all three groups (all p < 0.05), measured using one-way analysis of variance. Pre, preoperative; POD, postoperative day.
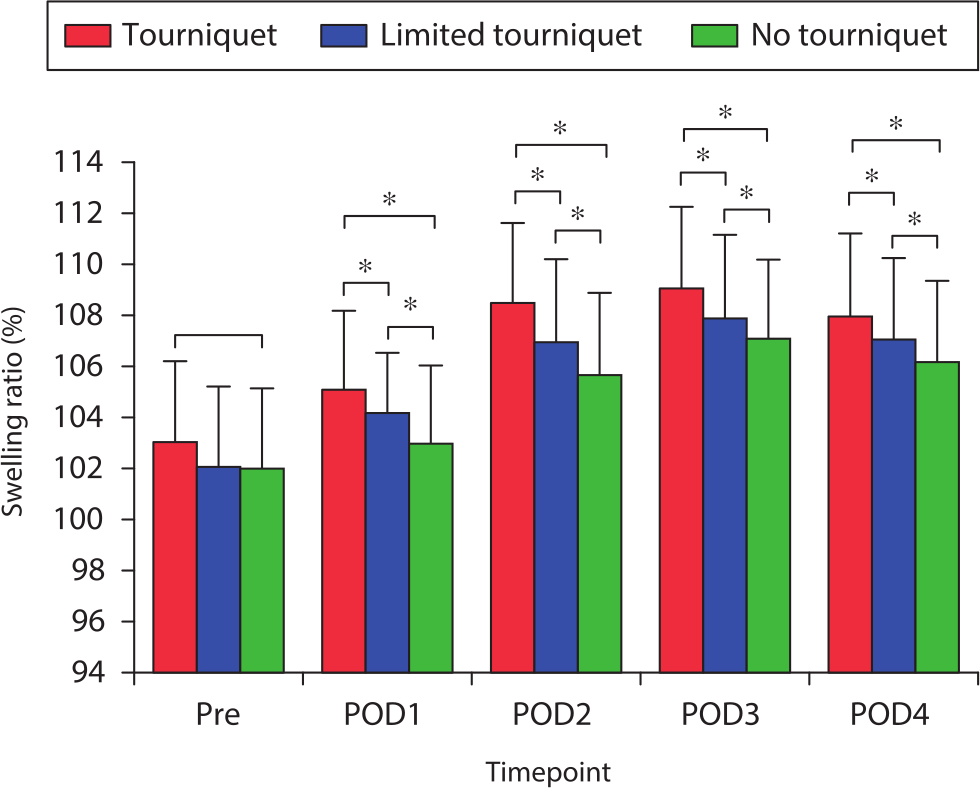
Fig. 5
Mean pre- and postoperative swelling ratio. *Significant differences (p < 0.05) among all three groups, measured using one-way analysis of variance. Pre, preoperative; POD, postoperative day.
Knee function was evaluated using quadriceps strength, the time intervals of straight leg raising, ROM, and American Knee Society Score (KSS) (Table III). There was a substantial improvement in knee function in all the groups at the time of discharge. Quadriceps strength, time interval of straight leg raising, ROM, and KSS were significantly better than in the other two groups at discharge and three weeks postoperatively (Table III). The differences among the three groups were not significant at three months postoperatively (Table III).
Table III.
Clinical outcome and postoperative rehabilitation.
| Parameter | Tourniquet (n = 60) | Limited-tourniquet (n = 60) | No-tourniquet (n = 60) | P1 value | P2 value | P3 value |
|---|---|---|---|---|---|---|
| Mean time intervals straight leg raising, hrs (SD) | 9.90 (1.50) | 8.01 (0.91) | 6.88 (1.13) | 0.001* | 0.001* | 0.001* |
| Mean hospital stay, days (SD) | 5.65 (1.14) | 5.51 (1.18) | 5.48 (1.54) | 0.880* | 0.921* | 0.942* |
| Mean American Knee Society Score (SD) | ||||||
| Preoperative | 50.71 (6.58) | 50.73 (6.78) | 51.11 (7.16) | 0.982* | 0.744* | 0.763* |
| Postoperative day 3 | 72.60 (2.28) | 74.93 (2.91) | 76.76 (3.45) | 0.001* | 0.001* | 0.001* |
| Postoperative week 3 | 80.20 (2.11) | 82.96 (2.46) | 84.88 (2.20) | 0.001* | 0.001* | 0.280* |
| Postoperative month 3 | 88.60 (2.35) | 89.15 (1.65) | 90.30 (1.74) | 0.120* | 0.110* | 0.090* |
| Mean quadriceps strength, grade (SD) | ||||||
| Preoperative | 4.93 (0.25) | 4.91 (0.29) | 4.95 (0.21) | 0.713* | 0.711* | 0.462* |
| Postoperative day 3 | 3.08 (0.16) | 3.38 (0.16) | 3.60 (0.14) | 0.001* | 0.001* | 0.001* |
| Postoperative week 3 | 3.42 (0.26) | 3.60 (0.20) | 3.82 (0.25) | 0.001* | 0.001* | 0.001* |
| Postoperative month 3 | 4.07 (0.29) | 4.22 (0.26) | 4.43 (0.27) | 0.050* | 0.060* | 0.051* |
| Mean range of motion, ° (SD) | ||||||
| Preoperative | 91.33 (10.24) | 92.16 (13.88) | 92.08 (12.08) | 0.701* | 0.730* | 0.971* |
| Postoperative day 3 | 99.91 (5.48) | 103.23 (9.23) | 106.50 (8.98) | 0.020* | 0.001* | 0.020* |
| Postoperative week 3 | 108.58 (12.14) | 112.50 (10.51) | 115.58 (11.39) | 0.041* | 0.001* | 0.032* |
| Postoperative month 3 | 122.00 (10.09) | 124.75 (9.93) | 126.91 (8.20) | 0.111* | 0.211* | 0.50* |
| Kellgren & Lawrence classification, n (%) | ||||||
| III | 22 (37) | 23 (38) | 21(35) | 0.130† | 0.121† | 0.111† |
| IV | 38 (63) | 37 (62) | 39 (65) | 0.311† | 0.301† | 0.323 |
-
*
One-way analysis of variance.
-
†
Chi-squared test or Fisher’s exact test.
-
P1, comparison between the tourniquet and limited-tourniquet groups; P2, comparison between the tourniquet and no-tourniquet groups; P3, comparison between the limited-tourniquet and no-tourniquet groups.
Complications
Postoperative deep vein thrombosis or other complications were not observed. No patient presented postoperative wound secretion, superficial or seep wound infection, or cerebrovascular events. Routine Doppler ultrasound identified intramuscular venous thrombosis without obvious symptoms in five patients in the tourniquet group, four in the limited-tourniquet group, and two in the no-tourniquet group.
Discussion
The main findings of this study were that among patients undergoing TKA with multiple doses of tranexamic acid, total blood loss and hidden blood loss were reduced when no tourniquet was used. Absence of a tourniquet was also associated with lower CK, CRP, and IL-6 levels as well as less pain, a lower swelling ratio, and better early function at three weeks postoperatively. However, these differences disappeared by three months after surgery. Absence of a tourniquet was associated with longer operating time but not with impaired visibility in the surgical field.
TKA is associated with substantial perioperative blood loss. Minimizing perioperative bleeding remains a primary goal in order to avoid the risks of blood transfusion and hidden blood loss following this type of surgery. Surgeons have implemented several strategies to achieve this goal, including the administration of tranexamic acid, the application of controlled hypotensive anaesthesia, and the use of a tourniquet.27-29 Patients in TKA with no tourniquet have been shown to present less perioperative blood loss and hidden blood loss as a result of improvement in surgical techniques, perioperative blood loss management, and the introduction of fast-track TKA.7,8,30
The application of a tourniquet in TKA has been a topic of debate. Although previous studies have associated tourniquets with a significant reduction in intraoperative blood loss, the effects on postoperative and total blood loss have been mixed.3,31 One study found that total blood loss and blood transfusion rate were not significantly different throughout the hospital course with or without a tourniquet.13 Another study reported that total blood loss and hidden blood loss were higher when a tourniquet was used, because of tourniquet-induced ischaemia.31 In summary, previous studies evaluating blood loss in TKA performed with or without tourniquet showed a high heterogeneity. Our results in this carefully controlled study involving only patients receiving multiple-dose tranexamic acid suggest that a tourniquet does not provide benefits beyond those of tranexamic acid, and is in fact associated with worse outcomes in the short term.
Our study found that not using a tourniquet was associated with smaller losses of total blood, hidden blood, Hb, and HCT during TKA involving multiple-dose tranexamic acid. We found that the negative effects of a tourniquet were mitigated in the limited-tourniquet use. We hypothesize that the ischaemia caused by tourniquet use can lead to sustained local reactive hyperaemia when the tourniquet is deflated, and that this hyperaemia can last several hours. This could lead to increased haemorrhage into adjacent traumatized tissue during the operation. Another potential explanation is postoperative hyperfibrinolysis, which may increase bleeding into adjacent soft tissue after surgery. This may explain why we were able to identify bleeding vascular structure in the no-tourniquet group but not in the tourniquet groups. A third possibility is that the tourniquet limits the delivery of the tranexamic acid to the soft tissue, leading to increased total blood loss and hidden blood loss in the joint and adjacent soft tissue.
The absence of a tourniquet was associated with lower levels of CK, CRP, and IL-6 in our patients. Several tissues release CK, which uses adenosine triphosphate (ATP) to generate energy, especially in the skeletal muscle. It may be that a tourniquet increases risk of soft tissue damage during TKA. Consistent with this idea, a prospective study showed that TKA using a tourniquet induced ischaemia that reduced levels of glucose and pyruvate below preoperative levels, while increasing levels of ischaemic byproducts such as lactate and glycerol.32 On the other hand, one study found that not using a tourniquet led to higher levels of CK and CRP, but lower pain score on postoperative days 1, 2, and 4. In the study, total blood loss was greater in the no-tourniquet group,5 as was soft tissue damage. These divergent findings highlight the need for further studies into the relationship between markers of muscle damage and inflammatory reaction and the use of a tourniquet in TKA.
Our department promotes early rehabilitation and activity: patients are encouraged to walk even on the day of surgery. Most patients achieve rehabilitation goals quickly and are discharged. Our results show that avoiding a tourniquet is associated with superior early recovery, greater quadriceps strength, shorter time interval of straight leg raising, less pain, lower limb swelling, and better early function recovery. Tourniquet use may weaken quadriceps because of muscle damage resulting from tourniquet-induced ischaemia or direct compressive injury.33 The higher postoperative pain with a tourniquet may also prevent greater use of quadriceps, keeping them weak. Our results are consistent with previous work linking tourniquet use in TKA to 20% lower quadriceps muscle volume at one month postoperatively, as well as lower quadriceps strength for up to three months postoperatively.10 We adopted the same rehabilitation programme and discharge criteria in the three groups. The standard rehabilitation programme consisting of weight bearing as tolerated with walking aid started the day after surgery. Patients were ready to be discharged when they had no signs of infection or other complications, were eating normally, and had 90° of flexion with full active extension of the knee. Therefore, there is no significant difference in length of stay in the study.
Although the application of tranexamic acid in TKA has been shown to reduce blood loss and blood transfusions in previous studies, a consensus has not been achieved about the optimal dosage, treatment duration, and mode of administration.34-36 According to the literature, TKA with a tourniquet can result in hyperfibrinolysis, which peaks at six hours and lasts for 24 hours after tourniquet deflation.37 One study showed no significant differences between one dose of tranexamic acid prior to incision and two doses of tranexamic acid (preoperative and postoperative, 10 mg/kg intravenously).38 On the contrary, other work revealed that multiple doses of intravenous tranexamic acid led to less blood loss than a single dose.39 This may be because administering repeated doses of tranexamic acid during 24 hours can inhibit the postoperative fibrinolysis lasting as long as 18 to 24 hours.24,37 These results suggest that, in order to inhibit fibrinolysis completely, a single dose of tranexamic acid is not enough. As a result, we decided to use multiple doses of tranexamic acid in our enhanced recovery management protocol for primary TKA.
Although tranexamic acid has antifibrinolytic properties, it has not been associated with an increase in thromboembolic complications40 and we observed no such complications in our study. There are several possible reasons for the low risk of such complications. First, we advanced the time of enoxaparin use by two hours (from eight to six hours postoperative). Second, chemical prophylaxis and physical approaches for TKA were routinely implemented. Third, all patients were encouraged to leave the bed as soon as possible.
The limitations of this study included a follow-up period of only three months. However, the early recovery of most of our patients means that this follow-up was sufficient to evaluate the effects of the tourniquet on early knee function. Second, we did not investigate prosthetic fixation or survival outcomes. A previous study showed no difference in cemented component migration between TKA performed in the presence or absence of tourniquet during two-year follow-up.41 Finally, our assessment of thromboembolic complications was limited to three months postoperatively. Although previous studies suggest that tranexamic acid does not increase risk of these complications, a larger study with sufficiently long follow-up is required to evaluate potential adverse events.
Despite these limitations, this study provides evidence that entirely avoiding a tourniquet in TKA reduces total blood loss and hidden blood loss when multiple doses of intravenous tranexamic acid are used. It is also associated with less pain, lower limb swelling, less inflammation and muscle damage, and faster recovery. On the other hand, no or limited use of a tourniquet is associated with higher intraoperative blood losses, although this was not associated with greater risk of negative outcomes. These results argue for not using a tourniquet in primary TKA involving multiple-dose intravenous tranexamic acid.
References
1. Tai TW , Lin CJ , Jou IM , et al. Tourniquet use in total knee arthroplasty: a meta-analysis . Knee Surg Sports Traumatol Arthrosc . 2011 ; 19 ( 7 ): 1121 – 1130 . Google Scholar
2. Huang ZY , Pei FX , Ma J , et al. Comparison of three different tourniquet application strategies for minimally invasive total knee arthroplasty: a prospective non-randomized clinical trial . Arch Orthop Trauma Surg . 2014 ; 134 ( 4 ): 561 – 570 . Crossref PubMed Google Scholar
3. Alcelik I , Pollock RD , Sukeik M , et al. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials . J Arthroplasty . 2012 ; 27 ( 3 ): 331 – 340 . Crossref PubMed Google Scholar
4. Touzopoulos P , Ververidis A , Mpogiatzis C , Chatzigiannakis A , Drosos GI . The use of tourniquet may influence the cement mantle thickness under the tibial implant during total knee arthroplasty . Eur J Orthop Surg Traumatol . 2019 ; 29 ( 4 ): 869 – 875 . Crossref PubMed Google Scholar
5. Tai TW , Chang CW , Lai KA , Lin CJ , Yang CY . Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial . J Bone Joint Surg Am . 2012 ; 94-A ( 24 ): 2209 – 2215 . Crossref PubMed Google Scholar
6. Bressi E , Longo UG , Mangiacapra F , et al. Impact of Tourniquet Use on Systemic Inflammatory Parameters, Functional Physical Recovery, and Cardiovascular Outcomes of Patients Undergoing Knee Arthroplasty: A case-control study . J Knee Surg . 2019 . (Epub ahead of print) PMID . Crossref PubMed Google Scholar
7. Schnettler T , Papillon N , Rees H . Use of a Tourniquet in Total Knee Arthroplasty Causes a Paradoxical Increase in Total Blood Loss . J Bone Joint Surg Am . 2017 ; 99-A ( 16 ): 1331 – 1336 . Crossref PubMed Google Scholar
8. Huang Z , Xie X , Li L , et al. Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: a prospective, randomized controlled trial . J Bone Joint Surg Am . 2017 ; 99-A ( 24 ): 2053 – 2061 . Crossref PubMed Google Scholar
9. Ejaz A , Laursen AC , Kappel A , et al. Faster recovery without the use of a tourniquet in total knee arthroplasty . Acta Orthop . 2014 ; 85 ( 4 ): 422 – 426 . Crossref PubMed Google Scholar
10. Dennis DA , Kittelson AJ , Yang CC , et al. Does Tourniquet Use in TKA Affect Recovery of Lower Extremity Strength and Function? A Randomized Trial . Clin Orthop Relat Res . 2016 ; 474 ( 1 ): 69 – 77 . Crossref PubMed Google Scholar
11. Tsubosaka M , Ishida K , Sasaki H , et al. Effects of Suture and Tourniquet on Intraoperative Kinematics in Navigated Total Knee Arthroplasty . J Arthroplasty . 2017 ; 32 ( 6 ): 1824 – 1828 . Crossref PubMed Google Scholar
12. Rathod P , Deshmukh A , Robinson J , et al. Does Tourniquet Time in Primary Total Knee Arthroplasty Influence Clinical Recovery? J Knee Surg . 2015 ; 28 ( 4 ): 335 – 342 . Crossref PubMed Google Scholar
13. Smith TO , Hing CB . Is a tourniquet beneficial in total knee replacement surgery? A meta-analysis and systematic review . Knee . 2010 ; 17 ( 2 ): 141 – 147 . Crossref PubMed Google Scholar
14. Aglietti P , Baldini A , Vena LM , et al. Effect of tourniquet use on activation of coagulation in total knee replacement . Clin Orthop Relat Res . 2000 ; 371 : 169 – 177 . Crossref PubMed Google Scholar
15. Mutlu S , Guler O , Mutlu H , et al. Tourniquet use during total knee arthroplasty does not offer significant benefit: A retrospective cohort study . Int J Surg . 2015 ; 18 : 123 – 127 . Google Scholar
16. Fan Y , Jin J , Sun Z , et al. The limited use of a tourniquet during total knee arthroplasty: a randomized controlled trial . Knee . 2014 ; 21 ( 6 ): 1263 – 1268 . Crossref PubMed Google Scholar
17. Tarwala R , Dorr LD , Gilbert PK , Wan Z , Long WT . Tourniquet use during cementation only during total knee arthroplasty: a randomized trial . Clin Orthop Relat Res . 2014 ; 472 ( 1 ): 169 – 174 . Crossref PubMed Google Scholar
18. Gillette BP , DeSimone LJ , Trousdale RT , Pagnano MW , Sierra RJ . Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty . Clin Orthop Relat Res . 2013 ; 471 ( 1 ): 150 – 154 . Crossref PubMed Google Scholar
19. Maniar RN , Kumar G , Singhi T , Nayak RM , Maniar PR . Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients . Clin Orthop Relat Res . 2012 ; 470 ( 9 ): 2605 – 2612 . Crossref PubMed Google Scholar
20. Alshryda S , Sarda P , Sukeik M , et al. Tranexamic acid in total knee replacement: a systematic review and meta-analysis . J Bone Joint Surg Br . 2011 ; 93-B ( 12 ): 1577 – 1585 . Crossref PubMed Google Scholar
21. Fillingham YA , Darrith B , Calkins TE , et al. Hip Society Research Group. 2019 Mark Coventry Award: A multicentre randomized clinical trial of tranexamic acid in revision total knee arthroplasty: does the dosing regimen matter? Bone Joint J . 2019 ; 101-B ( 7_Supple_C ): 10 – 16 . Google Scholar
22. Subramanyam KN , Khanchandani P , Tulajaprasad PV , Jaipuria J , Mundargi AV . Efficacy and safety of intra-articular versus intravenous tranexamic acid in reducing perioperative blood loss in total knee arthroplasty: a prospective randomized double-blind equivalence trial . Bone Joint J . 2018 ; 100-B ( 2 ): 152 – 160 . Crossref PubMed Google Scholar
23. Bradley KE , Ryan SP , Penrose CT , et al. Tranexamic acid or epsilon-aminocaproic acid in total joint arthroplasty? A randomized controlled trial . Bone Joint J . 2019 ; 101-B ( 9 ): 1093 – 1099 . Google Scholar
24. Xie J , Ma J , Yao H , Yue C , Pei F . Multiple Boluses of Intravenous Tranexamic Acid to Reduce Hidden Blood Loss After Primary Total Knee Arthroplasty Without Tourniquet: A Randomized Clinical Trial . J Arthroplasty . 2016 ; 31 ( 11 ): 2458 – 2464 . Crossref PubMed Google Scholar
25. Gross JB . Estimating allowable blood loss: corrected for dilution . Anesthesiology . 1983 ; 58 ( 3 ): 277 – 280 . Crossref PubMed Google Scholar
26. Nadler SB , Hidalgo JH , Bloch T . Prediction of blood volume in normal human adults . Surgery . 1962 ; 51 ( 2 ): 224 – 232 . PubMed Google Scholar
27. Sharrock NE , Salvati EA . Hypotensive epidural anesthesia for total hip arthroplasty: a review . Acta Orthop Scand . 1996 ; 67 ( 1 ): 91 – 107 . Crossref PubMed Google Scholar
28. Nielsen CS , Jans Øivind , Ørsnes T , et al. Combined Intra-Articular and Intravenous Tranexamic Acid Reduces Blood Loss in Total Knee Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial . J Bone Joint Surg Am . 2016 ; 98-A ( 10 ): 835 – 841 . Crossref PubMed Google Scholar
29. Alshryda S , Sukeik M , Sarda P , et al. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement . Bone Joint J . 2014 ; 96-B ( 8 ): 1005 – 1015 . Crossref PubMed Google Scholar
30. Arthur JR , Spangehl MJ . Tourniquet Use in Total Knee Arthroplasty . J Knee Surg . 2019 ; 32 ( 8 ): 719 – 729 . Crossref PubMed Google Scholar
31. Jiang FZ , Zhong HM , Hong YC , Zhao GF . Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials . J Orthop Sci . 2015 ; 20 ( 1 ): 110 – 123 . Crossref PubMed Google Scholar
32. Ejaz A , Laursen AC , Kappel A , et al. Tourniquet induced ischemia and changes in metabolism during TKA: a randomized study using microdialysis . BMC Musculoskelet Disord . 2015 ; 16 : 326 . Crossref PubMed Google Scholar
33. Leurcharusmee P , Sawaddiruk P , Punjasawadwong Y , Chattipakorn N , Chattipakorn SC . The Possible Pathophysiological Outcomes and Mechanisms of Tourniquet-Induced Ischemia-Reperfusion Injury during Total Knee Arthroplasty . Oxid Med Cell Longev . 2018 ; 2018 : 8087598 – 15 . Crossref PubMed Google Scholar
34. Levine BR , Haughom BD , Belkin MN , Goldstein ZH . Weighted versus uniform dose of tranexamic acid in patients undergoing primary, elective knee arthroplasty: a prospective randomized controlled trial . J Arthroplasty . 2014 ; 29 ( 9 Suppl ): 186 – 188 . Crossref PubMed Google Scholar
35. Irwin A , Khan SK , Jameson SS , et al. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures . Bone Joint J . 2013 ; 95-B ( 11 ): 1556 – 1561 . Crossref PubMed Google Scholar
36. Melvin JS , Stryker LS , Sierra RJ . Tranexamic Acid in Hip and Knee Arthroplasty . J Am Acad Orthop Surg . 2015 ; 23 ( 12 ): 732 – 740 . Google Scholar
37. Blanié A , Bellamy L , Rhayem Y , et al. Duration of postoperative fibrinolysis after total hip or knee replacement: a laboratory follow-up study . Thromb Res . 2013 ; 131 ( 1 ): e6 – e11 . Crossref PubMed Google Scholar
38. Lin PC , Hsu CH , Huang CC , Chen WS , Wang JW . The blood-saving effect of tranexamic acid in minimally invasive total knee replacement: is an additional pre-operative injection effective? J Bone Joint Surg Br . 2012 ; 94-B ( 7 ): 932 – 936 . Crossref PubMed Google Scholar
39. Xie J , Ma J , Kang P , et al. Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases . Thromb Res . 2015 ; 136 ( 2 ): 234 – 238 . Crossref PubMed Google Scholar
40. Shakur H , Roberts I , et al. , CRASH-2 trial collaborators . CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial . Lancet . 2010 ; 376 ( 9734 ): 23 – 32 . Google Scholar
41. Molt M , Harsten A , Toksvig-Larsen S . The effect of tourniquet use on fixation quality in cemented total knee arthroplasty a prospective randomized clinical controlled RSA trial . Knee . 2014 ; 21 ( 2 ): 396 – 401 . Crossref PubMed Google Scholar
Author contributions
H-Y. Zhao: Wrote the manuscript.
R. Yeersheng: Collected and assembled the data.
X-W. Kang: Designed the study.
Y-Y. Xia: Approved the manuscript.
P-D. Kang: Designed the study.
W-J. Wang: Took responsibility for the overall integrity of the work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
None declared.
Acknowledgements
The grouping of patients was completed by the department research secretaries, Dong-Hai Li and Qiu-Ru Wang.
Ethical review statement
This study was approved by the Institutional Review Board of the First Hospital of Lanzhou University, Lanzhou, China, and registered in the Chinese Clinical Trial Registry (ChiCTR-INR-17013110).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









