Abstract
Aims
To analyze the potential role of synovial fluid peptidase activity as a measure of disease burden and predictive biomarker of progression in knee osteoarthritis (KOA).
Methods
A cross-sectional study of 39 patients (women 71.8%, men 28.2%; mean age of 72.03 years (SD 1.15) with advanced KOA (Ahlbäck grade ≥ 3 and clinical indications for arthrocentesis) recruited through the (Orthopaedic Department at the Complejo Asistencial Universitario de León, Spain (CAULE)), measuring synovial fluid levels of puromycin-sensitive aminopeptidase (PSA), neutral aminopeptidase (NAP), aminopeptidase B (APB), prolyl endopeptidase (PEP), aspartate aminopeptidase (ASP), glutamyl aminopeptidase (GLU) and pyroglutamyl aminopeptidase (PGAP).
Results
Synovial fluid peptidase activity varied significantly as a function of clinical signs, with differences in levels of PEP (p = 0.020), ASP (p < 0.001), and PGAP (p = 0. 003) associated with knee locking, PEP (p = 0.006), ASP (p = 0.001), GLU (p = 0.037), and PGAP (p = 0.000) with knee failure, and PEP (p = 0.006), ASP (p = 0.001), GLU (p = 0.037), and PGAP (p < 0.001) with knee effusion. Further, patients with the greatest functional impairment had significantly higher levels of APB (p = 0.005), PEP (p = 0.005), ASP (p = 0.006), GLU (p = 0.020), and PGAP (p < 0.001) activity, though not of NAP or PSA, indicating local alterations in the renin-angiotensin system. A binary logistic regression model showed that PSA was protective (p = 0.005; Exp (B) 0.949), whereas PEP (p = 0.005) and GLU were risk factors (p = 0.012).
Conclusion
These results suggest synovial fluid peptidase activity could play a role as a measure of disease burden and predictive biomarker of progression in KOA.
Cite this article: Bone Joint Res 2020;9(11):789–797.
Article focus
-
We examined whether synovial fluid peptidase activity has a potential role as a measure of disease burden and predictive biomarker of progression in knee osteoarthritis (KOA).
Key messages
-
Synovial fluid peptidase levels varied significantly between patients requiring total knee arthroplasty (TKA) and others with advanced KOA managed conservatively.
Strengths and limitations
-
To our knowledge, these are the first data to suggest that synovial fluid peptidase activity could play a role as a measure of disease burden and predictive biomarker of progression in KOA.
-
Limitations of the study include failure to consider potential differences in conservative treatment, and the sample size being relatively small, limiting the reliability and validity of the results.
Introduction
Osteoarthritis (OA) is a chronic inflammatory joint disease that frequently coexists with other comorbidities, reducing joint range of motion and generating pain, functional limitations, and a high economic burden.1–3 Although there are some partially effective methods,4 it is generally accepted that treatments do not completely prevent disease progression,3 which is associated with cartilage erosion and joint inflammation, leading to disability.1
Several OA phenotypes have been described,5 and RNA expression patterns have been found in knee osteoarthritis (KOA) cartilage,6 indicating the importance of developing new biomarkers to improve the management of this condition. Regarding potential biomarkers, Bauer et al7 proposed the Burden of Disease, Investigative, Prognostic, Efficacy of Intervention and Diagnostic (BIPED) classification and this has been used in subsequent research;8 nonetheless, early biomarkers for clinical progression of OA have yet to be established.9
Several local renin-angiotensin systems have been described,10 including one in the synovial fluid and synovium.11 Animal models indicate its involvement in KOA,12 and that it promotes periarticular osteopenia by increasing bone resorption and decreasing bone formation.13 Moreover, research in humans suggests intra-articular renin and angiotensin-converting enzyme may contribute to progression in rheumatoid arthritis (RA).11 Indeed, aliskiren, a renin inhibitor, has had positive effects in rat models of OA14 and osteoporosis.15 Angiotensin II is a known potent proinflammatory mediator,16 and angiotensin receptor blockers show anti-inflammatory effects in animal models of arthritis.17 Further, articular chondrocyte angiotensin II type 1 receptor was implicated in KOA progression in a mechanical stress mouse model18 and activation of the renin-angiotensin system might be involved in the pathogenesis of this condition.12 Nonetheless, the association between angiotensin-converting enzyme and KOA remains controversial.19
Peptidases can degrade bioactive peptides, modifying their physiological actions, and consequently may regulate cell growth and differentiation, and signal transduction.20 Specifically, serum peptidases, such as angiotensin-converting enzyme (ACE), angiotensin II-converting enzyme (ACE2), neutral aminopeptidase (NAP), and aminopeptidase A (APA), are important elements of the renin-angiotensin system.21 For example, ACE is essential for degradation of angiotensin I, to obtain angiotensin II, and regulate blood pressure.22
Abnormal peptidase levels have been observed in a range of conditions, from renal cancer (ACE and NAP)23 to chronic tonsillitis (APA and dipeptidyl-peptidase IV).24 In OA, proteomic studies have shown inflammatory cytokines and proteases in synovial fluid,25,26 and evidence suggests the renin-angiotensin system may play a role in the pathophysiology of arthritis.13,27 Specifically, dipeptidyl-peptidase IV could be involved in OA inflammation.28 Other synovial peptidases implicated in inflammatory processes (e.g. aminopeptidase N, expressed by fibroblast-like synoviocytes) are present in synovial fluid and could play a role in OA.29 As de Silveira et al30 demonstrated, in an animal model of arthritis, joint inflammation was induced by activation of the renin-angiotensin system, but reduced by activation of angiotensin-converting enzyme-2/Ang-(1–7)/Mas receptor pathway.
Further, preoperatory predictive markers of knee joint infection have been proposed,31–33 but it remains unclear whether the activity of any specific enzymes can be considered a reliable measure of disease burden or predictor of progression in KOA. In this context, we considered that analyzing potential biomarkers could contribute to this line of research. In particular, we explored the potential role of synovial fluid peptidase activity as a measure of disease burden and predictive biomarker of progression in KOA, as this would be useful in clinical practice.
Methods
This cross-sectional study was approved by the Ethics Committee of the University of León, Spain (Agency code: AVPD; ref.: 280310015, 7 October 2011) and conducted in accordance with the Declaration of Helsinki (2013, revised May 5, 2015), ethical regulations and Spanish Laws for Data Protection (15/1999), and Biomedical Research in Human Participants (14/2007). All participants gave written informed consent to arthrocentesis and inclusion in this study.
The study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement.34 Patients with advanced KOA were recruited by non-probability sampling through the Orthopaedic Department at CAULE, Spain, between 2011 and 2019.
Inclusion criteria were a diagnosis of KOA (Ahlbäck grade ≥ 3)35 and clinical indications for arthrocentesis and related treatment, i.e. the extraction of synovial fluid and intra-articular injections for the local treatment of peripheral joint disease. We excluded patients with contraindications to arthrocentesis, biochemical markers of inflammatory activity, or inflammatory comorbidities. A sample size calculation indicated we needed to recruit at least 38 patients. Overall, we included 39 patients (women 71.8%, men 28.2%) with advanced KOA and a mean age of 72.03 years (SD 1.154; 69.69 to 74.36) (see Supplementary table i).
We assessed the activity of the following peptidases: puromycin-sensitive aminopeptidase (PSA) (EC 3.4.11.14, cytosolic form), NAP (EC 3.4.24.11), aminopeptidase B (APB) (EC 3.4.11.6), PEP (EC 3.4.21.26), aspartate aminopeptidase (ASP) (EC 3.4.11.21), glutamyl aminopeptidase (GLU) (EC 3.4.11.7), and pyroglutamyl aminopeptidase (PGAP) (EC 3.4.19.3). At enrolment, synovial fluid samples (blood-free and ≥ 10 ml) were collected from all patients by standard arthrocentesis, centrifuged, and frozen until blind analysis (see Supplementary Material 2). In all cases, samples were collected ≥ six months after the most recent intra-articular injections.
Further, we retrieved data on MRI and laboratory test results (e.g. knee cartilage thickness and blood counts) from health records and collected data on sociodemographic and clinical characteristics through clinical examinations and interviews. Lastly, patient-reported outcome measures ((PROMs) quality of life, physical status, and functional status) were assessed at the most recent visit (see Supplementary Material).
For secondary analysis, the sample was divided into a conservative treatment group (CTG) (n = 18; women 66.7%; mean age 71.33 years (SD 1.926; 67.31 to 75.35)) and knee arthroplasty group (KAG) (n = 21; women 77.8%, mean age 72.83 years (SD 1.135; 70.44 to 75.23)). The CTG contained patients who responded satisfactorily to conservative management, and the KAG those selected for knee arthroplasty following usual criteria: namely, knee locking, failure and/or effusion, pain and joint cartilage damage regardless of the presence of deformity, and others who did not respond satisfactorily to conservative management (see Supplementary Material). Figure 1 shows the flow of patients through the study.
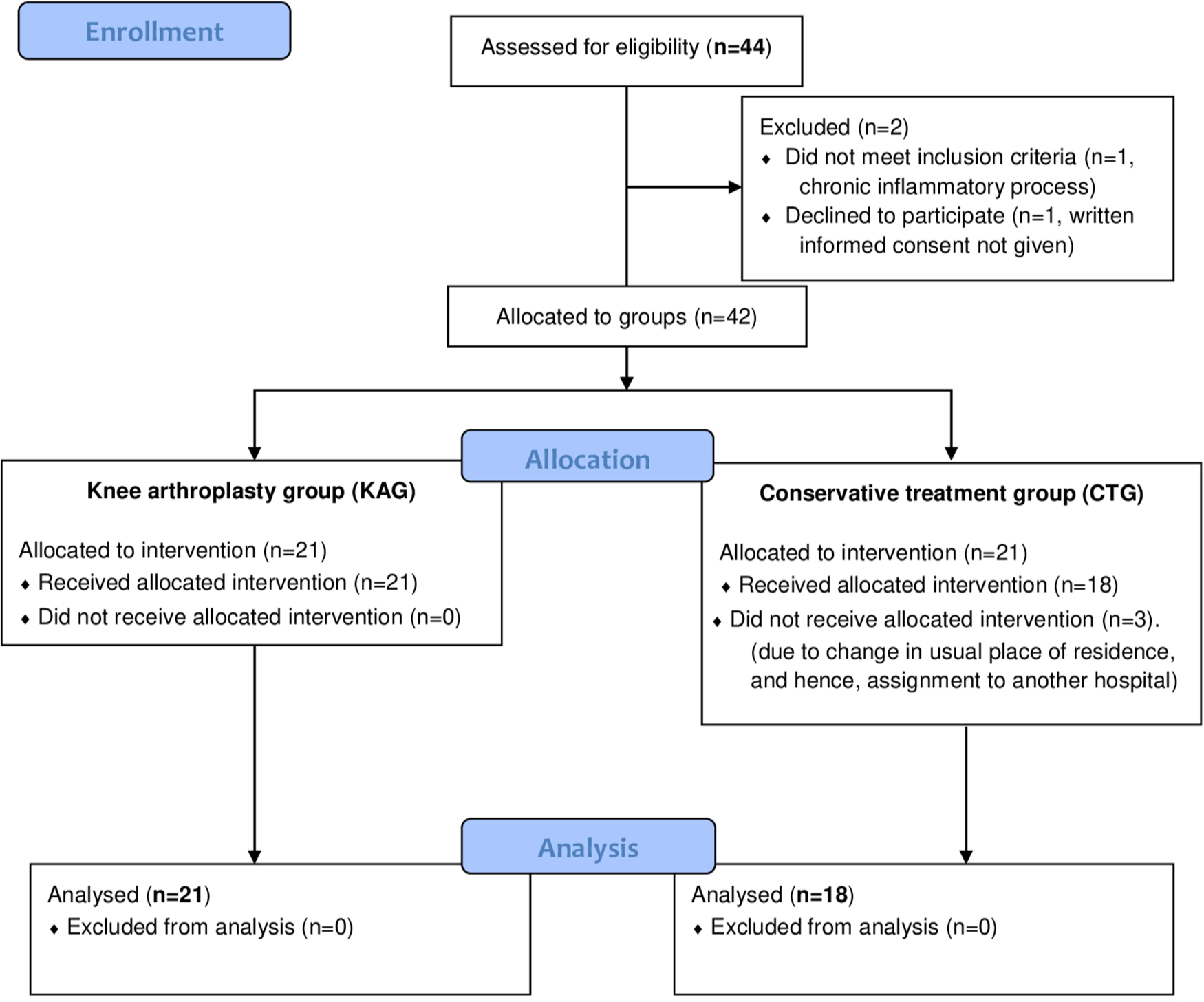
Fig. 1
Flow of patients through the study (Consolidated standards of reporting Trials (CONSORT) flow diagram).
Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows v25 (IBM, Armonk, New York, USA). The Shapiro-Wilk test was used to check whether quantitative data were normally distributed. Sociodemographic and clinical characteristics of all patients were summarized using descriptive statistics (medians, means, and SDs) and comparisons made using independent-samples t-test or Mann-Whitney U tests for quantitative variables, and chi-squared tests for qualitative variables.
Binary logistic regression models were built to explore the effects of the peptidase activities studied on the dependent binary variable of interest (namely, knee locking, failure, or effusion) with all the samples, and in secondary analysis, the need for arthroplasty (inclusion in the KAG). All enzymes were initially considered candidates and the best model was identified with a backward stepwise procedure, using the Wald statistic. Further, B coefficients were calculated for the predictive equation, as well as Exp(B) values and corresponding 95% confidence intervals for the peptidase activities included. Additionally, having performed Levene’s test to confirm homoscedasticity, independent-samples t-tests were used for between-group comparisons, and standardized means were calculated to assess effect sizes (Cohen’s d).
Results
The synovial fluid peptidase levels studied did not vary significantly with knee cartilage thickness as measured by MRI. Nonetheless, we observed clinically significant differences in NAP as a function of pain and range of motion (Table I). Further, peptidase levels did vary significantly as a function of clinical signs, with differences in levels of PEP, ASP, and PGAP associated with knee locking, PEP, ASP, GLU, and PGAP with knee failure, and PEP, ASP, GLU, and PGAP with knee effusion (Table II). Moreover, the knee locking model showed an influence of PSA, ASP, and GLU, while NAP and PGAP were significant in knee failure and effusion models (Table III). In addition, knee locking, failure, and effusion were all significantly associated with the need for knee arthroplasty (Table IV).
Table I.
Comparison between peptidase activity and patient-reported outcome measures (PROMs): state of the articular cartilage (as assessed by MRI) and pain on movement and range of motion (as assessed with the modified Knee Society Score) (n = 39).36
| Peptidase activity (U/mg prot)* | Median (IQR) | p-value† | p-value‡ | p-value§ |
|---|---|---|---|---|
| NAP | 87.8259 (23.42 to 338.79) | 0.565 | 0.051 | 0.051 |
| PSA | 173.0000 (40.56 to 367.44) | 0.502 | 0.154 | 0.154 |
| ABP | 46.8974 (9.00 to 276.54) | 0.776 | 0.103 | 0.103 |
| PEP | 15.3273 (3.00 to 64.15) | 0.266 | 0.103 | 0.103 |
| ASP | 13.7366 (4.93 to 32.99) | 0.734 | 0.205 | 0.205 |
| GLU | 14.0000 (5.00 to 46.88) | 0.738 | 0.564 | 0.564 |
| PGAP | 7.0000 (3.00 to 17.24) | 0.426 | 0.256 | 0.256 |
-
*
Peptidase activity reported as units of enzyme per milligram of protein; Mann-Whitney U test (p < 0.05).
-
†
Comparison between peptidase activity and state of the articular cartilage (assessed by MRI). Mann-Whitney U test.
-
‡
Comparison between peptidase activity and pain on movement (as assessed with the modified Knee Society Score).
-
§
Comparison between peptidase activity and range of motion (as assessed with the modified Knee Society Score).
-
APB, aminopeptidase B; ASP, aspartate aminopeptidase; GLU, glutamyl aminopeptidase; IQR, interquartile range; NAP, neutral aminopeptidase; PEP, prolyl endopeptidase; PGAP, pyroglutamyl aminopeptidase; PSA, puromycin-sensitive aminopeptidase.
Table II.
Comparison between peptidase activity (reported as units of enzyme per milligram of protein) and clinical signs: knee locking, knee failure, and knee effusion (n = 39).
| Peptidase activity (U/mg prot)* | Median (IQR) | p-value† | p-value‡ | p-value§ |
|---|---|---|---|---|
| NAP | 87.8259 (23.42 to 338.79) | 0.251 | 0.489 | 0.489 |
| PSA | 173.0000 (40.56 to 367.44) | 0.392 | 0.735 | 0.735 |
| ABP | 46.8974 (9.00 to 276.54) | 0.041 | 0.076 | 0.076 |
| PEP | 15.3273 (3.00 to 64.15) | 0.020 | 0.006 | 0.006 |
| ASP | 13.7366 (4.93 to 32.99) | < 0.001 | 0.001 | 0.001 |
| GLU | 14.0000 (5.00 to 46.88) | 0.071 | 0.037 | 0.037 |
| PGAP | 7.0000 (3.00 to 17.24) | 0.003 | < 0.001 | < 0.001 |
-
*
U/mg prot.: Peptidase activity reported as units of enzyme per milligram of protein; Mann-Whitney U test (p < 0.05).
-
†
Comparison between peptidase activity and knee locking.
-
‡
Comparison between peptidase activity and knee failure.
-
§
Comparison between peptidase activity and knee effusion.
-
APB, aminopeptidase B; ASP, aspartate aminopeptidase; GLU, glutamyl aminopeptidase; IQR, interquartile range; NAP, neutral aminopeptidase; PEP, prolyl endopeptidase; PGAP, pyroglutamyl aminopeptidase; PSA, puromycin-sensitive aminopeptidase.
Table III.
Binary logistic regression models.
| Category | B | SD | Wald | Sig. | Exp(B) | 95% CI for Exp(B) |
|---|---|---|---|---|---|---|
| Peptidase activity and knee locking * | ||||||
| PSA | −0.016 | 0.008 | 4.268 | 0.039 | 0.984 | 0.970 to 0.999 |
| ASP | 0.246 | 0.091 | 7.268 | 0.007 | 1.279 | 1.069 to 1.529 |
| GLU | 0.155 | 0.086 | 3.203 | 0.073 | 1.167 | 0.985 to 1.383 |
| Constant | −2.127 | 1.328 | 2.565 | 0.109 | 0.119 | N/A |
| Peptidase activity and knee failure † | ||||||
| NAP | −0.020 | 0.010 | 3.888 | 0.058 | 0.980 | 0.961 to 1.001 |
| PGAP | 0.930 | 0.337 | 7.643 | 0.006 | 2.536 | 1.311 to 4.904 |
| Constant | −3.273 | 1.529 | 4.581 | 0.032 | 0.038 | N/A |
| Peptidase activity and knee effusion ‡ | ||||||
| NAP | −0.020 | 0.010 | 3.888 | 0.058 | 0.980 | 0.961 to 1.001 |
| PGAP | 0.930 | 0.337 | 7.643 | 0.006 | 2.536 | 1.311 to 4.904 |
| Constant | −3.273 | 1.529 | 4.581 | 0.032 | 0.038 | N/A |
| Peptidase activity and inclusion in the KAG § | ||||||
| PSA | −0.052 | 0.019 | 8.012 | 0.005 | 0.949 | 0.915 to 0.984 |
| PEP | 0.414 | 0.149 | 7.738 | 0.005 | 1.513 | 1.130 to 2.026 |
| GLU | 0.423 | 0.169 | 6.250 | 0.012 | 1.527 | 1.096 to 2.128 |
| Constant | −3.625 | 2.077 | 3.046 | 0.081 | 0.027 | N/A |
-
*
Omnibus p < 0.005; Nagelkerke R2 = 0.577; Hosmer-Lemeshow p = 0.808.
-
†
Omnibus p < 0.005; Nagelkerke R2 = 0.520; Hosmer-Lemeshow p = 0.893.
-
‡
Omnibus p < 0.005; Nagelkerke R2 = 0.520; Hosmer-Lemeshow p = 0.893.
-
§
Omnibus p = 0.000; Nagelkerke R2 = 0.812; Hosmer-Lemeshow p = 0.222. This shows the influence of PSA, PEP, and GLU, as independent variables, on a dependent binary variable indicating inclusion in the Knee Arthroplasty Group. PSA was a protective factor, whereas PEP and GLU were risk factors.
-
ASP, aspartate aminopeptidase; CI, confidence interval; GLU, glutamyl aminopeptidase; KAG, Knee Arthroplasty Group; N/A, not applicable; NAP, neutral aminopeptidase; PEP, prolyl endopeptidase; PGAP, pyroglutamyl aminopeptidase; PSA, puromycin-sensitive aminopeptidase.
Table IV.
Between-group comparison of qualitative clinical variables commonly used in routine clinical practice as criteria for indicating knee arthroplasty (n = 39). All p-values were < 0.05 (chi-squared test).
| Qualitative clinical variable | Conservative treatment group (n = 18) | Knee arthroplasty group (n = 21) |
|---|---|---|
| Knee locking, n (%) | 4 (16) | 21 (84) |
| Knee failure, n (%) | 5 (19.2) | 21 (80.8) |
| Knee effusion, n (%) | 5 (19.2) | 21 (80.8) |
Regarding the secondary analysis by need for arthroplasty (CTG vs KAG), the groups were similar, with no significant differences in sociodemographic or the majority of clinical variables (Tables V and VI and Supplementary table iii). Nonetheless, as expected, there were significant differences in variables commonly used as criteria for indicating knee arthroplasty (Table VII). Specifically, KAG patients had not substantially benefited from previous treatment, such as hyaluronic acid injections, and had highly inflamed knee joints, with recurrent severe distention of the joint capsule, intense pain, and joint effusion, locking, and failure in all cases.
Table V.
Between-group comparison of quantitative sociodemographic and clinical variables.
| Variable | Conservative treatment group (n = 18) | Knee arthroplasty group (n = 21) | ||||||
| Average (median or mean (SD)) | IQR or 95% CI | p-value* | Average (median or mean (SD)) | IQR or 95% CI | p-value* | p-value† | p-value‡ | |
| Age, yrs | 72.83 (4.817) | 95% CI 70.44 to 75.23 | 0.518 | 71.33 (8.828) | 95% CI 67.31 to 75.35 | 0.317 | 0. 507 | 0.922 |
| Duration of pain, yrs | 9.50 | 2 to 30 | 0.007 | 9.00 | 6 to 22 | 0.005 | 0.563 | 0.282 |
| Pain VAS (1 to 10) | 8.00 | 4 to 10 | 0.003 | 8.00 | 6 to 10 | 0.029 | 0.205 | 0.174 |
| mKSS ROM (0 to 100) | 26.67 (13.284) | 95% CI 20.06 to 33.27 | 0.096 | 28.33 (15.838) | 95% CI 21.12 to 35.54 | 0.077 | 0.726 | 0.707 |
| mKSS pain on movement (0 to 100) | 22.17 (16.525) | 95% CI 13.95 to 30.38) | 0.091 | 13.00 | 0 to 69 | 0.018 | 0.919 | 0.967 |
-
*
Shapiro-Wilk test.
-
†
Independent-samples t-test.
-
‡
Mann-Whitney U test.
-
CI, confidence interval; IQR, interquartile range; mKSS, Insall’s modified Knee Society Score; ROM, range of motion; VAS, visual analogue scale.
Table VI.
Between-group comparison of qualitative sociodemographic and clinical variables.
| Variable | Conservative treatment group (n = 18) | Knee arthroplasty group (n = 21) | p-value* |
|---|---|---|---|
| Sex, n (%) | 0.442 | ||
| Female | 14 (77.8) | 14 (66.7) | |
| Male | 4 (22.4) | 7 (33.3) | |
| Surgical risk based on physical status (ASA class), n (%) | |||
| No systemic conditions | 6 (33.3) | 5 (23.8) | 0.700 |
| Mild systemic disease | 10 (55.6) | 12 (57.1) | |
| Severe systemic disease | 2 (11.1) | 4 (19.0) | |
| Laterality, n (%) | 0.882 | ||
| Right | 9 (50) | 10 (47.6) | |
| Left | 9 (50) | 11 (52.4) | |
| Contralateral pain, n (%) | 0.159 | ||
| No | 6 (33.3) | 3 (14.3) | |
| Yes | 12 (66.6) | 18 (85.7) | |
| mKSS pain on movement (Insall’s modified Knee Society Score), n (%) | |||
| Acceptable (60 to 69) | 0 (0) | 1 (4.8) | 0.348 |
| Poor (< 60) | 18 (100) | 20 (95.2) | |
| mKSS ROM, n (%) | |||
| Acceptable (60 to 69) | 0 (0) | 1 (4.8) | 0.348 |
| Poor (< 60) | 18 (100) | 20 (95.2) | |
| State of the articular cartilage (assessed by MRI), n (%) | |||
| Ulceration (depth < 50%) | 2 (11.1) | 0 (0) | 0.267 |
| Ulceration (depth ≥ 50%) | 8 (44.4) | 12 (57.1) | |
| Exposure of subchondral bone | 8 (44.4) | 9 (42.9) |
-
*
Chi-squared test.
-
ASA, American Society of Anesthesiologists; mKSS, Insall’s modified Knee Society Score;ROM, range of motion.
Table VII.
Between-group comparison of qualitative clinical variables.
| Clinical variable | Conservative treatment group (n = 18) | Knee arthroplasty group (n = 21) | p-value* |
|---|---|---|---|
| Degree of OA (Ahlbäck grade), n (%)† | |||
| 3 | 3 (16.7) | 4 (19.0) | 0.006 |
| 4 | 8 (44.4) | 17 (81.0) | |
| 5 | 7 | 0 | |
| Change in quality of life over 12 months (EuroQol EQ-5D)† | 18 (100) | 21 (100) | < 0.001 |
| BMI, kg/m2‡ | |||
| 18.5 to 24.9 (normal weight) | 4 (22.2) | 5 (23.8) | 0.981 |
| 25.0 to 29.9 (grade I and II overweight) | 10 (55.6) | 11 (52.4) | |
| 30.0 to 39.9 (grade I and II obesity) | 4 (22.2) | 5 (23.8) | |
| History of surgery for OA in contralateral knee, n (%) | 0.052 | ||
| No | 15 (83.3) | 21 (100) | |
| Yes | 3 (16.7) | 0 (0) | |
| History of surgical interventions in the knee, n (%) | 0.002 | ||
| No | 13 (72.2) | 5 (23.8) | |
| Yes | 5 (27.8) | 16 (76.2) | |
| Knee locking, n (%)† | < 0.001 | ||
| No | 14 (77.8) | 0 (0) | |
| Yes | 4 (22.2) | 21 (100) | |
| Knee failure, n (%)† | < 0.001 | ||
| No | 13 (72.2) | 0 (0) | |
| Yes | 5 (27.8) | 21 (100) | |
| Knee effusion, n (%)† | < 0.001 | ||
| No | 13 (72.2) | 0 (0) | |
| Yes | 5 (27.8) | 21 (100) | |
| History of treatment | |||
| Only oral analgesia | 11 (61.1) | 9 (42.9) | < 0.001 |
| Oral analgesia + physical therapy + intra-articular injections | 7 (38.9) | 12 (57.1) |
All peptidases studied were detected in all patients, and several between-group differences were found. Specifically, KAG patients showed significantly higher activity of APB, PEP, ASP, GLU, and PGLU, but not PNA or PSA (Table VIII).
Table VIII.
Between-group comparison of levels of activity of synovial fluid peptidases. Each column of p-values signify comparisons between groups.
| Peptidase activity (U/mg prot)* | Conservative treatment group (n = 18) | Knee arthroplasty group (n = 21) | p-value‡ | ||||
|---|---|---|---|---|---|---|---|
| Average (median or mean (SD)) | IQR or 95% CI | p-value† | Average (median or mean (SD)) | IQR or 95% CI | p-value† | ||
| NAP | 106.500 | 41.00 to 266.00 | 0.041 | 85.5089 | 23.42 to 338.79 | 0.001 | 0.223 |
| PSA | 202.2193 (87.06746) | 95% CI 158.9217 to 245.516 | 0.222 | 152.8595 | 40.56 to 367.44 | 0.013 | 0.183 |
| ABP | 17.500 | 9.00 to 242.00 | 0.000 | 55.5021 | 28.11 to 276.54 | 0.000 | 0.005 |
| PEP | 12.8358 (5.55793) | 95% CI 10.0719 to 15.5997 | 0.859 | 16.9785 | 9.06 to 64.15 | 0.000 | 0.006 |
| ASP | 8.500 | 5.00 to 22.40 | 0.023 | 19.0425 | 4.93 to 32.99 | 0.045 | < 0.001 |
| GLU | 11.000 | 5.00 to 30.88 | 0.037 | 16.3267 | 8.79 to 46.88 | 0.003 | 0.020 |
| PGAP | 6.000 | 3.00 to 13.00 | 0.012 | 9.4819 (3.21187) | 95% CI 8.0199 to 10.9439 | 0.080 | < 0.001 |
-
*
Peptidase activity is reported as units of enzyme per milligram of protein (U/mg prot).
-
†
Shapiro-Wilk test.
-
‡
Mann-Whitney U test.
-
APB, aminopeptidase B; ASP, aspartate aminopeptidase; CI, confidence interval; GLU, glutamyl aminopeptidase; IQR, interquartile range; NAP, neutral aminopeptidase; PEP, prolyl endopeptidase; PGAP, pyroglutamyl aminopeptidase; PSA, puromycin-sensitive aminopeptidase.
A binary logistic regression model showed effects of PSA, PEP, and GLU on the need for arthroplasty. PSA was a protective factor, whereas PEP and GLU were risk factors (Table III) (Nagelkerke R2 p = 0.812; Hosmer-Lemeshow p = 0.222; Omnibus p < 0.001).
There was a significant difference between groups (Levene’s p = 0.138; independent-samples t-test p = 0.011); specifically, the difference between means was −8.22763 (95% confidence interval (CI) −1.98798 to −14.46727), with higher levels in the KAG than the CTG. The effect sizes obtained were 0.36, 0.79, and 0.64 for PSA, PEP, and GLU, respectively.
Discussion
To our knowledge, this study provides the first evidence for the role of synovial fluid peptidase activity as a measure of disease burden and predictive biomarker of progression in KOA. Specifically, we found significant differences in biomarker levels as a function of pain and range of motion, with somewhat different patterns in each case.
To date, few studies have investigated synovial fluid aminopeptidase activity in intra-articular inflammatory processes in humans. Some years ago, human articular chondrocytes obtained from patients with OA undergoing arthroplasty were found to express angiotensin II receptors.39 In line with this, losartan showed anti-inflammatory action in human arthritis.40 Further, Cobankara et al11 have suggested that the local articular renin-angiotensin system is involved in joint destruction in RA, and recent studies support the view that this system plays a role in the pathophysiology of arthritis.13,27
We have found significant differences in synovial fluid levels of some biomarkers as a function of clinical signs: knee locking, failure, and effusion, although not as a function of PROMs (visual analogue scale, modified Knee Society Score, or EQ-5D score). Moreover, in patients with advanced KOA, synovial fluid peptidase analysis revealed significant differences between patients requiring TKA and those managed conservatively, the former (those with the greatest functional impairment) having significantly higher peptidase activities, indicating alterations in the local renin-angiotensin system in the cases of APB, PEP, ASP, GLU, and PGAP, but not NAP or PSA. This is consistent with the higher APB activity in synovial fluid from swollen knees in a rat model of RA41 and synovial fluid NAP activity inducing T-cell chemotaxis in a similar animal model.42 Further, NAP seems to be involved in the pathogenesis of RA43 and possibly also OA.29
The predictive model for knee locking showed the influence of PSA, ASP, and GLU, while APN and PGLU were significant in knee failure and effusion models (Table III). Further, the model for the need for arthroplasty (Table III) showed the influence of PSA, PEP, and GLU. Specifically, this model indicated that PSA activity was a protective factor, whereas PEP and GLU activities were risk factors. We could have studied other inflammatory cytokines and matrix metalloproteinases, but opted to focus on synovial soluble peptidases involved in the articular renin-angiotensin system, which has been associated with clinical progression in KOA.
Although between-group differences were not significant for NAP or PSA, both were considered candidates for inclusion in this model, given their clinical relevance, and the results indicated a role as a predictive factor for PSA, but not for NAP. The potential importance of NAP is related to its expression by fibroblast-like synoviocytes in inflamed synovial tissue in humans, suggesting a role in acute inflammatory arthritis.29 Regarding PSA, while there is a paucity of data, spinorphin, an endogenous enzyme inhibitor, has been reported to break down enkephalin and play a role in pain and inflammation.44 The possible role of PSA in pain strengthens its clinical relevance, in that, as underlined by scientific societies, pain is an important clinical feature in deciding whether to indicate arthroplasty, and this is what motivated us to include it in our study. Notably, patients with chronic pain associated with fibromyalgia have enzyme activity characterized by abnormally low serum enkephalin-degrading enzyme activity,45 suggesting that it may influence musculoskeletal pain and that PSA might be involved in pain neuromodulatory mechanisms in OA. More studies are needed to clarify these issues.
Given the high specificity in the clinical characterization of these patients, and in line with the BIPED classification,7,8 our results suggest a role for synovial fluid peptidase activity as a measure of disease burden and predictive biomarker of progression in KOA, which would be useful in clinical practice. Generally, patients with advanced KOA are seen by trauma and orthopaedic specialists every six months at outpatient appointments, in which arthrocentesis may be performed to drain synovial fluid, and intra-articular injections given. During such appointments, a small quantity of synovial fluid could be collected easily, inexpensively, and without causing bleeding, and sent for peptidase analysis, which is straightforward and quick. The use of PSA, PEP, and GLU activities as indicators of disease burden and predictors of progression could help clinicians decide whether to indicate knee arthroplasty. This might not only reduce delays, avoiding patients waiting a further year and potentially experiencing functional deterioration, but also the risk of complications (such as infection) should surgery become necessary,31–33 and the need for prosthesis arthroplasty, reducing surgical reintervention and failure rates.
This study has limitations, including failure to consider differences in conservative treatment, or other proteins and enzymes likely involved in KOA. Other limitations are the small sample size, and that we only analyzed samples from patients undergoing arthrocentesis. All the participants had a marked inflammatory component, and our results may not apply to less inflamed joints. Future studies are needed to explore whether enzyme activity is influenced by treatment type (anti-inflammatory corticosteroids, chondroprotective agents, or platelet-rich plasma), and confirm whether peptidase levels are indeed reliable bioindicators of KOA progression.
References
1. Ashkavand Z , Malekinejad H , Vishwanath BS . The pathophysiology of ostheoarthritis . J Pharm Res . 2013 ; 7 : 132 – 138 . Google Scholar
2. Herrero-Beaumont G , Roman-Blas JA , Bruyère O , et al. Clinical settings in knee osteoarthritis: pathophysiology guides treatment . Maturitas . 2017 ; 96 : 54 – 57 . Crossref PubMed Google Scholar
3. Glyn-Jones S , Palmer AJR , Agricola R , et al. Osteoarthritis . Lancet . 2015 ; 386 ( 9991 ): 376 – 387 . Crossref PubMed Google Scholar
4. Sánchez M , Delgado D , Pompei O , et al. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: an observational study . Cartilage . 2019 ; 10 ( 2 ): 245 – 253 . Crossref PubMed Google Scholar
5. Van Spil WE , Kubassova O , Boesen M , Bay-Jensen A-C , Mobasheri A . Osteoarthritis phenotypes and novel therapeutic targets . Biochem Pharmacol . 2019 ; 165 : 41 – 48 . Crossref PubMed Google Scholar
6. Li H , Yang HH , Sun ZG , Tang HB , Min JK . Whole-transcriptome sequencing of knee joint cartilage from osteoarthritis patients . Bone Joint Res . 2019 ; 8 ( 7 ): 290 – 303 . Crossref PubMed Google Scholar
7. Bauer DC , Hunter DJ , Abramson SB , et al. Classification of osteoarthritis biomarkers: a proposed approach . Osteoarthritis Cartilage . 2006 ; 14 ( 8 ): 723 – 727 . Crossref PubMed Google Scholar
8. Bay-Jensen AC , Reker D , Kjelgaard-Petersen CF , et al. Osteoarthritis year in review 2015: soluble biomarkers and the BIPED criteria . Osteoarthritis Cartilage . 2016 ; 24 ( 1 ): 9 – 20 . Crossref PubMed Google Scholar
9. Mobasheri A , Bay-Jensen A-C , van Spil WE , Larkin J , Levesque MC . Osteoarthritis year in review 2016: biomarkers (biochemical markers) . Osteoarthritis Cartilage . 2017 ; 25 ( 2 ): 199 – 208 . Crossref PubMed Google Scholar
10. Paul M , Poyan Mehr A , Kreutz R . Physiology of local renin-angiotensin systems . Physiol Rev . 2006 ; 86 ( 3 ): 747 – 803 . Crossref PubMed Google Scholar
11. Cobankara V , Oztürk MA , Kiraz S , et al. Renin and angiotensin-converting enzyme (ACE) as active components of the local synovial renin-angiotensin system in rheumatoid arthritis . Rheumatol Int . 2005 ; 25 ( 4 ): 285 – 291 . Crossref PubMed Google Scholar
12. Yamagishi K , Tsukamoto I , Nakamura F , Hashimoto K , Ohtani K , Akagi M . Activation of the renin-angiotensin system in mice aggravates mechanical loading-induced knee osteoarthritis . Eur J Histochem . 2018 ; 62 ( 3 ): 2930 . Crossref PubMed Google Scholar
13. Wang Y , Kou J , Zhang H , et al. The renin-angiotensin system in the synovium promotes periarticular osteopenia in a rat model of collagen-induced arthritis . Int Immunopharmacol . 2018 ; 65 : 550 – 558 . Crossref PubMed Google Scholar
14. Yan K , Shen Y . Aliskiren has chondroprotective efficacy in a rat model of osteoarthritis through suppression of the local renin-angiotensin system . Mol Med Rep . 2017 ; 16 ( 4 ): 3965 – 3973 . Crossref PubMed Google Scholar
15. Zhang Y , Wang L , Song Y , Zhao X , Wong MS , Zhang W . Renin inhibitor aliskiren exerts beneficial effect on trabecular bone by regulating skeletal renin-angiotensin system and kallikrein-kinin system in ovariectomized mice . Osteoporos Int . 2016 ; 27 ( 3 ): 1083 – 1092 . Crossref PubMed Google Scholar
16. Dagenais NJ , Jamali F . Protective effects of angiotensin II interruption: evidence for antiinflammatory actions . Pharmacotherapy . 2005 ; 25 ( 9 ): 1213 – 1229 . Crossref PubMed Google Scholar
17. Price A , Lockhart JC , Ferrell WR , Gsell W , McLean S , Sturrock RD . Angiotensin II type 1 receptor as a novel therapeutic target in rheumatoid arthritis: in vivo analyses in rodent models of arthritis and ex vivo analyses in human inflammatory synovitis . Arthritis Rheum . 2007 ; 56 ( 2 ): 441 – 447 . Crossref PubMed Google Scholar
18. Nakamura F , Tsukamoto I , Inoue S , Hashimoto K , Akagi M . Cyclic compressive loading activates angiotensin II type 1 receptor in articular chondrocytes and stimulates hypertrophic differentiation through a G-protein-dependent pathway . FEBS Open Bio . 2018 ; 8 ( 6 ): 962 – 973 . Crossref PubMed Google Scholar
19. Lin C , Chen H-C , Fang W-H , et al. Angiotensin-Converting enzyme insertion/deletion polymorphism and susceptibility to osteoarthritis of the knee: a case-control study and meta-analysis . PLoS One . 2016 ; 11 ( 9 ): e0161754 . Crossref PubMed Google Scholar
20. Carl-McGrath S , Lendeckel U , Ebert M , Röcken C . Ectopeptidases in tumour biology: a review . Histol Histopathol . 2006 ; 21 ( 12 ): 1339 – 1353 . Crossref PubMed Google Scholar
21. Fernández-Atucha A , Izagirre A , Fraile-Bermúdez AB , et al. Sex differences in the aging pattern of renin-angiotensin system serum peptidases . Biol Sex Differ . 2017 ; 8 : 5 . Crossref PubMed Google Scholar
22. Kuba K , Imai Y , Ohto-Nakanishi T , Penninger JM . Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters . Pharmacol Ther . 2010 ; 128 ( 1 ): 119 – 128 . Crossref PubMed Google Scholar
23. Errarte P , Beitia M , Perez I , et al. Expression and activity of angiotensin-regulating enzymes is associated with prognostic outcome in clear cell renal cell carcinoma patients . PLoS One . 2017 ; 12 ( 8 ): e0181711 . Crossref PubMed Google Scholar
24. Larrinaga G , Perez I , Sanz B , et al. Activity of soluble aminopeptidase A and dipeptidyl peptidase IV and membrane-bound aminopeptidase B and pyroglutamyl peptidase I in adenoid hyperplasia, tonsillar hyperplasia and chronic tonsillitis . Int J Pediatr Otorhinolaryngol . 2011 ; 75 ( 11 ): 1399 – 1403 . Crossref PubMed Google Scholar
25. Balakrishnan L , Nirujogi RS , Ahmad S , et al. Proteomic analysis of human osteoarthritis synovial fluid . Clin Proteomics . 2014 ; 11 ( 1 ): 6 . Crossref PubMed Google Scholar
26. Boris Chan PM , Zhu L , Wen CY , Chiu KY . Subchondral bone proteomics in osteoarthritis: current status and perspectives . J Orthop Translat . 2015 ; 3 ( 2 ): 71 – 77 . Crossref PubMed Google Scholar
27. Wu Y , Li M , Zeng J , et al. Differential expression of renin-angiotensin system-related components in patients with rheumatoid arthritis and osteoarthritis . Am J Med Sci . 2020 ; 359 ( 1 ): 17 – 26 . Crossref PubMed Google Scholar
28. Mantle D , Falkous G , Walker D . Quantification of protease activities in synovial fluid from rheumatoid and osteoarthritis cases: comparison with antioxidant and free radical damage markers . Clin Chim Acta . 1999 ; 284 ( 1 ): 45 – 58 . Crossref PubMed Google Scholar
29. Du Y , Lu C , Morgan RL , et al. Angiogenic and arthritogenic properties of the soluble form of CD13 . J Immunol . 2019 ; 203 ( 2 ): 360 – 369 . Crossref PubMed Google Scholar
30. da Silveira KD , Coelho FM , Vieira AT , et al. Anti-inflammatory effects of the activation of the angiotensin-(1-7) receptor, MAS, in experimental models of arthritis . J Immunol . 2010 ; 185 ( 9 ): 5569 – 5576 . Crossref PubMed Google Scholar
31. Tarabichi M , Shohat N , Goswami K , Parvizi J . Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Joint J . 2018 ; 100-B ( 2 ): 127 – 133 . Crossref PubMed Google Scholar
32. Kuo F-C , Lu Y-D , Wu C-T , You HL , Lee GB , Lee MS . Comparison of molecular diagnosis with serum markers and synovial fluid analysis in patients with prosthetic joint infection . Bone Joint J . 2018 ; 100-B ( 10 ): 1345 – 1351 . Crossref PubMed Google Scholar
33. Chen M-F , Chang C-H , Yang L-Y , et al. Synovial fluid interleukin-16, interleukin-18, and CRELD2 as novel biomarkers of prosthetic joint infections . Bone Joint Res . 2019 ; 8 ( 4 ): 179 – 188 . Crossref PubMed Google Scholar
34. No authors listed . STROBE statement: strengthening the reporting of observational studies in epidemiology . 2007 . https://www.strobe-statement.org/index.php?id=available-checklists Google Scholar
35. Ahlbäck S . Osteoarthrosis of the knee. A radiographic investigation . Acta Radiol Diagn . 1968 ; 277 ( Suppl ): 7 – 72 . PubMed Google Scholar
36. Insall JN , Dorr LD , Scott RD , Scott WN . Rationale of the Knee Society clinical rating system. Modified by: Stern SH, Becker MW, Insall JN. Unicondylar knee arthroplasty. An evaluation of selection criteria. Clin Orthop Relat Res 1993;(286):143-148 . Clin Orthop Relat Res . 1989 ; 248 : 13 – 14 . Google Scholar
37. No authors listed . Spanish Society for the Study of Obesity . 2020 . https://www.seedo.es/ (date last accessed 15 October 2020 ). Google Scholar
38. No authors listed . Federation of European Nutrition Societies (FENS) . 2020 . https://fensnutrition.org/ (date last accessed 15 October 2020 ). Google Scholar
39. Kawakami Y , Matsuo K , Murata M , et al. Expression of angiotensin II receptor-1 in human articular chondrocytes . Arthritis . 2012 ; 2012 : 648537 – 7 . Crossref PubMed Google Scholar
40. Silveira KD , Coelho FM , Vieira AT , et al. Mechanisms of the anti-inflammatory actions of the angiotensin type 1 receptor antagonist losartan in experimental models of arthritis . Peptides . 2013 ; 46 : 53 – 63 . Crossref PubMed Google Scholar
41. Mendes MT , Murari-do-Nascimento S , Torrigo IR , Alponti RF , Yamasaki SC , Silveira PF . Basic aminopeptidase activity is an emerging biomarker in collagen-induced rheumatoid arthritis . Regul Pept . 2011 ; 167 ( 2-3 ): 215 – 221 . Crossref PubMed Google Scholar
42. Morgan R , Endres J , Behbahani-Nejad N , et al. Expression and function of aminopeptidase N/CD13 produced by fibroblast-like synoviocytes in rheumatoid arthritis: role of CD13 in chemotaxis of cytokine-activated T cells independent of enzymatic activity . Arthritis Rheumatol . 2015 ; 67 ( 1 ): 74 – 85 . Crossref PubMed Google Scholar
43. Morgan RL , Behbahani-Nejad N , Endres J , et al. Localization, shedding, regulation and function of aminopeptidase N/CD13 on fibroblast like synoviocytes . PLoS One . 2016 ; 11 ( 9 ): e0162008 . Crossref PubMed Google Scholar
44. Yamamoto M , Chikuma T , Yajima R , et al. Axonal transport of puromycin-sensitive aminopeptidase in rat sciatic nerves . Neurosci Res . 2002 ; 42 ( 2 ): 133 – 140 . Crossref PubMed Google Scholar
45. Martínez-Martos JM , Correa-Rodríguez M , Rus A , Molina F , Ramírez-Expósito MJ , Aguilar-Ferrandiz ME . Altered serum oxytocinase and enkephalin-degrading aminopeptidase activities in patients with fibromyalgia . Biol Res Nurs . 2019 ; 21 ( 4 ): 431 – 439 . Crossref PubMed Google Scholar
Author contributions
J. Seco-Calvo: Drafted the first version of the study protocol, Contributed to the data gathering, Drafted and revised the manuscript.
S. Sánchez-Herráez: Contributed to the data gathering, Revised the manuscript.
L. Casis: Analyzed and interpreted the data, Revised the manuscript.
A. Valdivia: Analyzed and interpreted the data, Revised the manuscript.
I. Perez-Urzelai: Analyzed and interpreted the data, Revised the manuscript.
J. Gil: Analyzed and interpreted the data, Revised the manuscript.
E. Echevarría: Analyzed and interpreted the data, Revised the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
None declared.
Acknowledgements
We are grateful for help from Dr. J. A. Iglesias with synovial fluid sample processing, Dr. R. Ramos-Pascua with obtaining samples, and Arantza Perez Dobaran with enzyme substrate preparation.
Supplementary material
In the supplementary material, we provide more information about inclusion and exclusion criteria, and other key parts of the Methods section such as sample size calculation and assay conditions for the enzymes studied. Sociodemographic and clinical variables in the entire sample, and between-group comparison of qualitative sociodemographic and clinical variables, are also provided in the tables.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









