Abstract
Aims
Dystrophic calcification (DC) is the abnormal appearance of calcified deposits in degenerating tissue, often associated with injury. Extensive DC can lead to heterotopic ossification (HO), a pathological condition of ectopic bone formation. The highest rate of HO was found in combat-related blast injuries, a polytrauma condition with severe muscle injury. It has been noted that the incidence of HO significantly increased in the residual limbs of combat-injured patients if the final amputation was performed within the zone of injury compared to that which was proximal to the zone of injury. While aggressive limb salvage strategies may maximize the function of the residual limb, they may increase the possibility of retaining non-viable muscle tissue inside the body. In this study, we hypothesized that residual dead muscle tissue at the zone of injury could promote HO formation.
Methods
We tested the hypothesis by investigating the cellular and molecular consequences of implanting devitalized muscle tissue into mouse muscle pouch in the presence of muscle injury induced by cardiotoxin.
Results
Our findings showed that the presence of devitalized muscle tissue could cause a systemic decrease in circulating transforming growth factor-beta 1 (TGF-β1), which promoted DC formation following muscle injury. We further demonstrated that suppression of TGF-β signalling promoted DC in vivo, and potentiated osteogenic differentiation of muscle-derived stromal cells in vitro.
Conclusion
Taken together, these findings suggest that TGF-β1 may play a protective role in dead muscle tissue-induced DC, which is relevant to understanding the pathogenesis of post-traumatic HO.
Cite this article: Bone Joint Res 2020;9(11):742–750.
Article focus
-
Muscle death and dystrophic calcification (DC)/heterotopic ossification (HO).
Key messages
-
Presence of devitalized muscle tissue promoted DC following muscle injury.
-
Presence of devitalized muscle tissue caused a decrease in circulating transforming growth factor-beta 1 (TGF-β1) level.
-
Suppression of TGF-β1 signalling promoted osteogenesis.
Strengths and limitations
-
This study suggests muscle devitalization as a possible mechanistic explanation for the relationship between amputation level and incidence of HO. A limitation of the study is that the devitalized muscle tissue implantation model only produced DC, as a precursor event to HO.
Introduction
Dystrophic calcification (DC) refers to calcification observed in degenerating tissue due to injury. Extensive DC can lead to heterotopic ossification (HO), which is a pathological condition of ectopic bone formation. Recent studies have reported that the transition from calcification to ossification may be stages of a pathological continuum,1 and that the progression from DC to HO is considered a new mechanism leading to HO formation.2 The highest rate of HO is found in combat-related blast injuries, a polytrauma condition with severe muscle injury.3 Due to the abnormal mechanical effect of hard tissue located inside soft tissue, HO usually causes pain and restricted range of motion.4 It has been shown that HO is the single most significant obstacle for combat-injured military personnel to return to active duty.5
The mechanism of blast trauma-induced HO is still largely unknown; however, it has been noted that incidence of HO significantly increased in the residual limbs of combat-injured patients if the final amputation was performed within the zone of injury compared to that which was proximal to the zone of injury.6 While aggressive limb salvage strategies may maximize the function of residual limb, they may increase the possibility of retaining non-viable muscle tissue inside the body.7 It has been previously demonstrated that debridement of non-viable tissues diminishes HO formation.8 Our laboratory has previously characterized the cytokine expression profile in human blast-traumatized skeletal muscle tissue obtained during the debridement of high-energy wartime limb wounds, and found that dead muscle tissue elicited a strong immune response with elevated transforming growth factor-beta 1 (TGF-β1) signal.9 Also, we recently showed that TGF-β1 played a protective role in glucocorticoid-induced DC.10 Based on these findings, we hypothesized that residual dead muscle tissue at the zone of injury could promote HO formation through modulating TGF-β1 signalling.
To investigate the role of muscle tissue death in HO pathogenesis, devitalized muscle tissue was implanted into mouse muscle pouch in the presence of muscle injury induced by cardiotoxin (CTX) injection. The findings reported here show that the presence of devitalized muscle tissue caused a systemic decrease in circulating TGF-β1, which promoted DC formation following muscle injury. We further demonstrated that suppression of TGF-β signalling promoted DC in vivo, and potentiated osteogenic differentiation of muscle-derived stromal cells (MDSCs) in vitro. Taken together, these findings suggest that TGF-β1 could also be a key factor in modulating dead muscle tissue-induced DC, which is relevant to understanding the pathogenesis of post-traumatic HO.
Methods
Devitalized muscle tissue muscle pouch implantation and microCT imaging
The animal study protocol was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Protocol No. 18032493). Devitalized skeletal muscle tissue was harvested from the thigh muscles of a donor group of eight- to 12-week-old male C57BL/6 J mice (Jackson Laboratory, Bar Harbor, Maine, USA) and freeze-thawed three times to achieve devitalization, a common method found to be effective in devitalizing/decellularizing tissues and organs.10 Devitalized muscle tissues were then stored at -80°C until subsequent use in muscle implantation surgery.
To examine the effect of devitalized muscle in vivo, the experimental group of eight- to 12-week-old male C57BL/6 J mice were first injected at their hamstring muscles with 100 μl 10 μM CTX (Calbiochem, San Diego, California, USA).11 One day later, ~50 mg of cryostored devitalized skeletal muscle tissue was thawed at room temperature and implanted into a thigh muscle pouch according to a previously described surgical procedure.12 Briefly, the animal was first anaesthetized by isoflurane inhalation, and the inner thigh area was removed of hair and sterilized broadly with ethanol. The mouse was positioned onto the operative field facing up, and a 0.5 cm longitudinal incision was made first in the skin and then beneath the femoral artery in the muscle. The muscle pouch was created by blunt dissection of muscle fibres in the gracilis muscle. Next, a piece of ~50 mg devitalized muscle tissue was placed into the exposed muscle pouch. Muscle fibres were then closed by tension, and the skin was closed with 4-0 nylon sutures. CTX injection alone with sham surgery or devitalized muscle tissue implantation alone served as controls. Animals received a three-day course of buprenorphine (Covetrus, Portland, Maine, USA; 0.05 mg/kg administered subcutaneously twice a day) for pain management. To test for a systemic effect induced by the devitalized muscle tissue, CTX injection and muscle implantation were performed on the left leg and right leg of the animal, respectively. Mice were killed at a two-week timepoint for microCT analysis of ectopic mineralization. Scans were acquired using vivaCT 40 (Scanco Medical, Brüttisellen, Switzerland) at 45 kVp, 88 μA, and 300 ms integration time with an isotropic voxel size of 35 μm.
Blood sample and muscle tissue sample collection
To determine the systemic and local effect of dead muscle tissue on HO pathogenesis, blood samples and muscle tissue samples were collected at Day 3, Day 7, and Day 14 timepoints in animals with sham surgery or animals with dead muscle tissue implant. Blood samples were collected by cardiac puncture at the time of animal kill. Total TGF-β1 level in plasma was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, Massachusetts, USA). Muscle tissues were collected at Day 3, Day 7, and Day 14 timepoints in the same groups as described above. Specifically, gracilis muscle with the devitalized muscle tissue implant was collected together as the inner thigh muscle group, representing the tissue exhibiting local response induced by the dead muscle tissue, while biceps femoris muscle was collected as the outer thigh muscle group, representing the tissue exhibiting systemic response induced by the dead muscle tissue. Phosphorylated Smad3 level in the host muscle tissue was determined using a commercial ELISA kit (Abcam). Muscle tissue gene expression was determined by quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) analysis (see below).
Histology and immunohistochemistry
Muscle tissues were fixed in methanol, paraffin embedded, sectioned, and processed for routine haematoxylin and eosin, Trichrome, and Alizarin Red staining. Immunohistochemistry (IHC) was performed according to protocols described for the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, California, USA). Primary antibodies against phosphorylated Smad2, fibrin(ogen), as well as isotype control antibody were purchased from Abcam. Antibodies were used in a 1:100 to 200 dilution range at a concentration of 5 μg/ml. For immunofluorescence staining, primary antibody against platelet-derived growth factor receptor α (PDGFRα) (AF1062; R&D Systems, Minneapolis, Minnesota, USA) was used to detect the presence of MDSCs. The slides were mounted with 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting medium (Vector Laboratories), and were viewed using an inverted IX81 microscope (Olympus, Tokyo, Japan) equipped with a Retiga EXi cooled CCD camera (Teledyne Qimaging, Surrey, Canada) and MetaMorph software (Molecular Devices, San Jose, California, USA).
In vivo suppression of TGF-β signalling
To test the effect of suppressed TGF-β signalling on DC/HO pathogenesis, a TGF-β type I receptor inhibitor, SB431542 (10 mg/kg in corn oil vehicle containing 5% dimethyl sulfoxide (DMSO); Abcam) was administered by daily subcutaneous injection for seven days into eight- to 12-week-old male C57BL/6 J mice that received an injection of 50 μl of 10 μM CTX into the calf muscles at Day 1. Mice were killed at Day 7 for microCT analysis of ectopic mineralization. Mice injected daily with corn oil vehicle containing 5% DMSO combined with CTX treatment served as control.
In vitro suppression of TGF-β signalling
To test the effect of suppressed TGF-β signalling on osteogenic differentiation in vitro, MDSCs were harvested from another cohort of mice according to a previously described protocol.13 These cells have been previously characterized to be positive for the mesenchymal stem cell surface markers stem cells antigen-1 (Sca-1) and PDGFRα, have strong osteogenic potential, and are highly responsive to bone morphogenetic protein signal in vitro.14 MDSCs were maintained for three days in CTX-injured muscle tissue derived conditioned medium (CTX CM), with or without the supplementation of 10 μM TGF-β type I receptor inhibitor SB431542. CTX CM was collected by culturing CTX-injured muscle tissue in growth medium as described previously.14 On culture Day 3, MDSCs were collected for the measurement of TGF-β signalling inhibition using a phosphorylated Smad3 ELISA kit (Abcam), as well as osteogenic marker gene expression by qRT-PCR.
qRT-PCR analysis
Muscle tissues or cells were lysed in QIAzol Lysis Reagent and isolated using the RNeasy Mini Kit according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). RNA was then converted to complementary DNA (cDNA) using the SuperScript IV First-Strand Synthesis System (Invitrogen, Carlsbad, California, USA). Quantitative real-time PCR was performed with a StepOne Plus Realtime PCR system using PowerUp SYBR Green Master Mix (Applied Biosystems, Foster City, California, USA). The thermal cycling condition comprised an initial denaturation at 95°C for ten minutes, followed by 40 cycles of amplification consisting of 15 seconds of denaturation at 95°C and one minute extension at 60°C. The relative level of gene expression was calculated using the 2-ΔΔCt method. Primer sequences for actin, TGF-β1 (Tgfb1), activin receptor-like kinase-5 (Alk5), collagen type I (Col1), PAI-1 (Pai1), alkaline phosphatase (Alp), and bone sialoprotein I (Bsp1) are listed in Table I.
Table I.
Polymerase chain reaction primer sequences.
| Gene | GenBank accession number | Forward primer | Reverse primer | Primer efficiency, % |
|---|---|---|---|---|
| Actin | NM_007393.5 | 5’-GGCTGTATTCCCCTCCATCG-3’ | 5’-CCAGTTGGTAACAATGCCATGT-3’ | 106.965 |
| Tgfb1 | NM_011577.2 | 5’-CACCTGCAAGACCATCGACA-3’ | 5’-CATAGTAGTCCGCTTCGGGC-3’ | 101.098 |
| Alk5 | NM_009370.3 | 5’- CCTCGAGACAGGCCATTTGT-3’ | 5’- GCCAGCTGACTGCTTTTCTG-3’ | 113.534 |
| Col1 | NM_007742.4 | 5’-TTCTCCTGGCAAAGACGGACTCAA-3’ | 5’-AGGAAGCTGAAGTCATAACCGCCA-3’ | 113.257 |
| Pai1 | NM_008871.2 | 5’-TCTGGGAAAGGGTTCACTTTACC-3’ | 5’-GACACGCCATAGGGAGAGAAG-3’ | 107.895 |
| Alp | NM_007431.3 | 5’-ATCGGAACAACCTGACTGACCCTT-3’ | 5’-ACCCTCATGATGTCCGTGGTCAAT-3’ | 119.631 |
| Bsp1 | NM_001204203.1 | 5’-TGCCCTCTGATCAGGACAAC-3’ | 5’-ATCCGACTGATCGGCACTCT-3’ | 113.257 |
-
Alk5, activin receptor-like kinase-5; Alp, alkaline phosphatase; Bsp1, bone sialoprotein I; Col1, collagen type I; Pai1, PAI-1; Tgfb1, transforming growth factor-beta 1.
Statistical analysis
All data were expressed as mean and SD, with n ≥ 4 for each group of in vivo animal experiments. An independent-samples t-test was used to make comparisons between two groups upon validation of Gaussian distribution of the data by D'Agostino & Pearson normality test. Statistical significance (*) was considered at p < 0.05. Statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad Software, San Diego, California, USA).
Results
Dead muscle tissue promoted DC formation in the presence of muscle injury
MicroCT imaging showed that combined devitalized muscle tissue implantation and CTX injection caused a greater amount of DC in two weeks (Figure 1a), while either sham surgery with CTX injection or devitalized muscle tissue implantation alone did not cause DC at implantation/injection sites in two weeks (Figures 1b and 1c). Histology revealed that DC was always formed some distance away from the implant (Figure 1d), indicating that dead muscle tissue did not calcify by itself but possibly elicited a systemic effect that resulted in muscle injury-induced DC formation. MicroCT and histology images also demonstrated DC formation when devitalized muscle tissue implantation and CTX injection were performed on different legs of a single animal, supporting our hypothesis of a systemic effect caused by dead muscle tissue (Supplemental Figure a). Tissues containing DC appeared basophilic, staining positive for haematoxylin (Figure 1e) and Alizarin Red, indicating calcium deposition (Figure 1f).
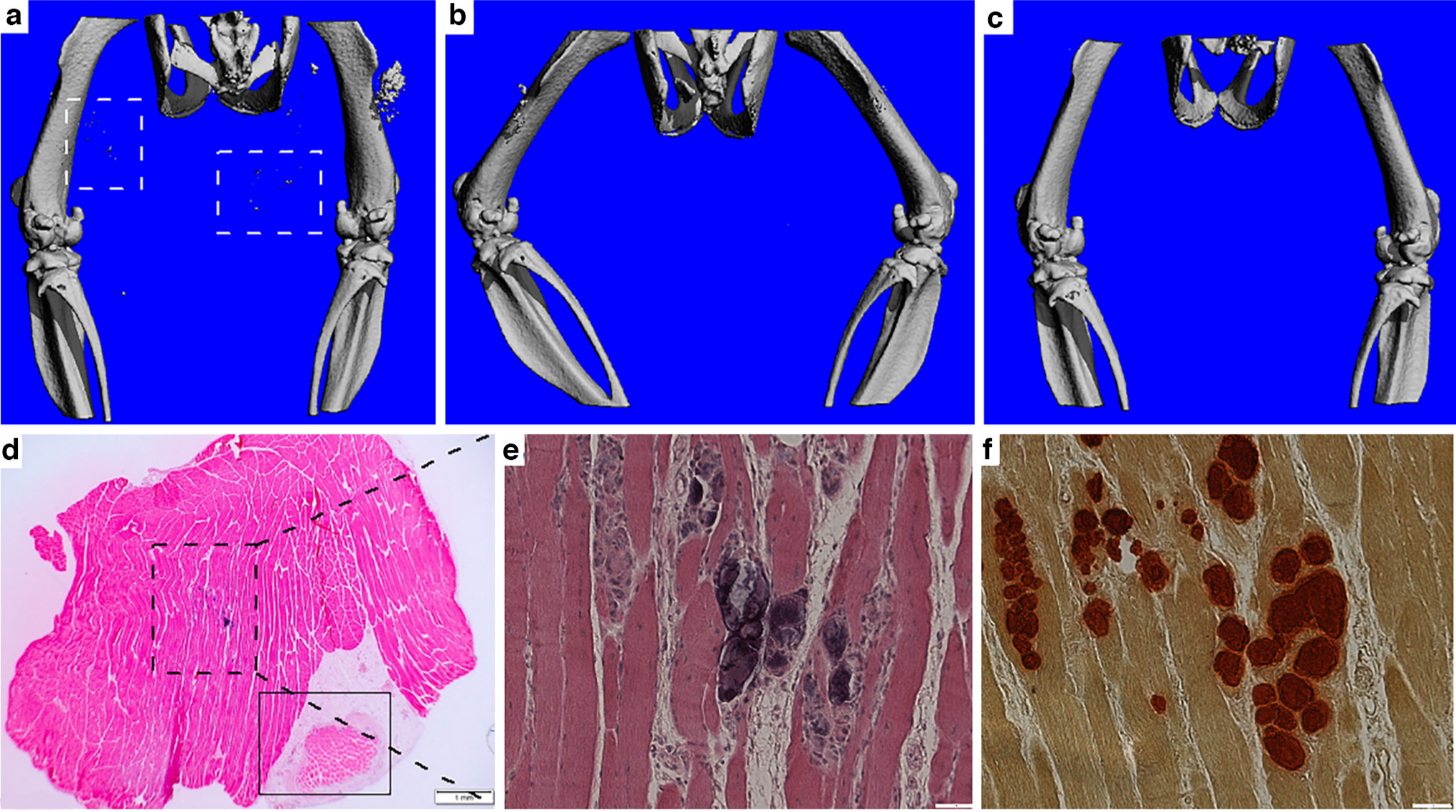
Fig. 1
Dead muscle tissue promoted dystrophic calcification (DC) formation in the presence of muscle injury. a) to c) MicroCT imaging showed DC formation (dashed line squares) in a) the implantation plus cardiotoxin (CTX) injection group, but not in b) the sham surgery plus CTX injection group nor c) the devitalized muscle tissue implantation alone group at Day 14 timepoint. d) Haematoxylin and eosin (H&E) staining showing the location of the implant (solid line square) relative to the DC site (dashed line square). Bar = 1 mm. e) and f) Higher magnification of the DC site. e) H&E staining; f) Alizarin Red staining demonstrating calcium deposition. Bar = 50 μm.
Implantation of dead muscle tissue caused a decrease in circulating level of TGF-β1
To determine how dead muscle tissue would affect DC formation, we collected blood samples from mice with or without devitalized muscle tissue implantation, and measured circulating TGF-β1 levels at Day 3, Day 7, and Day 14 timepoints after implantation. Significantly decreased plasma levels of TGF-β1 were found in mice with devitalized muscle tissue implant at the later timepoints (Figure 2), which coincided with DC formation. We also confirmed the effect of the changes in circulating TGF-β1 level on muscle tissue by analyzing the level of TGF-β1 signalling target protein in the outer thigh muscle tissue. Starting from Day 7 timepoint onwards, decreased phosphorylated Smad3 level was observed in the outer thigh muscle of mice implanted with devitalized muscle tissue implant (Figures 3b, 3d and 3f).
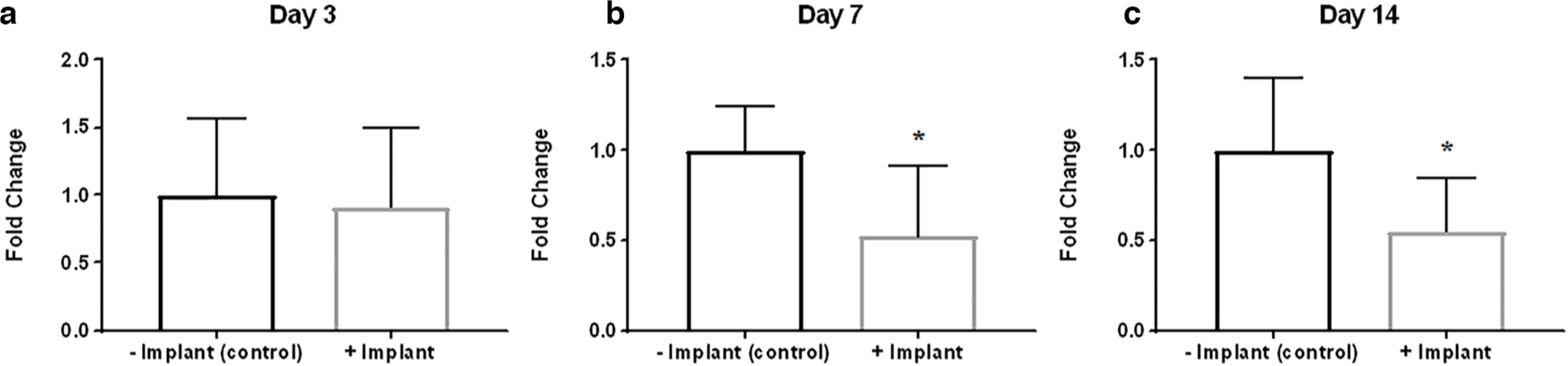
Fig. 2
Implantation of dead muscle tissue caused a decrease in circulating level of transforming growth factor-beta 1 (TGF-β1) at later timepoints. Blood sample analysis of plasma TGF-β1 level in animals with or without dead muscle tissue implantation at: a) Day 3; b) Day 7; and c) Day 14 timepoints. *p < 0.05; n = 6 to 8.
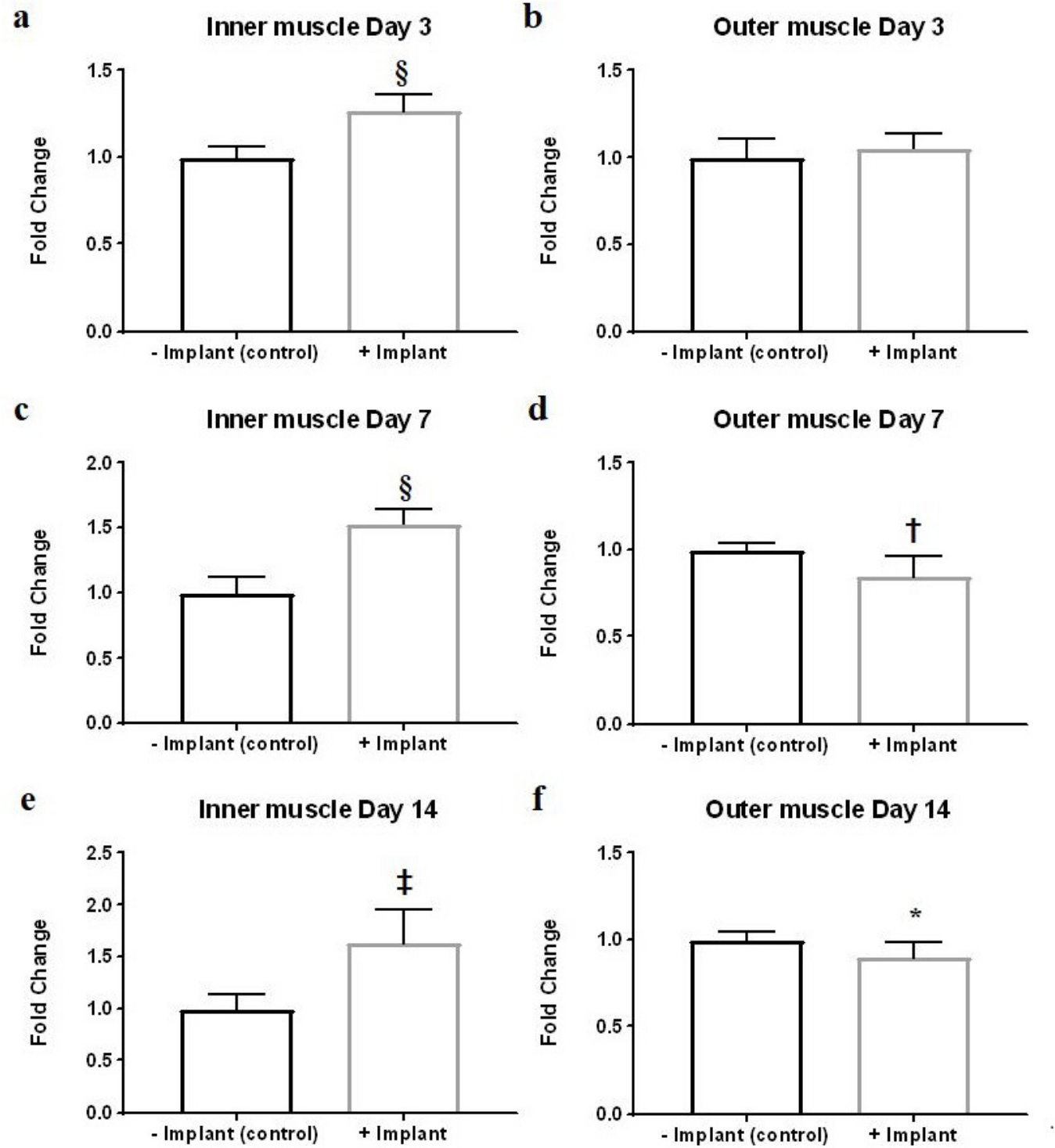
Fig. 3
Effect of implantation of dead muscle tissue on muscle tissue phosphorylated Smad3 level. Analysis of phosphorylated Smad3 level in a), c), and e) inner and b), d), and f) outer muscle tissues of animals with or without dead muscle tissue implantation at: a) and b) Day 3; c) and d) Day 7; and e) and f) Day 14 timepoints. *p < 0.05; †p < 0.01; ‡p < 0.001; §p < 0.0001; n = 6 to 8.
Local fibrotic response towards implanted dead muscle tissue
We also examined the level of phosphorylated Smad3 in the inner thigh muscle, representing local response induced by the dead muscle tissue. Significantly higher levels of phosphorylated Smad3 were found in mice harbouring devitalized muscle tissue implant starting from Day 3 onwards (Figures 3a, 3c and 3e). Histology confirmed this finding, showing positive staining of phosphorylated Smad2 surrounding the implant, as well as collagen deposition typical of a fibrotic response as revealed by Trichrome staining (Figure 4). Strong fibrin accumulation was also found in dead muscle tissue, also typical of a fibrotic response (Figure 4).15
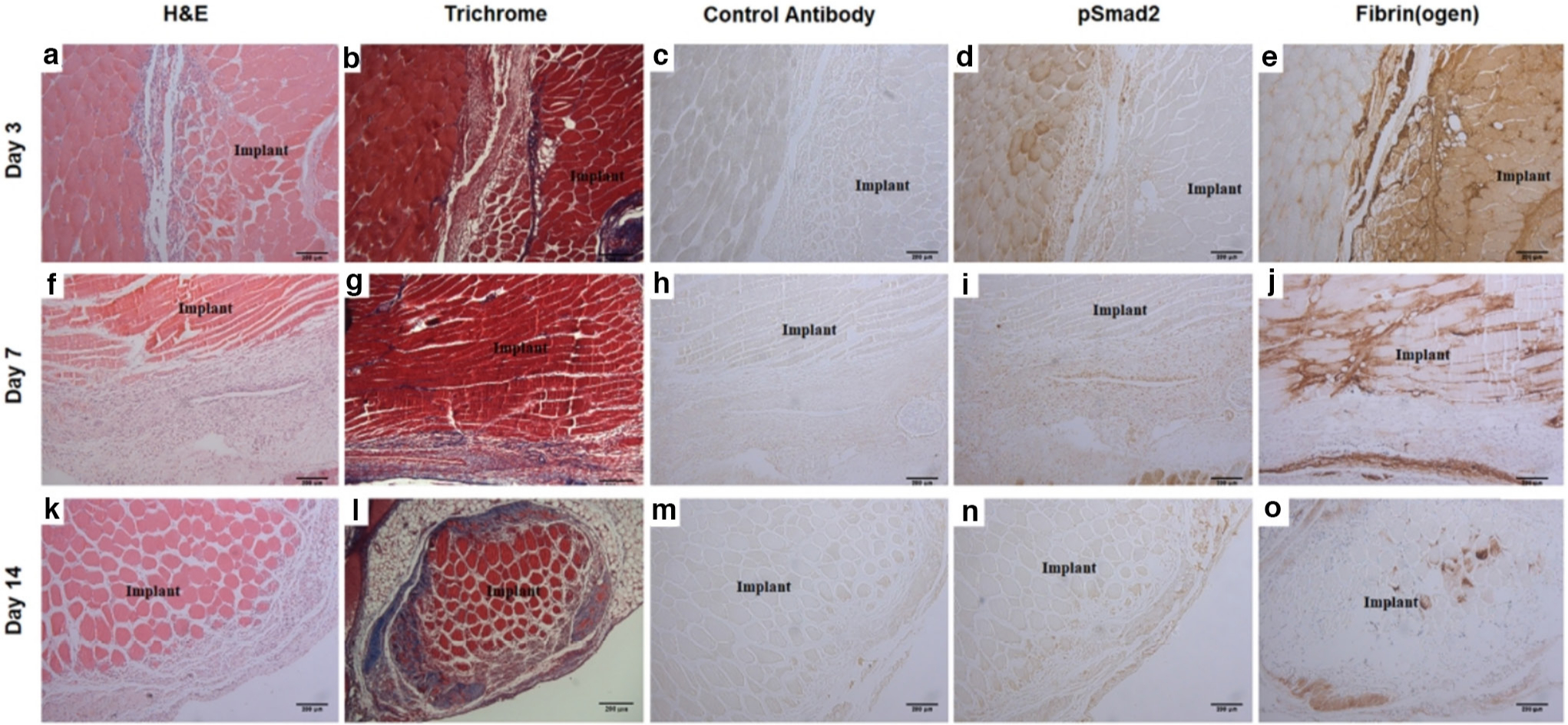
Fig. 4
Local fibrotic response towards implanted dead muscle tissue. Time course at: a) to e) Day 3; f) to j) Day 7; and k) to o) Day 14 timepoints. Haematoxylin and eosin (H&E) histology of devitalized muscle tissue implant site at: a) Day 3; f) Day 7; and k) Day 14. Trichrome staining demonstrating collagen deposition at: b) Day 3; g) Day 7; and l) Day 14. Control immunostaining using isotype control antibody at: c) Day 3; h) Day 7; and m) Day 14. Immunostaining of phosphorylated Smad2 at: d) Day 3; i) Day 7; and n) Day 14. Immunostaining of fibrin(ogen) at: e) Day 3; j) Day 7; and o) Day 14. The location of implant is as indicated. Bar = 200 μm.
In the inner thigh muscle, qRT-PCR analysis showed: increased collagen type I and PAI-1 expression at Day 7 timepoint, which are downstream targets of TGF-β1 signalling (Figures 5e and 5g) and decreased TGF-β1 receptor ALK5 expression was observed at Day 3 and Day 7 timepoints but not increased TGF-β1 expression (Figures 5a and 5c). In the outer thigh muscle tissue, slightly decreased TGF-β1 expression was found at Day 7 timepoint (Figure 5b).
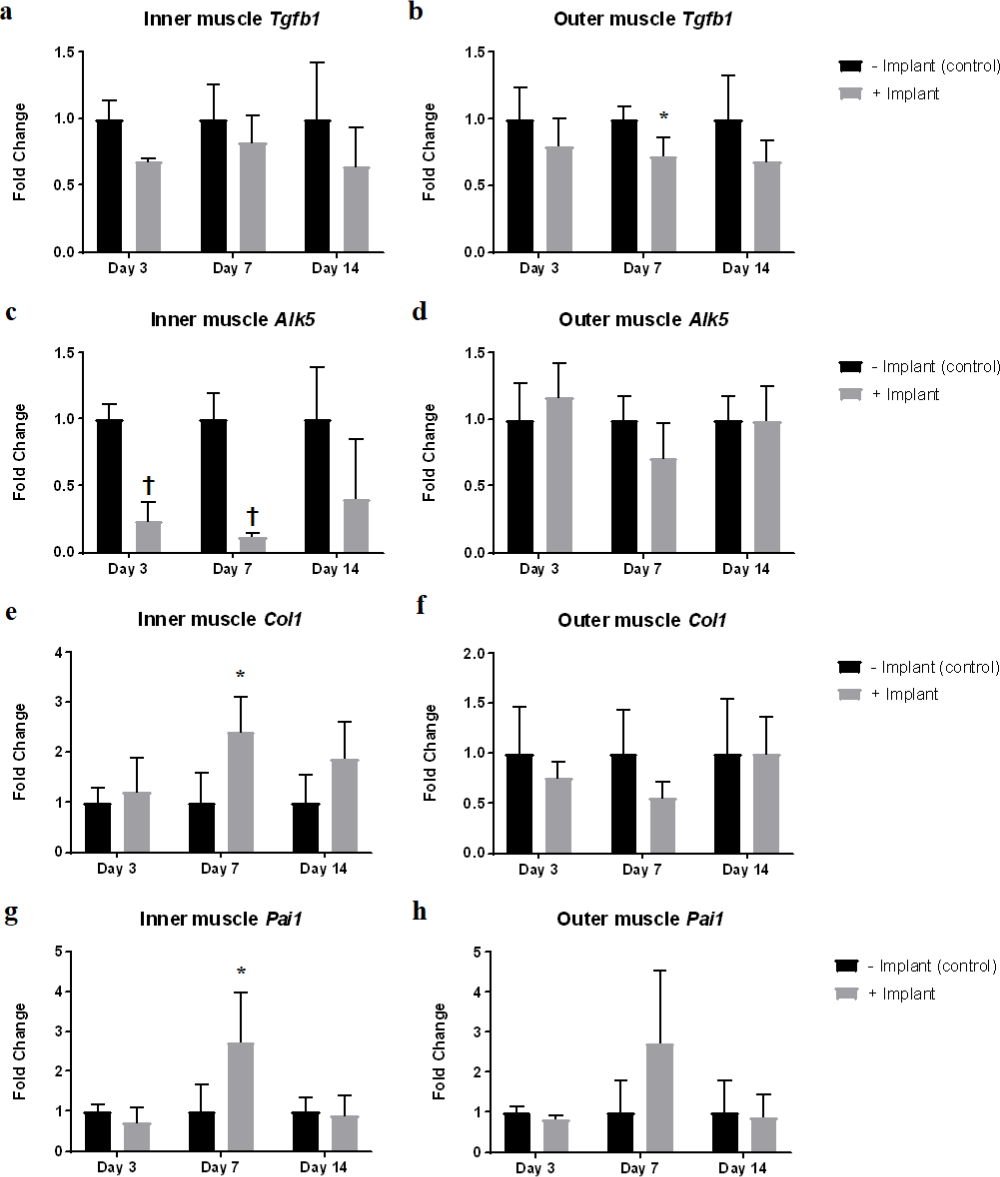
Fig. 5
Effect of implantation of dead muscle tissue on expression of transforming growth factor-beta 1 (TGF-β1) signalling related genes. Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) analysis of gene expression level of different genes related to TGF-β1 signalling pathway at different timepoints in the a), c), e), and g) inner thigh muscles and b), d), f), and h) outer thigh muscles. a) and b) Tgfb1, c) and d) activin receptor-like kinase-5 (Alk5), e) and f) collagen type I (Col1), and g) and h) PAI-1 (Pai1). *p < 0.05; †p < 0.001; n = 4.
Suppression of TGF-β signalling promoted DC in vivo and potentiated osteogenic differentiation of MDSCs in vitro
To test the effect of systemic suppression of TGF-β signalling on DC formation in vivo, mice were subcutaneously injected daily with a specific small molecule inhibitor of TGF-β type I receptor, SB431542, in the presence of muscle injury induced by CTX injection. The results demonstrated that suppression of TGF-β signalling promoted DC formation (Figure 6b), which was not observed in the vehicle injection with muscle injury control group (Figure 6a). Interestingly, upon suppression of TGF-β signalling, PDGFRα positive cells were detected near the calcium deposits, suggesting the possibility that MDSCs participated in DC formation (Figures 6c to 6e).
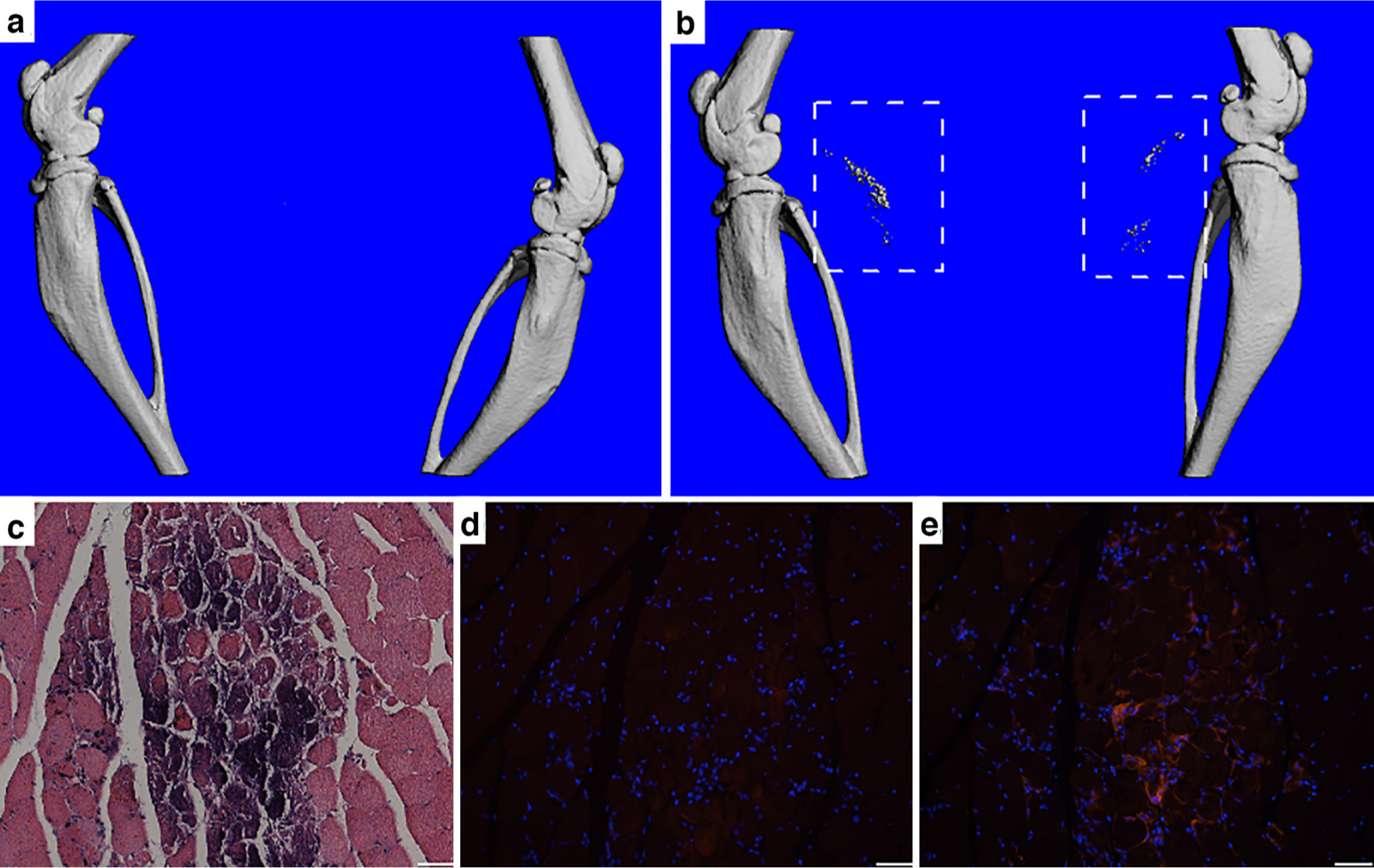
Fig. 6
Suppression of transforming growth factor-beta 1 (TGF-β) signalling in the presence of muscle injury promoted dystrophic calcification (DC) in vivo. a) and b) MicroCT imaging showed no DC formation in a) control group without TGF-β signalling suppression, and DC formation (dashed line square) in b) TGF-β signalling suppression by SB431542 group, both groups with muscle injury induced by cardiotoxin (CTX) injection into calf muscles. c) to e) Histological analysis of DC site. c) Haematoxylin and eosin (H&E) staining showing calcium deposition. d) Control immunostaining without primary antibody showing no positive staining surrounding the calcium deposition. e) Immunostaining for platelet-derived growth factor receptor α (PDGFRα) (red) showing positively stained muscle-derived stromal cells (MDSCs) surrounding the calcium deposits, suggesting their participation in DC formation. Bar = 50 μm.
We next tested directly the effect of suppressed TGF-β signalling on MDSC osteogenesis in vitro. Upon treatment with SB431542, inhibition of TGF-β signalling in MDSCs was indicated by significantly reduced Smad3 phosphorylation, although Smad1 phosphorylation was not affected (Figures 7A and 7). Concomitantly, significant decrease in the expression of PAI-1, a TGF-β1 target gene, was observed (Figure 7c). Importantly, expression of osteogenic marker genes was greatly enhanced in the treated MDSCs (Figure 7d). Taken together, these data provided strong evidence that suppressed TGF-β signalling potentiated MDSC osteogenesis.
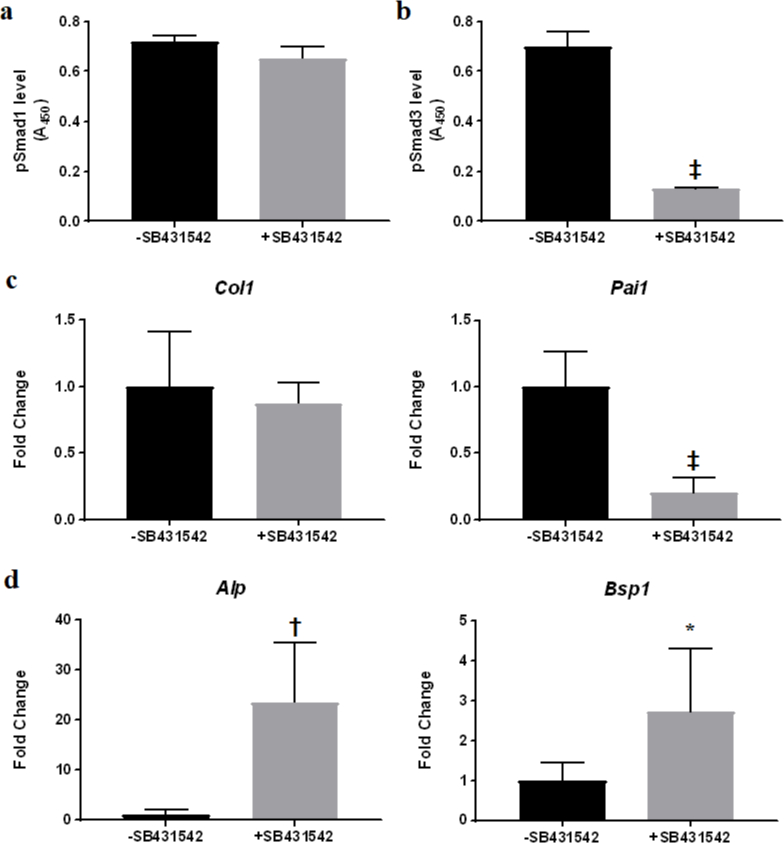
Fig. 7
Suppression of transforming growth factor-beta (TGF-β) signalling potentiated muscle-derived stromal cell (MDSC) osteogenesis in vitro. a) and b) Effect of SB431542 treatment on Smad phosphorylation measured by enzyme-linked immunosorbent assay (ELISA). a) Phosphorylated Smad1 level was not affected; b) phosphorylated Smad3 level was significantly reduced. ‡p < 0.0001; n = 3. c) Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) analysis of TGF-β1 target gene expression in MDSCs treated with SB431542. ‡p < 0.0001; n = 3. d) Polymerase chain reaction (PCR) analysis of expression of osteogenic marker genes in MDSCs treated with SB431542. *p < 0.05; †p < 0.001; n = 3. Alp, alkaline phosphatase; Bsp1, bone sialoprotein I; Col1, collagen type I; Pai1, PAI-1.
Discussion
In this study, we have attempted to address the underlying cause for DC, which often leads to pathological ossification, known as HO. We hypothesized that residual dead muscle tissue remaining in the zone of injury could promote HO formation in the presence of muscle injury, which may be the basis for the different incidence of HO associated with different amputation levels in the course of limb salvage after limb blast trauma. Our results support this hypothesis by showing that the presence of dead muscle tissue promotes DC formation in CTX-injured muscle tissue via a systemic effect, i.e. decreased circulating TGF-β1 level. We have previously shown that supplementation of TGF-β1 inhibits osteogenesis in vivo and in vitro.11 Here, we demonstrated that systemic suppression of TGF-β signalling promoted DC in vivo in the presence of muscle injury, while direct inhibition of TGF-β signalling potentiated osteogenic differentiation of MDSCs in vitro, further confirming that TGF-β1 acts as a negative regulator of osteogenesis. The importance of MDSCs to HO pathogenesis is strengthened by the recent finding by Eisner et al,16 who showed that murine tissue-resident PDGFRα+fibro-adipogenic progenitors are able to spontaneously acquire osteogenic phenotype in an altered inflammatory environment. Taken together, these findings strongly implicate TGF-β1 as a protective factor to prevent pathological ectopic mineralization following muscle injury.
We also demonstrated that implantation of dead muscle tissue induces a local fibrotic response, resulting in increased fibrin accumulation and increased TGF-β signalling (Figure 4). Fibrin accumulation usually leads to subsequent fibrosis via TGF-β1 signalling.15 qRT-PCR results confirmed this fibrotic response by showing the upregulation of TGF-β1 target gene expression in the inner thigh muscle tissue (Figures 5e and 5g). Interestingly, qRT-PCR analysis of inner thigh muscle tissue did not reveal increased TGF-β1 gene expression but significantly decreased TGF-β1 receptor gene expression instead (Figures 5a and 5c). These findings suggest that circulating TGF-β1 is being consumed locally – the feedback mechanism thus greatly downregulated TGF-β1 receptor gene expression in the inner thigh muscle, and since there is no increase in TGF-β1 production, the circulating TGF-β1 level should decrease.
How TGF-β1 is depleted from the circulating pool by dead muscle tissue is not explored in this study and until Day 7 timepoint after muscle implantation, there is no decrease in circulating TGF-β1 level nor phosphorylated Smad3 level in the outer thigh muscle, indicating that the local immune response takes some time to affect systemic response. Recently, decreased plasmin level has been reported to be associated with DC/HO formation;17,18 however, the plasmin target(s) responsible for this effect remain(s) to be identified. TGF-β1 is a well-known target of plasmin, which releases active TGF-β1 from its latency-associated peptide.19 The finding reported here showed fibrin accumulation in dead muscle tissue (Figure 4), possibly leading to local hyperfibrinolysis, thereby exhausting circulating plasmin. We speculate that, as a result, TGF-β1 activation will be suppressed systemically given the low level of circulating plasmin, while elevated TGF-β1 activation will be seen locally at the dead muscle tissue site. Our results support this notion by showing that a local fibrotic response towards dead muscle tissue happened as early as Day 3, and suppressed TGF-β signalling in tissue sites away from the dead muscle tissue implant at later timepoints (Figures 2b, 2d and 2f).
In conclusion, we demonstrated that immune response towards dead muscle tissue resulted in decreased systemic circulating TGF-β1 levels and local suppressed TGF-β signalling, which facilitated muscle injury-induced DC/HO formation. The limitation of this study is that the devitalized muscle tissue implantation model only produced DC, as a precursor event to HO. However, the mechanism of action reported here contributes to the understanding of the pathogenesis of post-traumatic HO following severe muscle trauma, and informs the design of surgical approaches to reduce the risk for HO, such as thorough debridement or minimally invasive procedures. A particular relevant example is hip surgery, where it has been proposed that surgical trauma to the gluteal muscles triggers HO.20 Therefore, either a complete debridement of devitalized gluteus minimus muscle in case of acetabular fractures,8 or avoiding muscle trauma by choosing an anterior approach over a posterior approach in total hip arthroplasty, is likely to reduce the incidence of HO.21,22
References
1. Moore-Lotridge SN , Li Q , Gibson BHY , et al. Trauma-induced nanohydroxyapatite deposition in skeletal muscle is sufficient to drive heterotopic ossification . Calcif Tissue Int . 2019 ; 104 ( 4 ): 411 – 425 . Crossref PubMed Google Scholar
2. Meyers C , Lisiecki J , Miller S , et al. Heterotopic ossification: a comprehensive review . JBMR Plus . 2019 ; 3 ( 4 ): e10172 . Crossref PubMed Google Scholar
3. Forsberg JA , Pepek JM , Wagner S , et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors . J Bone Joint Surg Am . 2009 ; 91-A ( 5 ): 1084 – 1091 . Crossref PubMed Google Scholar
4. Eisenstein N , Stapley S , Grover L . Post-traumatic heterotopic ossification: an old problem in need of new solutions . J Orthop Res . 2018 ; 36 ( 4 ): 1061 – 1068 . Crossref PubMed Google Scholar
5. Alfieri KA , Forsberg JA , Potter BK . Blast injuries and heterotopic ossification . Bone Joint Res . 2012 ; 1 ( 8 ): 174 – 179 . Crossref PubMed Google Scholar
6. Potter BK , Burns TC , Lacap AP , Granville RR , Gajewski DA . Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision . J Bone Joint Surg Am . 2007 ; 89-A ( 3 ): 476 – 486 . Crossref PubMed Google Scholar
7. Brown KV , Dharm-Datta S , Potter BK , et al. Comparison of development of heterotopic ossification in injured US and UK Armed Services personnel with combat-related amputations: preliminary findings and hypotheses regarding causality . J Trauma . 2010 ; 69 ( Suppl 1 ): S116 – S122 . Crossref PubMed Google Scholar
8. Rath EMS , Russell GV , Washington WJ , Routt MLC . Gluteus minimus necrotic muscle debridement diminishes heterotopic ossification after acetabular fracture fixation . Injury . 2002 ; 33 ( 9 ): 751 – 756 . Crossref PubMed Google Scholar
9. Jackson WM , Aragon AB , Onodera J , et al. Cytokine expression in muscle following traumatic injury . J Orthop Res . 2011 ; 29 ( 10 ): 1613 – 1620 . Crossref PubMed Google Scholar
10. Gilbert TW , Sellaro TL , Badylak SF . Decellularization of tissues and organs . Biomaterials . 2006 ; 27 ( 19 ): 3675 – 3683 . Crossref PubMed Google Scholar
11. Li L , Xiang S , Wang B , et al. TGF-β1 plays a protective role in glucocorticoid-induced dystrophic calcification . Bone . 2020 ; 136 : 115355 . Crossref PubMed Google Scholar
12. Asatrian G , Chang L , James AW . Muscle pouch implantation: an ectopic bone formation model . Methods Mol Biol . 2014 ; 1213 : 185 – 191 . Crossref PubMed Google Scholar
13. Glass GE , Chan JK , Freidin A , et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells . Proc Natl Acad Sci U S A . 2011 ; 108 ( 4 ): 1585 – 1590 . Crossref PubMed Google Scholar
14. Li L , Jiang Y , Lin H , et al. Muscle injury promotes heterotopic ossification by stimulating local bone morphogenetic protein-7 production . J Orthop Translat . 2019 ; 18 : 142 – 153 . Crossref PubMed Google Scholar
15. Vidal B , Serrano AL , Tjwa M , et al. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway . Genes Dev . 2008 ; 22 ( 13 ): 1747 – 1752 . Crossref PubMed Google Scholar
16. Eisner C , Cummings M , Johnston G , et al. Murine tissue-resident PDGFRα+ Fibro-Adipogenic progenitors spontaneously acquire osteogenic phenotype in an altered inflammatory environment . J Bone Miner Res . 2020 ; 35 ( 8 ): 1525 – 1534 . Crossref PubMed Google Scholar
17. Mignemi NA , Yuasa M , Baker CE , et al. Plasmin prevents dystrophic calcification after muscle injury . J Bone Miner Res . 2017 ; 32 ( 2 ): 294 – 308 . Crossref PubMed Google Scholar
18. Yuasa M , Mignemi NA , Nyman JS , et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification . J Clin Invest . 2015 ; 125 ( 9 ): 3723 . Crossref PubMed Google Scholar
19. Annes JP , Munger JS , Rifkin DB . Making sense of latent TGFbeta activation . J Cell Sci . 2003 ; 116 ( Pt 2 ): 217 – 224 . Crossref PubMed Google Scholar
20. Papavasiliou AV , Bardakos NV . Complications of arthroscopic surgery of the hip . Bone Joint Res . 2012 ; 1 ( 7 ): 131 – 144 . Crossref PubMed Google Scholar
21. Newman EA , Holst DC , Bracey DN , et al. Incidence of heterotopic ossification in direct anterior vs posterior approach to total hip arthroplasty: a retrospective radiographic review . Int Orthop . 2016 ; 40 ( 9 ): 1967 – 1973 . Crossref PubMed Google Scholar
22. Bergin PF , Doppelt JD , Kephart CJ , et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers . J Bone Joint Surg Am . 2011 ; 93-A ( 15 ): 1392 – 1398 . Crossref PubMed Google Scholar
Author contributions
L. Li: Designed and performed the research, Analyzed the data, Wrote the original manuscript.
S. Xiang: Designed and performed the research, Analyzed the data, Wrote the original manuscript.
B. Wang: Performed the research, Analyzed the data.
H. Lin: Performed the research, Analyzed the data.
G. Cao: Performed the research, Analyzed the data.
P. G. Alexander: Wrote the original manuscript.
R. S. Tuan: Designed the research, Revised the manuscript, Approved the submitted final version.
L. Li and S. Xiang contributed equally to this work.
Funding statement
This study was supported in part by funding from the Commonwealth of Pennsylvania Department of Health (SAP4100062224, SAP4100050913) and China Scholarship Council (awarded to La Li). No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
We thank Alyssa D. Falcione (University of Pittsburgh) for assistance with animal experiments and for advice regarding animal medication treatments.
Ethical review statement
The animal study protocol was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Protocol No. 18032493).
Supplementary material
Figure showing the systemic effect caused by devitalized muscle tissue implantation.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









