Abstract
Aims
This study aimed to examine the effects of tumour necrosis factor-alpha (TNF-α) on osteoblasts in metal wear-induced bone loss.
Methods
TNF-α immunoexpression was examined in periprosthetic tissues of patients with failed metal-on-metal hip arthroplasties and also in myeloid MM6 cells after treatment with cobalt ions. Viability and function of human osteoblast-like SaOs-2 cells treated with recombinant TNF-α were studied by immunofluorescence, terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay, western blotting, and enzyme-linked immunosorbent assay (ELISA).
Results
Macrophages, lymphocytes, and endothelial cells displayed strong TNF-α immunoexpression in periprosthetic tissues containing metal wear debris. Colocalization of TNF-α with the macrophage marker CD68 and the pan-T cell marker CD3 confirmed TNF-α expression in these cells. Cobalt-treated MM6 cells secreted more TNF-α than control cells, reflecting the role of metal wear products in activating the TNF-α pathway in the myeloid cells. While TNF-α did not alter the immunoexpression of the TNF-receptor 1 (TNF-R1) in SaOs-2 cells, it increased the release of the soluble TNF-receptor 1 (sTNF-R1). There was also evidence for TNF-α-induced apoptosis. TNF-α further elicited the expression of the endoplasmic reticulum stress markers inositol-requiring enzyme (IRE)-1α, binding-immunoglobulin protein (BiP), and endoplasmic oxidoreductin1 (Ero1)-Lα. In addition, TNF-α decreased pro-collagen I α 1 secretion without diminishing its synthesis. TNF-α also induced an inflammatory response in SaOs-2 cells, as evidenced by the release of reactive oxygen and nitrogen species and the proinflammatory cytokine vascular endothelial growth factor.
Conclusion
The results suggest a novel osteoblastic mechanism, which could be mediated by TNF-α and may be involved in metal wear debris-induced periprosthetic bone loss.
Cite this article: Bone Joint Res 2020;9(11):827–839.
Article focus
-
While many studies in the context of metal wear-induced implant loosening have examined osteoclastic bone resorption, less is known about the role of osteoblasts in periprosthetic bone loss.
-
Investigations into the effects of tumour necrosis factor-alpha (TNF-α) on osteoblasts in metal wear-induced bone loss, with a particular focus on the TNF-α overexpression in periprosthetic tissues of patients with failed hip arthroplasties.
Key messages
-
Exogenous TNF-α provokes endoplasmic reticulum (ER) stress, reduces collagen secretion, and induces apoptosis and an inflammatory response in human osteoblast-like SaOs-2 cells.
Strengths and limitations
-
Data suggest a novel osteoblastic mechanism that may be involved in metal wear debris-induced periprosthetic bone loss.
-
Findings may also be relevant in other diseases that are characterized by inflammatory bone loss.
-
More work is needed to establish a cause-effect relationship between TNF-α and ER stress in inflammatory bone loss.
Introduction
Implant loosening due to periprosthetic bone loss is a major problem in joint arthroplasty surgery and necessitates patients to undergo complex revision surgery. Central to the current understanding of periprosthetic bone loss are wear products that originate from the implant components. Metal wear may exist in particulate form or as metal ions.1 It is commonly thought that these wear products elicit inflammatory reactions that ultimately result in bone loss, thus implant instability.
One prominent inflammatory mediator thought to be involved in periprosthetic bone loss is TNF-α.2-4 This cytokine has been reported to be expressed at substantially higher levels in tissue samples obtained from patients who underwent revision surgery for failed hip arthroplasties, as compared to tissue samples from patients who underwent primary implantation of a total hip arthroplasty.2 Along with that, notably higher TNF-α levels were detected in the synovial fluid of patients with loose hip arthroplasties as compared to patients with osteoarthritis (OA).3 Significantly higher plasma TNF-α levels were also measured in patients with loose prostheses than in patients with stable prostheses, patients with OA, and control subjects.4 Various cell types including monocytes/macrophages,5-7 preosteoblasts,8 osteocytes,9 and osteoclasts10 have been reported to upregulate TNF-α mRNA/protein secretion in vitro upon stimulation with metal wear products.
TNF-α mediates inflammatory reactions and cell injury.11 It elicits its action through two cell surface receptors, TNF-R1 (p55), a death-domain containing protein, and TNF-R2 (p75), both of which are expressed in many tissues including the bone.12 Upon binding to the TNF-Rs, soluble forms (sTNF-Rs) are shed in the extracellular compartment and are thought to modulate inflammation by controlling TNF-α activity.11 In bone, TNF-α has been reported to stimulate osteoclast differentiation, activation, and bone resorption.13-15 Literature further suggests that TNF-α inhibits osteoblast differentiation,16 but the effect of TNF-α on bone formation mediated by osteoblasts is not well understood.
Recently, inflammation has been linked to endoplasmic reticulum (ER) stress,17 and TNF-α has been reported to induce ER stress in cultured cells18 and in animals.19 The ER is a cell organelle involved in protein synthesis, folding, assembly, and trafficking, but it is also essential in sensing cellular stress.17 Upon perturbation of the ER function, accumulation of unfolded or misfolded newly synthesized proteins culminates in ER stress, which can be sensed by ER stress sensors such as inositol-requiring enzyme (IRE)-1α.20 ER stress is counteracted by the unfolded protein response (UPR) that activates ER chaperones such as binding immunoglobulin protein (BiP) in the attempt to restore homeostasis in the ER.21 Endoplasmic oxidoreductin1 (Ero1)-Lα is a redox-sensitive protein responsible for oxidative protein folding that is essential for many secreted proteins.22 Prolonged and unresolved ER stress may eventually result in apoptotic cell death.
Considering that TNF-α is overexpressed in periprosthetic tissues of patients with failed hip arthroplasties, this study aimed to investigate the effects of TNF-α on osteoblasts.
Methods
Patients’ samples
To examine the expression of TNF-α in periprosthetic tissue, hip capsular tissue was obtained from six patients with failed metal-on-metal hip arthroplasties. Patients with inflammatory arthritis were not included in this study. Institutional Review Board approval was obtained from Otto von Guericke University Magdeburg, Magdeburg, Germany and from National University of Singapore, Singapore (Approval No. 150/2 and NHG DSRB reference number: 2016/00080). Written consent was obtained from patients, whose tissue samples were used for histology work, and local and international guidelines were followed.
The mean time to revision was 149 months (108 to 228). All patients (aged 59 to 73 years) underwent revision surgery for aseptic implant loosening. Infection was excluded according to routine protocols involving clinical examination, preoperative blood analysis, and microbiological and histological analysis of the obtained specimens. All tissue samples were immediately fixed in 4% formalin (Merck, Darmstadt, Germany).
Immunohistochemistry
To examine the presence of TNF-α in periprosthetic tissue, immunohistochemistry was performed using hip capsular tissue obtained from patients who underwent revision surgery for failed hip arthroplasties. After embedding the tissue samples into paraffin, they were cut into 4 μm-thick sections using a microtome. The sections were processed as described23 using anti-TNF-α antibody. To confirm the specificity of the antibodies, some sections were incubated with isotype control antibody and processed in an identical manner. A list of all primary antibodies used in the present study is provided in Table I.
Table I.
List of primary antibodies used for western blotting, immunofluorescence, and double-immunofluorescence.
| Antibody | Commercial source (Catalogue No.) | Dilution |
|---|---|---|
| TNF-α | Abcam (ab1793)* Santa Cruz (sc-1348)† |
1:200 for immunohistochemistry 1:200 for immunofluorescence |
| CD68 | Santa Cruz (sc-70761) | 1:50 for double-immunofluorescence |
| CD3 | Abcam (ab5690) | 1:50 for double-immunofluorescence |
| TNF-R1 | Santa Cruz (sc-7895) | 1:50 for immunofluorescence |
| IRE-1α | Cell Signaling Technology (#3294)‡ | 1:500 for western blotting |
| BiP | Cell Signaling Technology (#3177 S) | 1:500 for western blotting |
| Ero1-Lα | Cell Signaling Technology (#3264 S) | 1:500 for western blotting |
| ß-actin | Santa Cruz (sc-47778) | 1:5000 for western blotting |
| Isotypic control | ||
| IgG mouse | Abcam (ab37355) | N/A |
| IgG rabbit | Abcam (ab27478) | N/A |
-
*
Cambridge, Massachusetts, USA.
-
†
Dallas, Texas, USA.
-
‡
Danvers, Massachusetts, USA.
-
BiP, binding-immunoglobulin protein; Ero1-Lα, endoplasmic oxidoreductin1-Lα; IgG, immunoglobulin G; IRE-1α, inositol-requiring enzyme-1α; N/A, not applicable; TNF-α, tumour necrosis factor-alpha; TNF-R1, tumour necrosis factor-receptor 1.
Double-immunofluorescence
Double-immunofluorescence was performed using paraffin-embedded samples for cellular localization of TNF-α in periprosthetic tissue. Antigen retrieval was performed using 0.01 M citrate buffer and heat. Quenching of endogenous peroxidise activity was performed with 3% hydrogen peroxide (H2O2) in methanol. The sections were incubated with a mixture of primary antibodies (anti-TNF-α and anti-CD68 or anti-CD3) in a humidified chamber at 4°C overnight. Subsequently, the sections were incubated with the respective fluorescent-tagged secondary antibody for one hour. The sections were mounted using a fluorescent mounting medium containing the nuclear marker 4′,6’-diamidino-2-phenylindole (DAPI) (DAKO Cytomation, Glostrup, Denmark). To confirm the specificity of the antibodies, some sections were incubated with isotype control antibodies and processed in an identical manner. Cellular colocalization was examined by confocal microscopy using sequential mode to avoid cross-talk.
Cell culture
Human macrophage-like MM6 cells were maintained in Roswell Park Memorial Institute (RPMI-1640) medium (Thermo Fisher Scientific, Waltham, Massachusetts, USA), supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM non-essential amino acids, 1 mM sodium pyruvate, and 1% penicillin/streptomycin (Merck) at 37°C and in 5% CO2 in a humidified atmosphere. Cells were maintained at a density of 0.1 to 1.0 × 106 cells/ml, and the medium was changed every two to three days. To study the effect of cobalt ions on TNF-α secretion in MM6 cells, the cells were seeded at a density of 0.5 × 106 cells/ml and treated with 0, 1, 10, 50, and 100 μM cobalt(II) chloride (CoCl2) (Merck) for 24 hours. This cell line is well characterized24 and has been used for research in the context of implant failure before.25
Human osteoblast-like SaOs-2 cells were maintained in 85% McCoy’s 5A culture medium (Thermo Fisher Scientific) and supplemented with 15% FBS, 1% penicillin/streptomycin (Merck) at 37°C and in 5% CO2 in a humidified atmosphere. The cells were grown to 80% to 90% confluence and detached by mild trypsinization. The medium was changed every two to three days. To study the effects of TNF-α on the expression of TNF-R1, sTNF-R, pro-collagen I α 1, and the formation of reactive oxygen and nitrogen species (ROS/RNS) as well as vascular endothelial growth factor (VEGF) in SaOs-2 cells, the cells were treated with 0, 1, 10, 50, and 100 ng/ml of human recombinant TNF-α (Cat No. 210-TA; R&D Systems, Minneapolis, Minnesota, USA) in serum-free medium for 24 hours. After overnight adherence of the cells, the medium was changed and the cells were treated with the given concentrations of recombinant TNF-α. This cell line resembles mature osteoblasts26 is well characterized, commonly used for osteoblastic models, and has been used in studies on TNF-α-stimulation before.7,27
Immunofluorescence
To study the effect of cobalt ions on the immunoexpression of TNF-α in myeloid cells, MM6 cells were seeded on poly-L-lysine-coated glass coverslips. After adherence, the MM6 cells were cultured for 24 hours in the absence or presence of CoCl2. Immediately after treatment, the cultured cells were fixed with 4% paraformaldehyde (Merck) and antigens were blocked with 5% bovine serum albumin (BSA) (Merck) for one hour. Next, the cells were incubated with an anti-TNF-α antibody at 4°C overnight. The cells were then incubated with a Cyc3-conjugated secondary antibody for one hour in the dark. The coverslips containing the cells were mounted using a fluorescent mounting medium containing the nuclear marker DAPI onto glass slides. To study the effect of TNF-α on osteoblasts, SaOs-2 cultures were treated with recombinant TNF-α for 24 hours and processed as above using an anti-TNF-R1 primary antibody and a fluorescein isothiocyanate (FITC)-conjugated secondary antibody.
TUNEL staining
To detect apoptosis in SaOs-2 cells following treatment with recombinant TNF-α for 24 hours in serum-free medium, fluorescent terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay was performed using the In Situ Cell Death Detection Kit, Fluorescein (Cat No. 11684795910; Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. The coverslips containing the cells were mounted with a fluorescent mounting medium containing DAPI. Confocal images from four random microscopic fields of each biological triplicate were captured at a magnification level of 10× and the proportion of TUNEL-positive SaOs-2 cells was calculated.
Western blotting
Following treatment with recombinant TNF-α for 24 hours in serum-free medium, protein was extracted from SaOs-2 cells at 4°C using a mammalian protein extraction reagent (M-PER; Cat No.78501; Thermo Fisher Scientific) containing protein inhibitors. The protein concentration was estimated according to the Bradford’s method using BSA as a standard.28 Equal amounts of protein were heated to 95°C for five minutes and subsequently separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein bands were electroblotted onto 0.45 μm polyvinylidene difluoride membranes (Bio-Rad, Hercules, USA). The membranes were blocked with 5% BSA for one hour and subsequently incubated with anti-IRE-1α, anti-Bip, anti-Ero1-Lα, and anti-ß-actin antibodies overnight at 4°C. The membranes were subsequently incubated with horseradish peroxidase conjugated secondary antibodies. The immunoreactive bands were visualized using an enhanced chemiluminescence kit (Pico PLUS, Cat No.34580; Thermo Fisher Scientific) .
Measurement of TNF-α using ELISA
The amount of TNF-α in culture medium of MM6 cells following exposure to cobalt ions for 24 hours was measured using the TNF-α ELISA Kit (Cat No. KHC3011; Thermo Fisher Scientific) according to the manufacturer’s instructions. The optical density of samples and standards was read at 450 nm using a microplate reader (Infinite 200 PRO; Tecan, Männedorf, Switzerland). The TNF-α concentration was measured using the standard curve obtained. All values were normalized for total protein content.
Measurement of cellular and secreted pro-collagen I alpha 1 using ELISA
The cellular pro-collagen I α 1 concentration from SaOs-2 cells and the pro-collagen I α 1 content secreted in the culture medium of SaOs-2 cells following treatment with recombinant TNF-α for 24 hours was measured using the Human Pro-Collagen I α 1 ELISA Kit (Cat No. ab210966; Abcam, Cambridge, Massachusetts, USA) according to the manufacturer’s instructions. The optical density of samples and standards was read at 450 nm using a microplate reader (Infinite 200 PRO; Tecan). The pro-collagen I α 1 concentration was measured using the standard curve obtained.
Measurement of sTNF-R1 and VEGF using ELISA
The concentration of sTNF-R1 and VEGF in culture medium in SaOs-2 cells treated with recombinant TNF-α for 24 hours in serum-free medium was measured using a Human sTNF-R (60 kDa) ELISA kit (cat no BMS203; Thermo Fisher Scientific) and a VEGF Human ELISA kit (cat no. KHG0111; Thermo Fisher Scientific) following the manufacturer’s instructions. The optical density of samples and standards was read at 450 nm using a microplate reader (Infinite 200 PRO; Tecan). The sTNF-R1 and VEGF concentrations were measured using the standard curve obtained.
Measurement of extracellular ROS/RNS
The ROS/RNS levels released by SaOs-2 cells that were treated with recombinant TNF-α for 24 hours in serum-free medium were measured using Oxiselect In Vitro ROS/RNS Assay Kit (cat no. STA-347; Cell Biolabs, San Diego, California, USA) following the manufacturer’s instructions. The relative fluorescence of the samples and the standards was read at 480 nm excitation/530 nm emission using SpectraMaxM5 microplate reader (Molecular Devices, San Jose, California, USA).
Statistical analysis
Data are presented as mean ± SD. Boxplots were used to present sTNF-R1 data. Statistical significance was evaluated by one-way analysis of variance followed by post hoc analysis using Dunnett’s multiple comparisons test (GraphPad Prism 7 software, San Diego, California, USA). Results were considered as statistically significant at p < 0.05.
Results
Cellular localization of TNF-α protein expression in periprosthetic tissues
Strong TNF-α immunoexpression was observed in periprosthetic tissues containing metal debris obtained from patients who underwent revision surgery for failed hip arthroplasties. TNF-α-expressing cells were identified as macrophages, lymphocytes, and endothelial cells lining the blood vessels (Figure 1). No specific staining was noted in the respective IgG controls. Double-immunofluorescence demonstrated complete colocalization of the macrophage marker CD68 and TNF-α, supporting the finding that macrophages express TNF-α (Figure 2). Colocalization of TNF-α with the pan-T cell marker CD3 suggested that T-lymphocytes also express TNF-α (Figure 2).
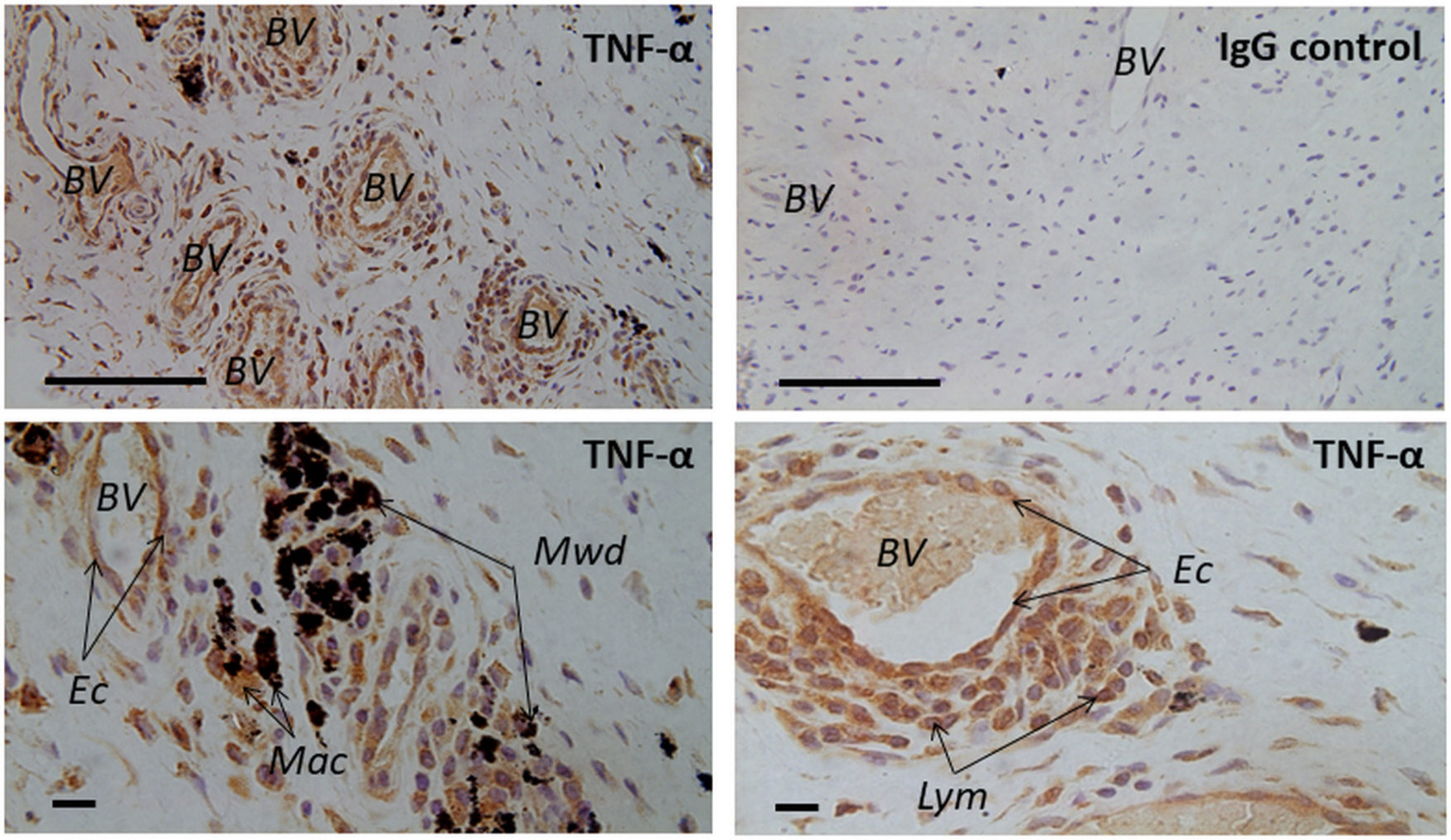
Fig. 1
Light microscopic images displaying hip capsular tissue from patients who underwent revision surgery for a failed hip arthroplasty. Tissues were stained for tumour necrosis factor-alpha (TNF-α) using anti-TNFα antibody, as part of the immunohistochemical staining (see Table I for more details of this antibody). Note the strong immunoexpression in the revision samples containing metal wear products. TNF-α-expressing cells were identified as macrophages (Mac), lymphocytes (Lym), and endothelial cells (Ec) lining the blood vessels (BV). No specific staining was detected in the isotypic controls. IgG, immunoglobulin G; Mwd, metal wear debris. Scale bars: upper panel = 100 μm, lower panel = 10 μm.
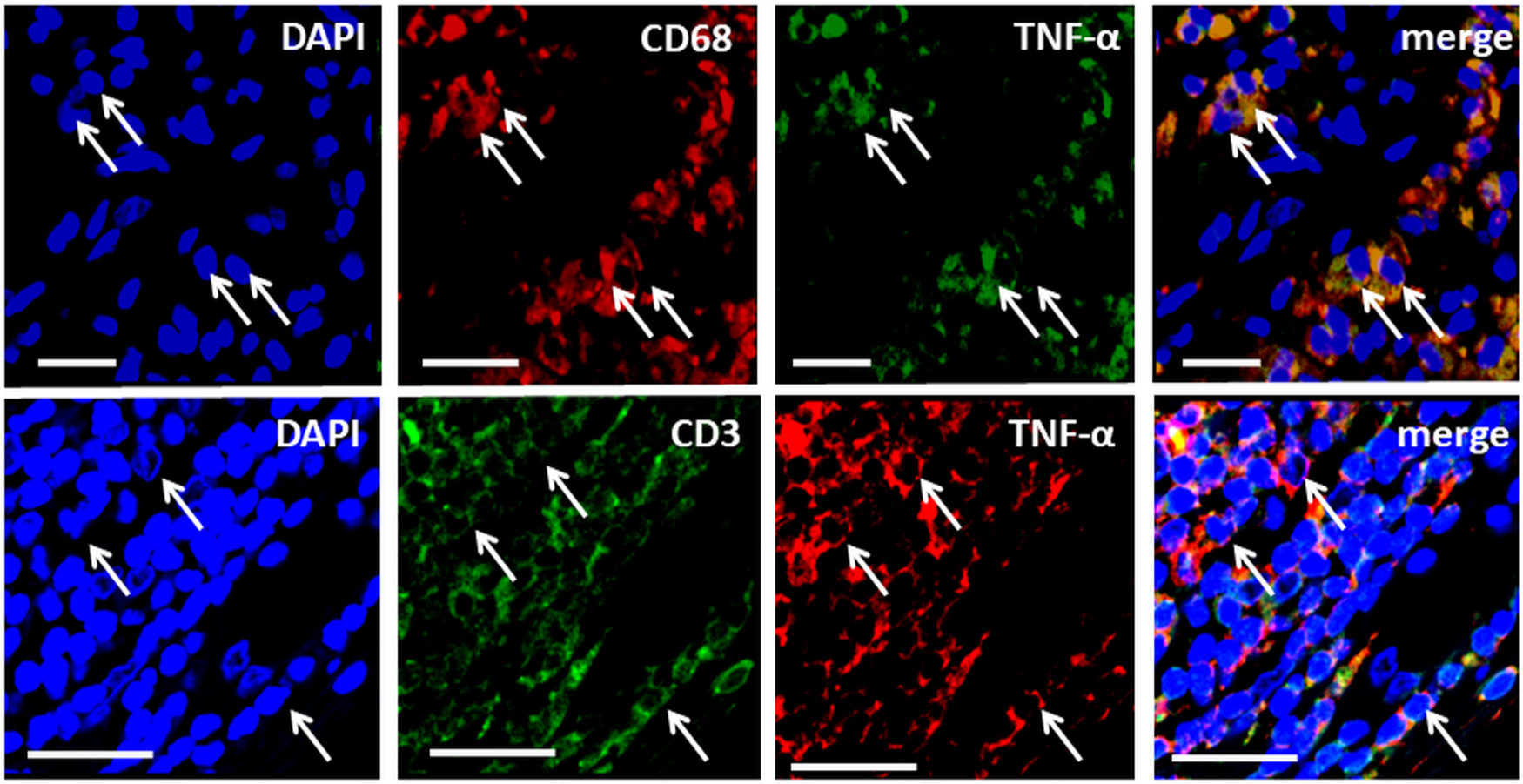
Fig. 2
Double-immunofluorescence demonstrating colocalization of tumour necrosis factor-alpha (TNF-α) with the macrophage marker Cluster of Differentiation 68 (CD68) (upper panel) and the pan T-cell marker Cluster of Differentiation 3 (CD3) (lower panel). Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars = 50 μm.
Metal-enhanced secretion of TNF-α in human MM6 cells
Using human MM6 cells, we demonstrated that cobalt ions significantly increased the secretion of TNF-α (Figure 3a). This finding was supported by an enhanced immunoexpression of TNF-α in these cells following treatment with cobalt ions (Figure 3b).
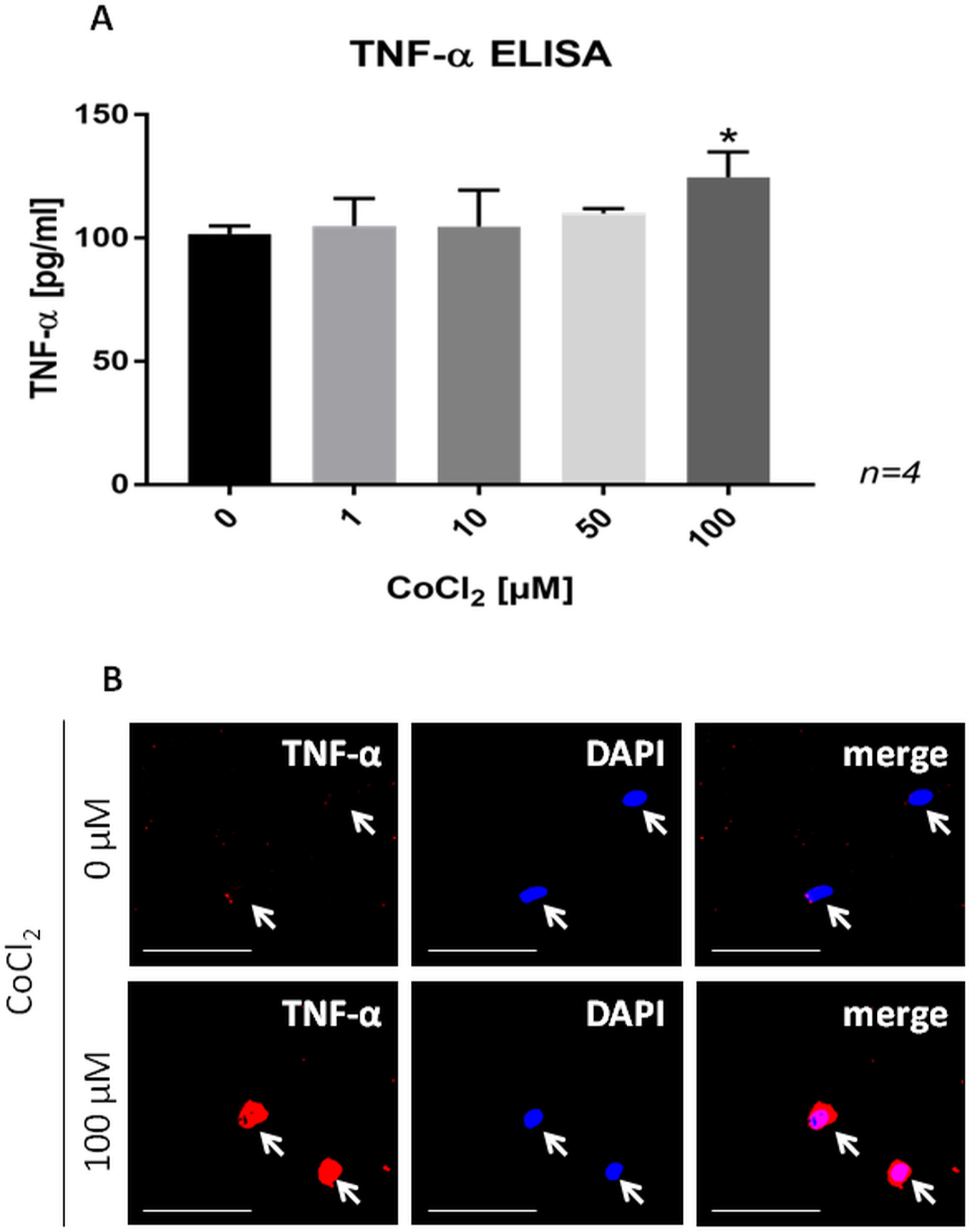
Fig. 3
a) Bar chart showing a dose-dependent increase in the secretion of tumour necrosis factor-alpha (TNF-α) in MM6 cells following 24 hours of cobalt ion treatment. Data are presented as the mean ± SD. *p < 0.05 versus control group, evaluated by one-way analysis of variance followed by post-hoc analysis using Dunnett’s multiple comparisons test. b) Confocal images of TNF-α-stained MM6 cells in control cells and cells treated with 100 μM cobalt ions for 24 hours. Note the increased immunoexpression of TNF-α in MM6 cells treated with cobalt ions. Scale bars = 50 μm. CoCl2, cobalt(II)chloride; DAPI, 4′,6’-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay.
Protein expression of the cell surface receptor TNF-R1 and its soluble form sTNF-R1 by SaOs-2 cells
Immunofluorescence labelling confirmed TNF-R1 protein expression in human osteoblast-like SaOs-2 cells. While the TNF-R1 immunoexpression did not appear to be changed in response to exogenous TNF-α (Supplementary Figure a), significantly higher sTNF-R1 levels were measured in the culture medium of TNF-α treated SaOs-2 cells (Figure 4).
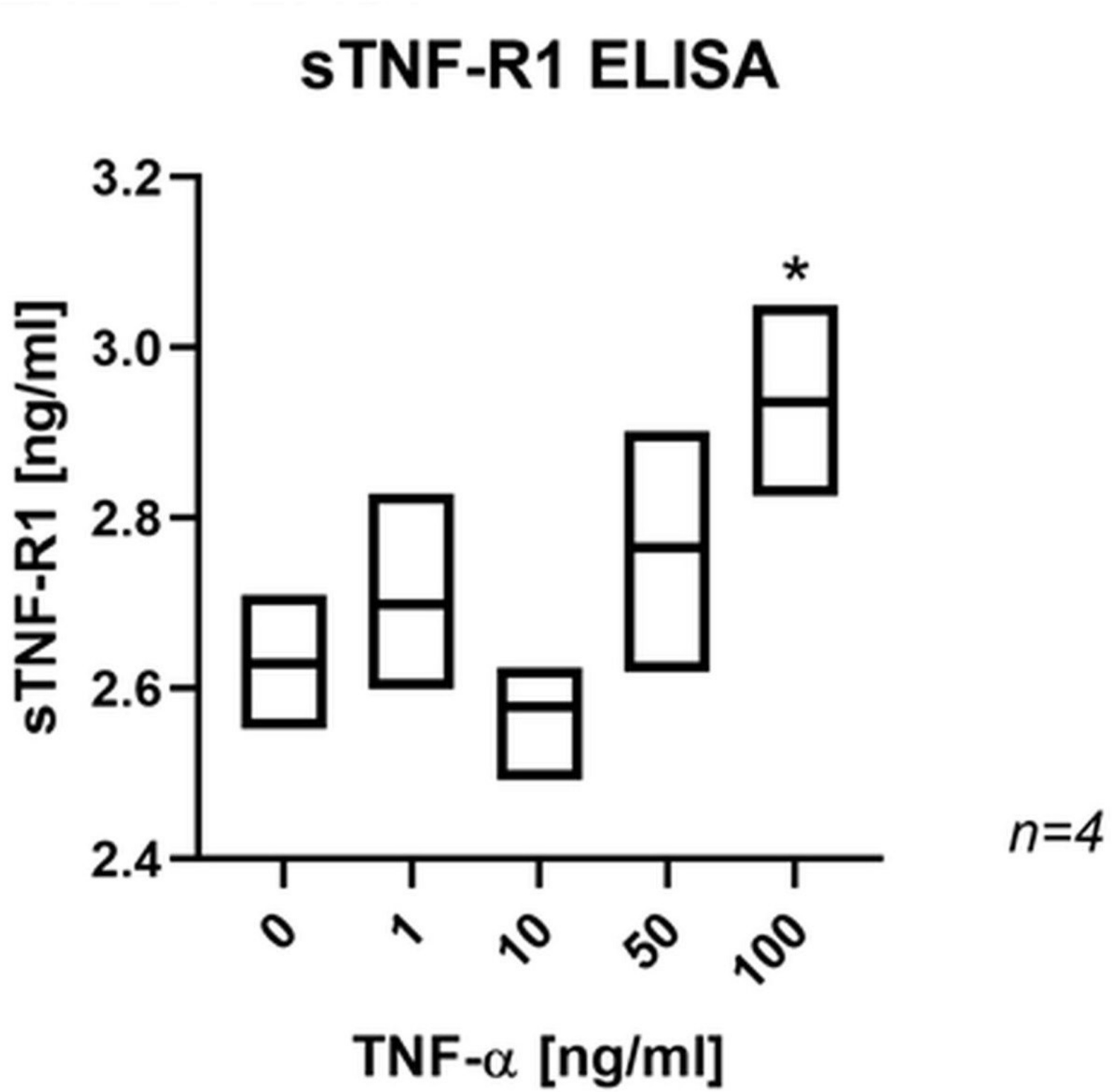
Fig. 4
Box plot showing a significant effect of tumour necrosis factor-alpha (TNF-α) on the release of soluble tumour necrosis factor-receptor 1 (sTNF-R1) in SaOs-2 cells. *p < 0.01 versus control group, evaluated by one-way analysis of variance followed by post-hoc analysis using Dunnett’s multiple comparisons test. ELISA, enzyme-linked immunosorbent assay.
TNF-α-induced ER stress in SaOs-2 cells
TNF-α had a significant effect on the protein expression of IRE-1α, BiP, and Ero1-Lα in the SaOs-2 cells (Figure 5). The immunoreactive band for IRE-1α appeared at 130 kDa and was significantly increased by treatment with 10, 50, and 100 ng/ml TNF-α (Figure 5a). The immunoreactive band for BiP appeared at 78 kDa and was significantly increased by treatment with 50 ng/ml and 100 ng/ml TNF-α (Figure 5b). The immunoreactive band for Ero1-Lα appeared at 60 kDa and was significantly increased by treatment with 100 ng/ml TNF-α (Figure 5c). The upregulation of these proteins suggests that TNF-α induces ER stress in osteoblasts.
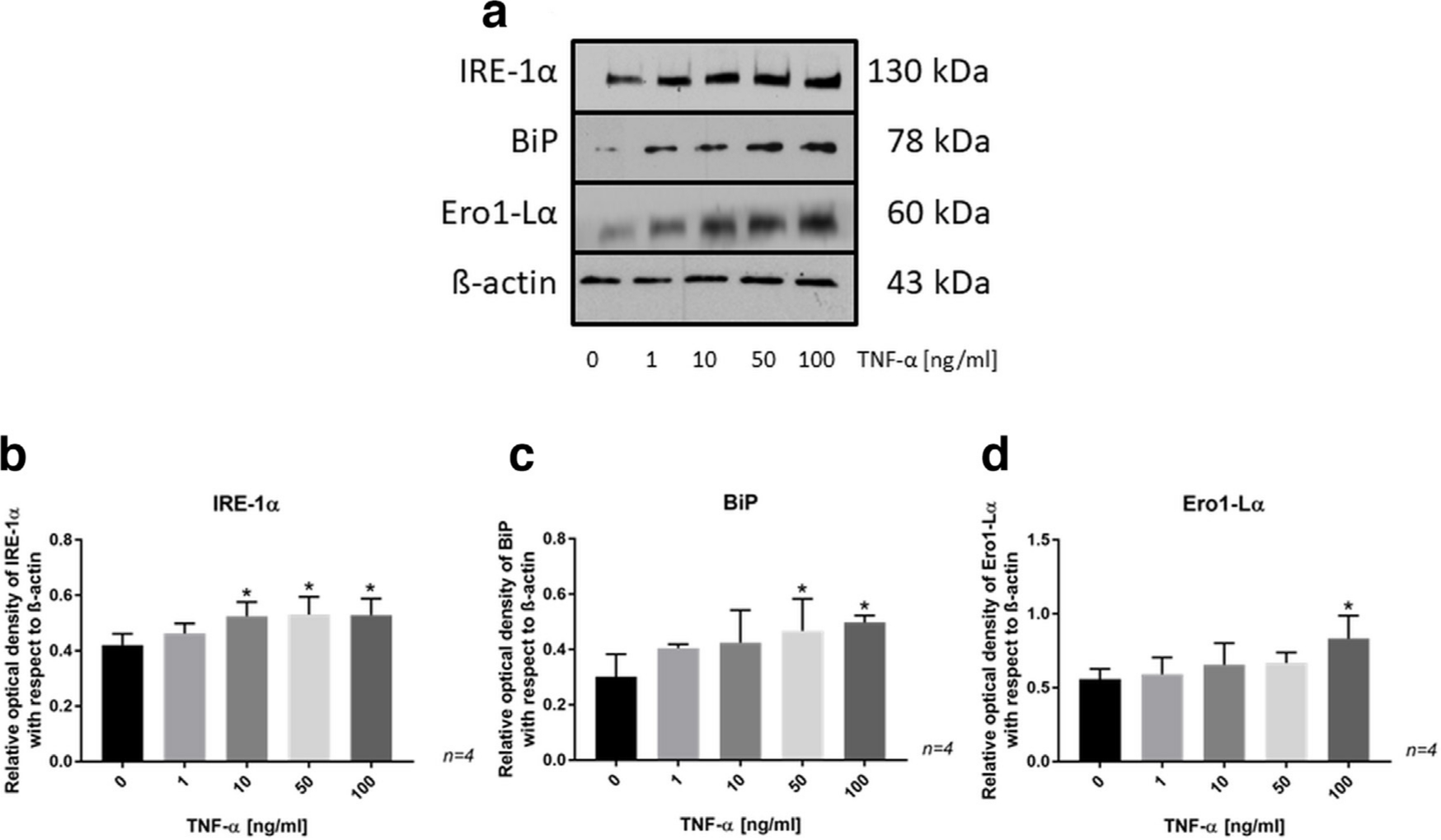
Fig. 5
a) Western blot showing the protein expression of inositol-requiring enzyme (IRE)-1α, binding-immunoglobulin protein (BiP), and endoplasmic reticulum (ER)-residing protein endoplasmic oxidoreductin1–Lα (Ero1-Lα) in SaOs-2 cells treated with tumour necrosis factor-alpha (TNF-α) for 24 hours. b) to d) Bar charts from protein analysis by western blotting showing significant changes in protein expression between control and TNF-α-treated SaOs-2 cells. Data are presented as the mean ± SD. *p < 0.05 versus control group, evaluated by one-way analysis of variance followed by post-hoc analysis using Dunnett’s multiple comparisons test.
TNF-α-induced apoptosis in SaOs-2 cells
Exogenous TNF-α increased the number of TUNEL-positive SaOs-2 cells, which was significant for treatment with 10, 50, and 100 ng/ml TNF-α (Figure 6). This suggests that TNF-αinduces apoptosis of osteoblasts.
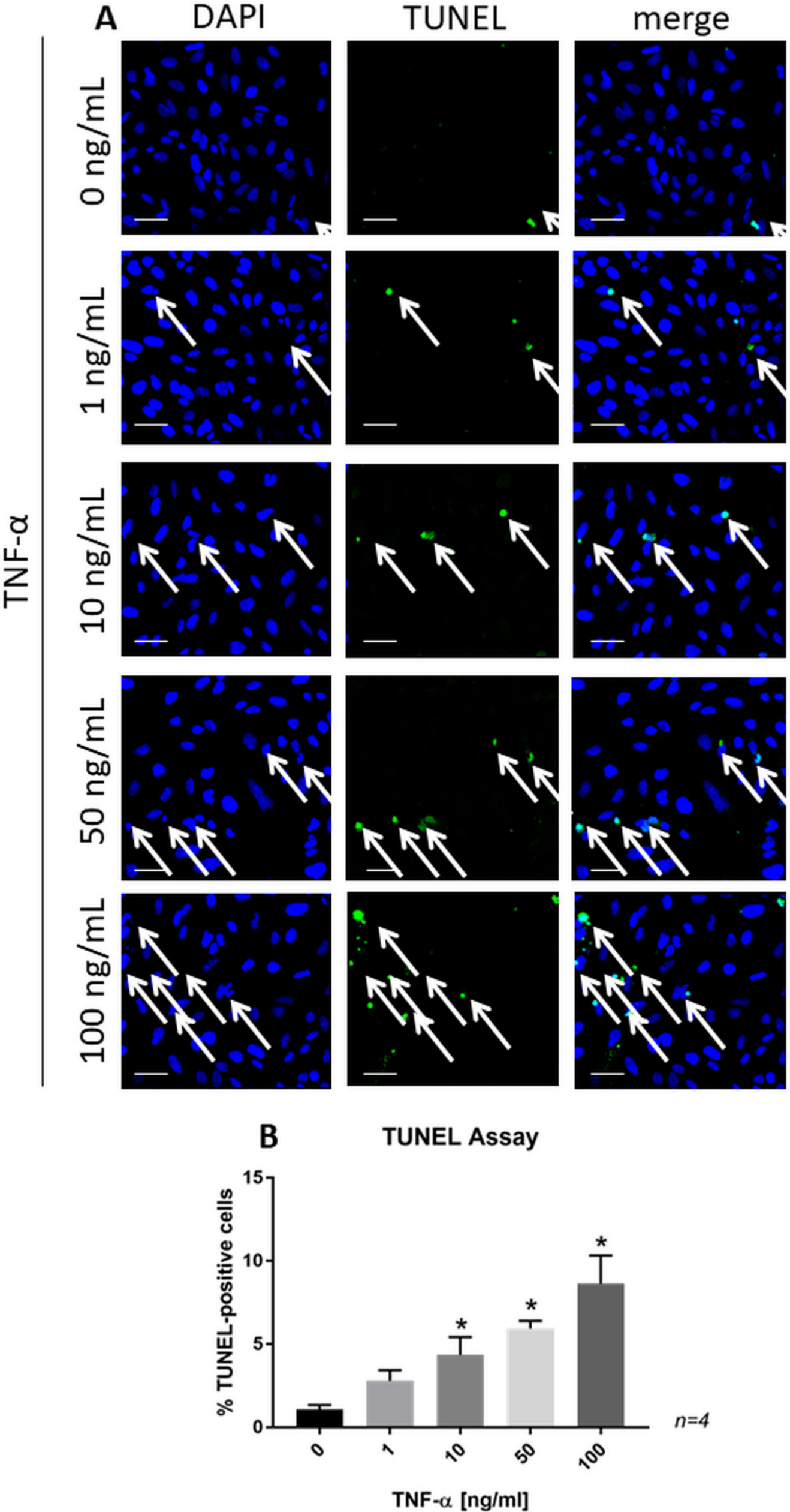
Fig. 6
a) Confocal images of terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL)-stained SaOs-2 cells treated with recombinant tumour necrosis factor-alpha (TNF-α) for 24 hours. Scale bars = 50 μm. b) Bar chart representing the significant changes in the percentage of TUNEL-positive cells. Data are presented as the mean ± SD. *p < 0.01 versus control group, evaluated by one-way analysis of variance followed by post-hoc analysis using Dunnett’s multiple comparisons test. DAPI, 4′,6-diamidino-2-phenylindole.
TNF-α-decreased secretion of pro-collagen I alpha 1 but not its synthesis in SaOs-2 cells
Pro-collagen I α 1 secretion was significantly decreased in osteoblast-like SaOs-2 cells following exposure to 10, 50, and 100 ng/ml recombinant TNF-α in comparison with control cells without diminishing pro-collagen I α 1 synthesis, as indicated by similar levels of cellular pro-collagen I α 1 at the highest concentration of TNF-α treatment (Figure 7).
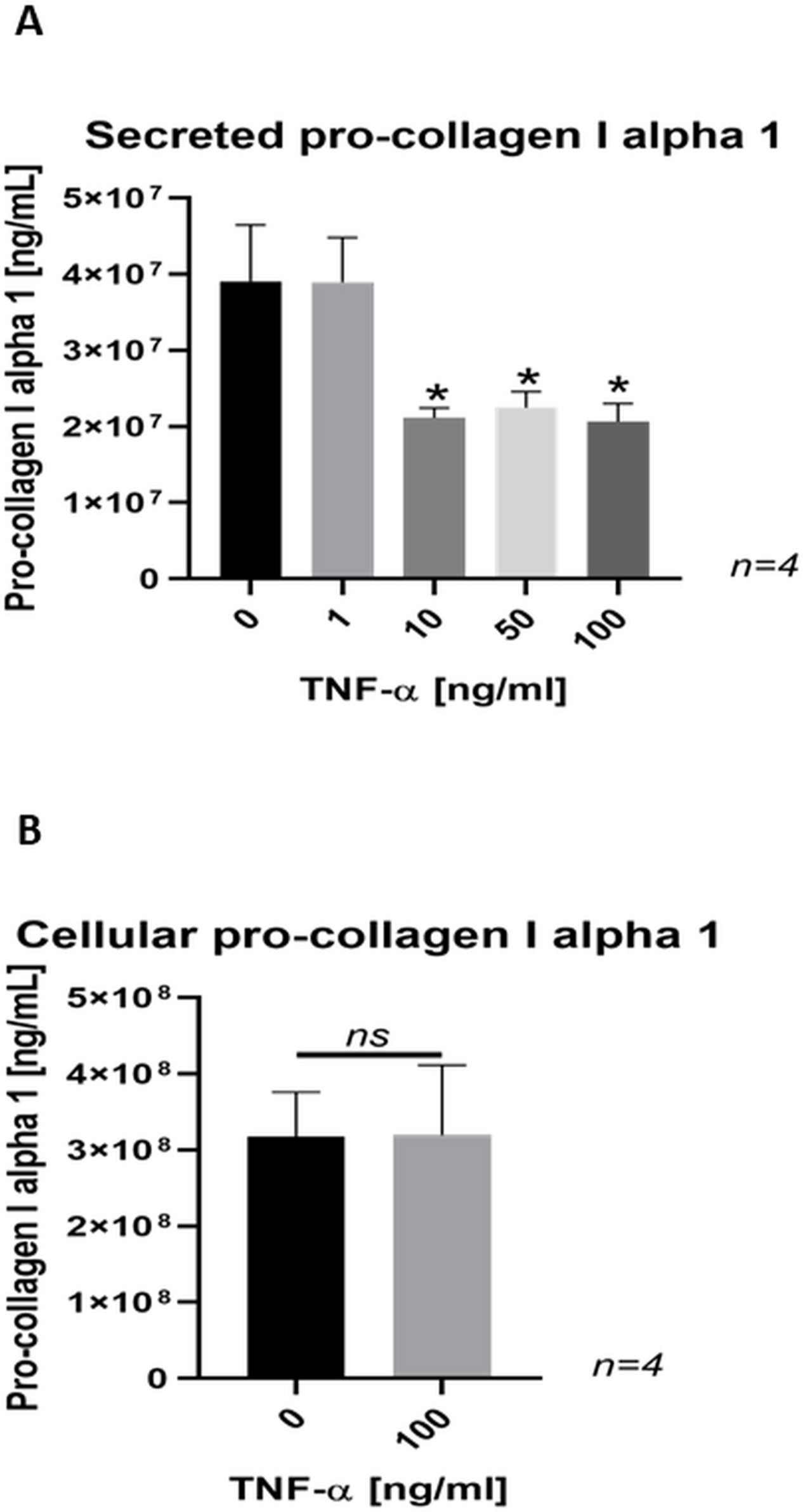
Fig. 7
a) Bar chart showing a dose-dependent decrease in the secretion of pro-collagen I α 1 in SaOs-2 cells treated with tumour necrosis factor-alpha (TNF-α) for 24 hours. Data are presented as the mean ± SD. *p < 0.001 versus control group, evaluated by one-way analysis of variance followed by post-hoc analysis using Dunnett’s multiple comparisons test. b) Bar chart showing no difference in cellular pro-collagen I alpha 1 content in SaOs-2 cells treated with TNF-α for 24 hours. Data are presented as the mean ± SD. p > 0.05 versus control group. ns, not significant.
TNF-α-induced inflammatory response by SaOs-2 cells
Significantly higher levels of total ROS/RNS (Figure 8a) and VEGF (Figure 8b) were measured in the culture medium of SaOs-2 cells after treatment with exogenous TNF-α.
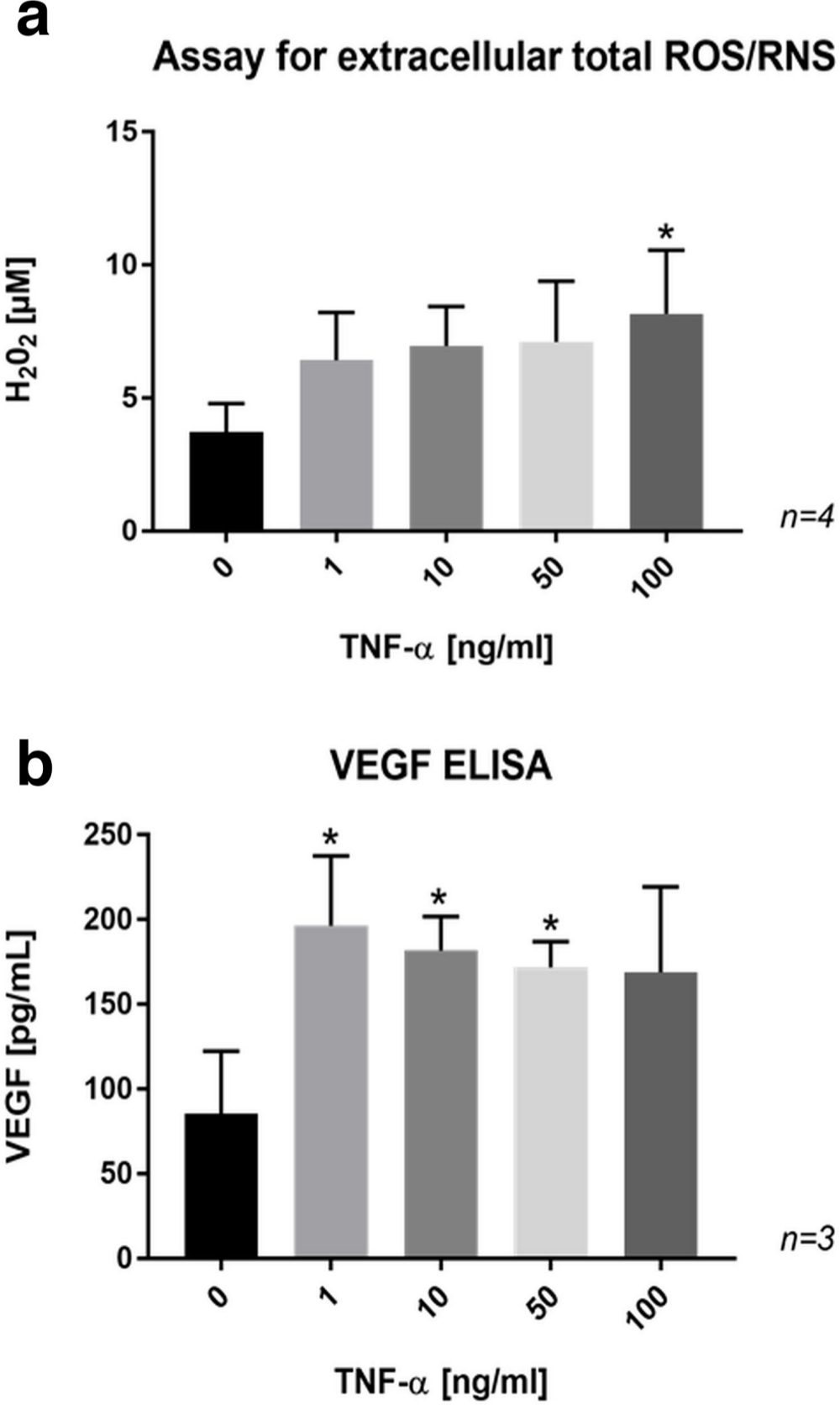
Fig. 8
a) Bar chart showing a dose-dependent increase in the formation of extracellular reactive oxygen and nitrogen species (ROS/RNS) by SaOs-2 cells treated with tumour necrosis factor-alpha (TNF-α) for 24 hours. b) Bar chart showing enhanced release of vascular endothelial growth factor (VEGF) in the culture medium of SaOs-2 cells treated with TNF-α for 24 hours. Data are presented as the mean ± SD. *p < 0.05 versus control group, evaluated by one-way analysis of variance followed by post-hoc analysis using Dunnett’s multiple comparisons test. ELISA, enzyme-linked immunosorbent assay; H2O2, hydrogen peroxide.
Discussion
Consistent with the notion that TNF-α is expressed by various cell types, we observed that macrophages, lymphocytes, and endothelial cells were immunopositive for TNF-α in periprosthetic tissue containing metal wear debris obtained from patients with failed hip arthroplasty. Others have also noted TNF-α immunoexpression in macrophages,29,30 fibroblasts,29,30 and endothelial cells29 in periprosthetic tissues. Using MM6 cells, we confirmed that metal wear products such as cobalt ions enhance the secretion of TNF-α in cells from the myeloid cell lineage, which is in agreement with results by others.6 This suggests that the enhanced TNF-α immunoexpression by the inflammatory cells in the patients’ tissue samples could be in response to stimulation with metal wear products.
We then examined the effect of the inflammatory environment, here simulated by the addition of exogenous TNF-α, on the viability and function of osteoblasts. Since inflammatory responses are mainly attributed to activation of TNF-α upon binding to the transmembrane TNF-R1 receptor,31 we first confirmed TNF-R1 expression in human osteoblast-like SaOs-2 cells. TNF-R1 immunoexpression did not appear to be altered upon treatment with exogenous TNF-α, which is in line with the literature.32 However, we observed enhanced sTNF-R1 levels in the culture medium obtained from these cells following exposure to exogenous TNF-α, which may indicate the activation of the TNF-α signalling pathway through their respective receptors.33 It remains unclear as to whether the enhanced release of sTNF-Rs is sufficient to counteract TNF-α activity through TNF-decay. Notably, moderately enhanced sTNF-R levels, as opposed to very high sTNF-R concentrations, have been suggested to stabilize TNF-α bioactivity by preserving its structure in complexes and hence amplify TNF-α activity.34
The significant upregulation of IRE-1α and BiP in the SaOs-2 cells in our study following exposure to TNF-α suggests that TNF-α induces ER stress in these cells. Increased expression of BiP is known to reflect irregular folding of secretory proteins.35 To avoid misfolded proteins to form aggregates within the ER, BiP binds misfolded proteins and facilitates proper refolding.35 It is also among the first proteins triggering the pro-survival UPR.21 Activation of the UPR is further represented in our study by the upregulation of IRE-1α. IRE-1α is an ER stress sensor and cell fate executor.20 Under irremediable ER stress, however, signalling through IRE-1α can trigger apoptosis.36
In our study, TNF-α significantly increased the number of TUNEL-positive SaOs-2 cells, suggesting that TNF-α promotes apoptosis in these cells. Although apoptosis of osteoblasts may reflect the endpoint of unresolved ER stress, we are unable to exclude the possibility that apoptosis may be due to activation of the extrinsic pathway that is mediated by the death receptor TNF-R1.37 The increased number of apoptotic osteoblasts in our study is, however, in line with a few other reports documenting that TNF-α induces apoptosis in osteoblasts.38,39
Our finding of significantly decreased pro-collagen I α 1 protein secretion without diminishing pro-collagen I α 1 synthesis is in agreement with other reports.40,41 This suggests that TNF-α may predominantly affect post-translational collagen modifications rather than pro-collagen synthesis, which is consistent with the upregulation of ER stress markers since the ER is the cell organelle, in which intracellular post-translational modifications occur. In this connection, the TNF-α-induced upregulation of the ER-localized Ero1-Lα protein expression in SaOs-2 cells in our study is of note, since this enzyme is responsible for oxidative protein folding, namely the formation of disulfide bonds, which also occurs in pro-collagen.42 Ero1-Lα has further been shown to be critical for collagen secretion by hepatic stellate cells.43
Finally, we sought to investigate the effect of TNF-α on the formation of inflammatory mediators such as ROS/RNS and VEGF by osteoblasts. These molecules have been reported to be involved in osteoclast recruitment, differentiation, and bone resorption.44,45 In our study, significantly higher levels of ROS/RNS and VEGF were measured in the culture medium of TNF-α-treated SaOs-2 cells. Similarly, others reported that TNF-α increases the release of ROS and VEGF in various cell types.46-49 These findings support the notion that exogenous TNF-α can elicit an inflammatory response in osteoblasts. It is tempting to speculate that TNF-α-induced release of inflammatory factors by osteoblasts could contribute to osteoclastic bone resorption.
We acknowledge that a costimulation experiment investigating the combined effect of cobalt ions and TNF-α might be a better reflection of the likely in vivo scenario and would provide additional information as to whether the combination has an additive effect on apoptosis, collagen synthesis, and release of proinflammatory mediators in osteoblasts.
This study focused on inflammatory factors in the context of implant loosening. Investigations on the effect of mechanical factors on osteoblast viability and function50 were beyond the scope of this study. We acknowledge that experiments using patient-derived cells could have strengthened this study. Cell lines were used to ensure homogeneity and consistency in response to treatment.
In conclusion, the present study suggests a novel osteoblastic mechanism involved in metal wear debris-induced periprosthetic bone loss. TNF-α may promote periprosthetic bone loss in three ways: apoptosis of osteoblasts; reduced collagen secretion; and potentially through enhanced osteoclastic bone resorption regulated by osteoblasts through the release of inflammatory mediators. The results highlight the adverse effects of TNF-α on osteoblast-mediated bone homeostasis and may also be relevant in other diseases that are characterized by inflammatory bone loss such as rheumatoid arthritis, periprosthetic joint infection, or periodontal disease. More detailed work is needed to establish a cause-effect relationship between TNF-α and ER stress in inflammatory bone loss.
References
1. Lohmann CH , Singh G , Willert H-G , Buchhorn GH . Metallic debris from metal-on-metal total hip arthroplasty regulates periprosthetic tissues . World J Orthop . 2014 ; 5 ( 5 ): 660 – 666 . Crossref PubMed Google Scholar
2. Christiansen RJ , Münch HJ , Bonefeld CM , et al. Cytokine profile in patients with aseptic loosening of total hip replacements and its relation to metal release and metal allergy . J Clin Med . 2019 ; 8 ( 8 ): 1259 . Crossref PubMed Google Scholar
3. Nivbrant B , Karlsson K , Kärrholm J . Cytokine levels in synovial fluid from hips with well-functioning or loose prostheses . J Bone Joint Surg Br . 1999 ; 81-B ( 1 ): 163 – 166 . Crossref PubMed Google Scholar
4. Hundrić-Haspl Z , Pecina M , Haspl M , Tomicic M , Jukic I . Plasma cytokines as markers of aseptic prosthesis loosening . Clin Orthop Relat Res . 2006 ; 453 : 299 – 304 . Crossref PubMed Google Scholar
5. Blaine TA , Rosier RN , Puzas JE , et al. Increased levels of tumor necrosis factor-alpha and interleukin-6 protein and messenger RNA in human peripheral blood monocytes due to titanium particles . J Bone Joint Surg Am . 1996 ; 78-A ( 8 ): 1181 – 1192 . Crossref PubMed Google Scholar
6. Garrigues GE , Cho DR , Rubash HE , et al. Gene expression clustering using self-organizing maps: analysis of the macrophage response to particulate biomaterials . Biomaterials . 2005 ; 26 ( 16 ): 2933 – 2945 . Crossref PubMed Google Scholar
7. Nakashima Y , Sun DH , Trindade MC , et al. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro . J Bone Joint Surg Am . 1999 ; 81-A ( 5 ): 603 – 615 . Crossref PubMed Google Scholar
8. Yang S , Zhang K , Li F , et al. Biological responses of preosteoblasts to particulate and ion forms of Co-Cr alloy . J Biomed Mater Res A . 2015 ; 103 ( 11 ): 3564 – 3571 . Crossref PubMed Google Scholar
9. Kanaji A , Caicedo MS , Virdi AS , et al. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation . Bone . 2009 ; 45 ( 3 ): 528 – 533 . Crossref PubMed Google Scholar
10. Cadosch D , Gautschi OP , Chan E , Simmen H-P , Filgueira L . Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts . J Biomed Mater Res A . 2010 ; 92 ( 2 ): 475 – 483 . Crossref PubMed Google Scholar
11. Sedger LM , McDermott MF . TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future . Cytokine Growth Factor Rev . 2014 ; 25 ( 4 ): 453 – 472 . Crossref PubMed Google Scholar
12. Nanes MS . Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology . Gene . 2003 ; 321 : 1 – 15 . Crossref PubMed Google Scholar
13. Kobayashi K , Takahashi N , Jimi E , et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction . J Exp Med . 2000 ; 191 ( 2 ): 275 – 286 . Crossref PubMed Google Scholar
14. Fuller K , Murphy C , Kirstein B , Fox SW , Chambers TJ . Tnfalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL . Endocrinology . 2002 ; 143 ( 3 ): 1108 – 1118 . Crossref PubMed Google Scholar
15. Azuma Y , Kaji K , Katogi R , Takeshita S , Kudo A . Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts . J Biol Chem . 2000 ; 275 ( 7 ): 4858 – 4864 . Crossref PubMed Google Scholar
16. Osta B , Benedetti G , Miossec P . Classical and paradoxical effects of TNF-α on bone homeostasis . Front Immunol . 2014 ; 5 : 48 . Crossref PubMed Google Scholar
17. Zhang K , Kaufman RJ . From endoplasmic-reticulum stress to the inflammatory response . Nature . 2008 ; 454 ( 7203 ): 455 – 462 . Google Scholar
18. Xue X , Piao J-H , Nakajima A , et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha . J Biol Chem . 2005 ; 280 ( 40 ): 33917 – 33925 . Crossref PubMed Google Scholar
19. Denis RG , Arruda AP , Romanatto T , et al. TNF-α transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus . Neuroscience . 2010 ; 170 ( 4 ): 1035 – 1044 . Crossref PubMed Google Scholar
20. Chen Y , Brandizzi F . Ire1: ER stress sensor and cell fate executor . Trends Cell Biol . 2013 ; 23 ( 11 ): 547 – 555 . Crossref PubMed Google Scholar
21. Quinones QJ , de Ridder GG , Pizzo SV . Grp78: a chaperone with diverse roles beyond the endoplasmic reticulum . Histol Histopathol . 2008 ; 23 ( 11 ): 1409 – 1416 . Crossref PubMed Google Scholar
22. Sevier CS , Kaiser CA . Ero1 and redox homeostasis in the endoplasmic reticulum . Biochim Biophys Acta . 2008 ; 1783 ( 4 ): 549 – 556 . Crossref PubMed Google Scholar
23. Hameister R , Lohmann CH , Dheen ST , Singh G , Kaur C . Bone biology in postnatal Wistar rats following hypoxia-reoxygenation . Histol Histopathol . 2020 ; 35 ( 1 ): 111-124 . Crossref PubMed Google Scholar
24. Ziegler-Heitbrock HW , Thiel E , Fütterer A , et al. Establishment of a human cell line (mono Mac 6) with characteristics of mature monocytes . Int J Cancer . 1988 ; 41 ( 3 ): 456 – 461 . Crossref PubMed Google Scholar
25. Lawrence H , Deehan D , Holland J , Kirby J , Tyson-Capper A . The immunobiology of cobalt: demonstration of a potential aetiology for inflammatory pseudotumours after metal-on-metal replacement of the hip . Bone Joint J . 2014 ; 96-B ( 9 ): 1172 – 1177 . Crossref PubMed Google Scholar
26. Pautke C , Schieker M , Tischer T , et al. Characterization of osteosarcoma cell lines MG-63, SaOS-2 and U-2 OS in comparison to human osteoblasts . Anticancer Res . 2004 ; 24 ( 6 ): 3743 – 3748 . PubMed Google Scholar
27. David MS , Kelly E , Cheung I , et al. Saos-2 osteosarcoma cells bind fibroblasts via ICAM-1 and this is increased by tumour necrosis factor-α . PLoS One . 2014 ; 9 ( 6 ): e101202 . Crossref PubMed Google Scholar
28. Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem . 1976 ; 72 ( 1-2 ): 248 – 254 . Crossref PubMed Google Scholar
29. Xu JW , Konttinen YT , Lassus J , et al. Tumor necrosis factor-alpha (TNF-alpha) in loosening of total hip replacement (Thr) . Clin Exp Rheumatol . 1996 ; 14 ( 6 ): 643 – 648 . PubMed Google Scholar
30. Jones LC , Frondoza C , Hungerford DS . Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components . J Biomed Mater Res . 1999 ; 48 ( 6 ): 889 – 898 . Crossref PubMed Google Scholar
31. MacEwan DJ . TNF receptor subtype signalling: differences and cellular consequences . Cell Signal . 2002 ; 14 ( 6 ): 477 – 492 . Crossref PubMed Google Scholar
32. Vandenabeele P , Declercq W , Beyaert R , Fiers W . Two tumour necrosis factor receptors: structure and function . Trends Cell Biol . 1995 ; 5 ( 10 ): 392 – 399 . Crossref PubMed Google Scholar
33. Aderka D . The potential biological and clinical significance of the soluble tumor necrosis factor receptors . Cytokine Growth Factor Rev . 1996 ; 7 ( 3 ): 231 – 240 . Crossref PubMed Google Scholar
34. Aderka D , Engelmann H , Maor Y , Brakebusch C , Wallach D . Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors . J Exp Med . 1992 ; 175 ( 2 ): 323 – 329 . Crossref PubMed Google Scholar
35. Gething MJ . Role and regulation of the ER chaperone BiP . Semin Cell Dev Biol . 1999 ; 10 ( 5 ): 465 – 472 . Crossref PubMed Google Scholar
36. Szegezdi E , Logue SE , Gorman AM , Samali A . Mediators of endoplasmic reticulum stress-induced apoptosis . EMBO Rep . 2006 ; 7 ( 9 ): 880 – 885 . Crossref PubMed Google Scholar
37. Guicciardi ME , Gores GJ . Life and death by death receptors . Faseb J . 2009 ; 23 ( 6 ): 1625 – 1637 . Crossref PubMed Google Scholar
38. Chae HJ , Chae SW , Kang JS , et al. Dexamethasone suppresses tumor necrosis factor-alpha-induced apoptosis in osteoblasts: possible role for ceramide . Endocrinology . 2000 ; 141 ( 8 ): 2904 – 2913 . Crossref PubMed Google Scholar
39. Jilka RL , Weinstein RS , Bellido T , Parfitt AM , Manolagas SC . Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines . J Bone Miner Res . 1998 ; 13 ( 5 ): 793 – 802 . Crossref PubMed Google Scholar
40. Pischon N , Darbois LM , Palamakumbura AH , Kessler E , Trackman PC . Regulation of collagen deposition and lysyl oxidase by tumor necrosis factor-alpha in osteoblasts . J Biol Chem . 2004 ; 279 ( 29 ): 30060 – 30065 . Crossref PubMed Google Scholar
41. Panagakos FS , Hinojosa LP , Kumar S . Formation and mineralization of extracellular matrix secreted by an immortal human osteoblastic cell line: modulation by tumor necrosis factor-alpha . Inflammation . 1994 ; 18 ( 3 ): 267 – 284 . Crossref PubMed Google Scholar
42. Byers PH , Click EM , Harper E , Bornstein P . Interchain disulfide bonds in procollagen are located in a large nontriple-helical COOH-terminal domain . Proc Natl Acad Sci U S A . 1975 ; 72 ( 8 ): 3009 – 3013 . Crossref PubMed Google Scholar
43. Fujii M , Yoneda A , Takei N , et al. Endoplasmic reticulum oxidase 1α is critical for collagen secretion from and membrane type 1-matrix metalloproteinase levels in hepatic stellate cells . J Biol Chem . 2017 ; 292 ( 38 ): 15649 – 15660 . Crossref PubMed Google Scholar
44. Nakagawa M , Kaneda T , Arakawa T , et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts . FEBS Lett . 2000 ; 473 ( 2 ): 161 – 164 . Crossref PubMed Google Scholar
45. Agidigbi TS , Kim C . Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases . Int J Mol Sci . 2019 ; 20 ( 14 ): 3576 . Crossref PubMed Google Scholar
46. Honorati MC , Cattini L , Facchini A . Il-17, IL-1beta and TNF-alpha stimulate VEGF production by dedifferentiated chondrocytes . Osteoarthritis Cartilage . 2004 ; 12 ( 9 ): 683 – 691 . Crossref PubMed Google Scholar
47. Paleolog EM , Young S , Stark AC , et al. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis . Arthritis Rheum . 1998 ; 41 ( 7 ): 1258 – 1265 . Crossref PubMed Google Scholar
48. Babbar N , Casero RA . Tumor Necrosis Factor- Increases Reactive Oxygen Species by Inducing Spermine Oxidase in Human Lung Epithelial Cells: A Potential Mechanism for Inflammation-Induced Carcinogenesis . Cancer Res . 2006 ; 66 ( 23 ): 11125 – 11130 . Google Scholar
49. Yang D , Elner SG , Bian ZM , et al. Pro-Inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells . Exp Eye Res . 2007 ; 85 ( 4 ): 462 – 472 . Crossref PubMed Google Scholar
50. Ziebart J , Fan S , Schulze C , et al. Effects of interfacial micromotions on vitality and differentiation of human osteoblasts . Bone Joint Res . 2018 ; 7 ( 2 ): 187 – 195 . Crossref PubMed Google Scholar
Author contributions
R. Hameister: Conceptualized the study, Performed the investigation, visualization, and project administration, Wrote, reviewed, and edited the manuscript.
C. H. Lohmann: Acquired the resources and funding, Supervised the study, Wrote, reviewed, and edited the manuscript.
S. T. Dheen: Acquired the resources, Supervised the study, Wrote, reviewed, and edited the manuscript.
G. Singh: Conceptualized and supervised the study, Performed the investigation and project administration, Acquired the resources and funding, Wrote, reviewed, and edited the manuscript.
C. Kaur: Conceptualized and supervised the study, Performed the investigation, visualization, and project administration, Acquired the resources, Wrote, reviewed, and edited the manuscript.
Funding statement
The study received funding from the National Medical Research Council in Singapore under grant agreement NMRC/CNIG/1147/2016 and the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement 602398.No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
C. H. Lohmann reports an institutional grant (paid to Otto von Guericke University Magdeburg) from the European Commission (FP7), related and not related to this study, respectively.
G. Singh reports an institutional grant (paid to National University of Singapore and National University Hospital, Singapore) from the National Medical Research Council, Singapore, related to this study.
Ethical review statement
Institutional Review Board approval was obtained from Otto von Guericke University Magdeburg, Magdeburg, Germany and from National University of Singapore, Singapore (Approval No. 150/2 and NHG DSRB reference number: 2016/00080). Written consent was obtained from patients, whose tissue samples were used for histology work, and local and international guidelines were followed.
Supplementary material
Figure displaying tumour necrosis factor-receptor 1 (TNF-R1) immunoexpression in SaOs-2 cells in response to exogenous tumour necrosis factor-alpha (TNF-α).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









