Abstract
Objectives
Venous thromboembolism (VTE) is a major potential complication following orthopaedic surgery. Subcutaneously administered enoxaparin has been used as the benchmark to reduce the incidence of VTE. However, concerns have been raised regarding the long-term administration of enoxaparin and its possible negative effects on bone healing and bone density with an increase of the risk of osteoporotic fractures. New oral anticoagulants such as rivaroxaban have recently been introduced, however, there is a lack of information regarding how these drugs affect bone metabolism and post-operative bone healing.
Methods
We measured the migration and proliferation capacity of mesenchymal stem cells (MSCs) under enoxaparin or rivaroxaban treatment for three consecutive weeks, and evaluated effects on MSC mRNA expression of markers for stress and osteogenic differentiation.
Results
We demonstrate that enoxaparin, but not rivaroxaban, increases the migration potential of MSCs and increases their cell count in line with elevated mRNA expression of C-X-C chemokine receptor type 4 (CXCR4), tumor necrosis factor alpha (TNFα), and alpha-B-crystallin (CryaB). However, a decrease in early osteogenic markers (insulin-like growth factors 1 and 2 (IGF1, IGF2), bone morphogenetic protein2 (BMP2)) indicated inhibitory effects on MSC differentiation into osteoblasts caused by enoxaparin, but not by rivaroxaban.
Conclusions
Our findings may explain the adverse effects of enoxaparin treatment on bone healing. Rivaroxaban has no significant impact on MSC metabolism or capacity for osteogenic differentiation in vitro.
Cite this article: Dr H. Pilge. Enoxaparin and rivaroxaban have different effects on human mesenchymal stromal cells in the early stages of bone healing. Bone Joint Res 2016;5:95–100. DOI: 10.1302/2046-3758.53.2000595.
Article focus
-
Long-term administration of enoxaparin negatively affects post-operative bone healing.
-
Do new oral anticoagulants such as rivaroxaban have the same effects on bone healing?
Key messages
-
Enoxaparin treatment increases migration and cell count of mesenchymal stem cells, but inhibits their osteogenic differentiation capacity.
-
The new oral anticoagulant rivaroxaban has fewer effects on mesenchymal stromal cells during post-operative bone healing.
Strengths and limitations
-
Strengths: Cells of eight patients were each evaluated during treatment with and without three different concentrations of two different anticoagulants in parallel.
-
Limitations: As an in vitro study, our data may not reflect what is actually occurring during bone healing in vivo.
Introduction
Following major orthopaedic or trauma surgery, venous thromboembolism (VTE) is a severe complication that can lead to mortality. In patients undergoing major extremity orthopaedic surgery without VTE prophylaxis, the prevalence of deep vein thrombosis has been reported to be 40% to 60%1,2 and the prevalence of fatal pulmonary embolism has been reported to be 2% to 12%.2,3 The incidence of VTE in cases of fractures of the lower extremity has been reported to be 28%.4 Current treatments for the prevention of VTE include heparin and low-molecular-weight heparin (LMWH). By using enoxaparin (a commonly used LMWH), the risk can be reduced to 2%, but there are reports that long-term administration negatively affects bone healing and density, and increases the risk of osteoporotic fractures.5-10
Rivaroxaban (Xarelto, Bayer Schering Pharma AG, Wuppertal, Germany) is a new oral factor Xa inhibitor which was granted market approval in 2008 by the European Commission as an antithrombotic drug following hip and knee arthroplasty. It has a 10 000-fold greater selectivity for factor Xa than for other related serine proteases, and unlike LMWHs and similar agents, rivaroxaban does not require antithrombin as a cofactor and can inhibit free and clotted factor Xa and factor Xa bound to the prothrombinase complex.11,12 The clinical efficacy of rivaroxaban in reducing post-operative VTE has been proven and is comparable with that of LMWHs.13
In the post-operative process, bone healing starts with recruitment and cell migration to the local trauma site, followed by proliferation and osteogenic differentiation of mesenchymal stromal cells (MSCs). Finally, osteoblasts promote the calcification of the extracellular matrix. Although the use of rivaroxaban is increasing, there is a lack of information regarding the drug’s effect on post-operative bone healing. To understand better the effect of rivaroxaban and enoxaparin on the early stages of bone healing before MSCs progress towards osteogenic differentiation, we focused on MSC migration and proliferation under rivaroxaban and enoxaparin treatment. We also studied the relative mRNA expression of accompanying marker genes for both processes in addition to markers for the induction of osteogenic differentiation.
Materials and Methods
The study protocol was approved and authorised by the local Institutional Review Board according to the Helsinki Declaration. All patients provided written informed consent to participate in this study prior to surgery. During elective surgery, bone marrow was harvested from the femoral head or the iliac crest of eight patients (four male, four female) aged 30 years (sd 8) who presented without a history of bone marrow pathologies.
MSC isolation and expansion
Bone marrow mononuclear cells were separated by density-gradient centrifugation (Biocoll 1.077 g/mL, Biochrom GmbH, Berlin, Germany) and seeded in DMEM (Sigma-Aldrich, St. Louis, Missouri) containing 20% foetal bovine serum (FBS Superior, Biochrom GmbH), 1% penicillin/streptomycin/L-glutamine (Sigma-Aldrich) in a humidified atmosphere at 37°C and 5% CO2. Non-adherent cells were removed after five days, and the medium was changed twice weekly. Adherent cells were passaged weekly and seeded at 5000 cells/cm². All experiments were carried out using MSCs of eight different donors each derived from passage 3. Before cells were used, the MSC character of the cultures was determined and possible contamination with haematopoietic cells excluded by flow cytometry (⩾ 95% expression of CD73, CD90, and CD105, while lacking CD34 and CD45) as previously described.14
MSC cell count
Passage 3 MSCs of the eight donors were cultured with three different concentrations of enoxaparin (2, 10, 50 µg/mL dissolved and pre-diluted in phosphate buffered saline (PBS)), rivaroxaban (20, 100, 500 ng/mL dissolved and pre-diluted in dimethyl sulfoxide (DMSO)), each spanning the median serum concentrations measured in vivo,15,16 and carrier controls (carrier end concentration in each culture 0.1%). The cells were trypsinised (Trypsin-EDTA solution, Sigma-Aldrich) weekly, and the cell count and viability were determined after seven, 14 and 21 days.
Migration assay
After seven days of drug treatment, 105 trypsinised MSCs of eight different donors were resuspended in DMEM and placed in cell culture inserts with an 8 µm pore size (Greiner Bio-One GmbH, Frickenhausen, Germany). The inserts were placed in 12 well plates with growth medium containing 50 ng/mL stromal cell-derived factor 1α (SDF-1α) (PeproTech, Rocky Hill, New Jersey) as a chemoattractant. After 20 hours of incubation in a humidified atmosphere at 37°C and 5% CO2, cells that migrated to the lower chamber were trypsinised and counted using a haemocytometer.
Quantitative real-time PCR
RNA of the treated MSCs of eight different donors was isolated using the RNeasy Mini Kit in combination with the RNase-free DNase Set, and cDNA was synthesised by the QuantiTect Reverse Transcription Kit according to the manufacturer’s instructions (all Qiagen, Hilden, Germany). Ct values were measured in duplicate on a StepOne Real-Time PCR System using the SYBR Green PCR Master Mix (both Applied Biosystems, Life Technologies, Carlsbad, California). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the reference control, and relative gene expression levels (CXCR4, TNFa, CryaB, IGF1, IGF2, BMP2) were calculated by the ΔΔCt method (primer sequences are available as supplementary material alongside this article online).
Statistical analysis
This was performed using GraphPad Prism (version 5.01, GraphPad Software Inc., San Diego, California). Data are given as mean and standard error of the mean (sem). Kolmogorov-Smirnov tests for sample distribution and Friedman one-way analysis of variance (ANOVA) tests for paired samples with post hoc Dunn’s multiple comparison were used for statistical examination. Adjusted p-values are provided in the text, and asterisks are used throughout the figures to indicate the levels of statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Effects on MSC migration
After one week of drug treatment, the migratory capacity of MSCs was tested and compared with the respective carrier controls. In the PBS-treated controls, of the 105 MSCs in the upper chamber, 15 570 cells (sd 3265) migrated to the lower chamber. As shown in Figure 1, enoxaparin treatment resulted in a higher number of migrating cells in a dose-dependent manner (2 µg/mL: 17 571 cells (sd 4131), 10 µg/mL: 23 214 cells (sd 5648), 50 µg/mL: 24 071 cells (sd 3601); p = 0.018) with post hoc Dunn’s multiple comparison showing statistical significance between the highest enoxaparin dose and the PBS-treated controls. Compared with the DMSO-treated MSCs (19 971 cells (sd 2796)), rivaroxaban treatment did not cause significantly more cells to migrate to the lower chamber (20 ng/mL: 19 729 cells (sd 3390), 100 ng/mL: 21 071 cells (sd 1968), 500 ng/mL: 23 571 cells (sd 4499)).
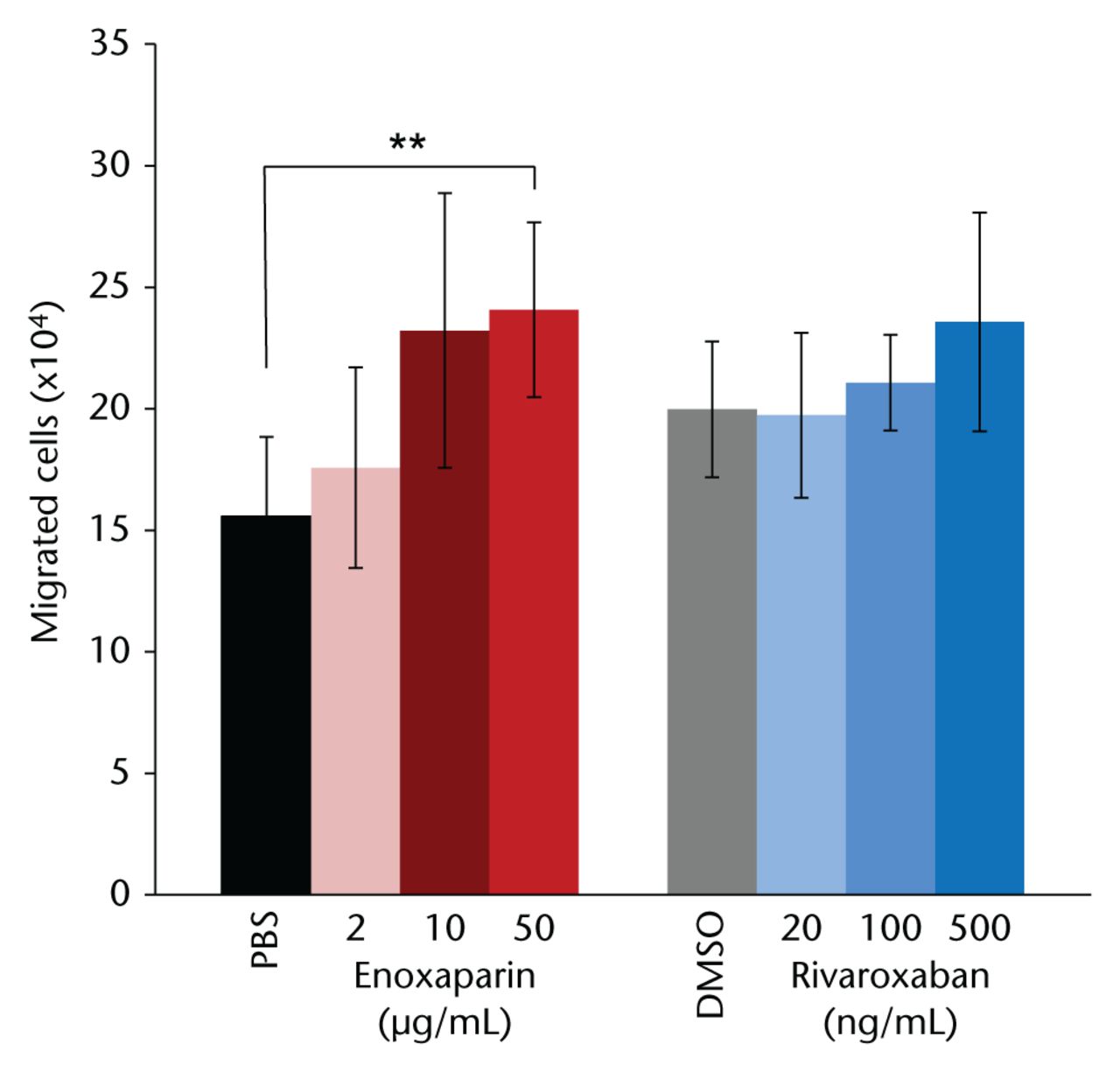
Fig. 1
Mesenchymal stromal cells treated with the highest dose of enoxaparin for seven days showed a significantly increased migratory potential towards stromal cell-derived factor 1α . Dimethyl sulfoxide (DMSO)-resolved rivaroxaban did not have a significant effect on migration. Asterisks show significance levels of Dunn’s multiple comparison post hoc tests (n = 8) (PBS, phosphate buffered saline).
Effects on MSC cell count
As shown in Figure 2, PBS- and DMSO-treated controls, as well as cells treated with the lowest rivaroxaban concentration, did not differ in their cell count during the assay. Rivaroxaban treatment in the two higher concentrations lowered the cell count to 95% at all three time points, but without statistical significance. However, enoxaparin treatment, even after the first week, resulted in a significantly increased cell count (2 µg/mL: 115%, 10 µg/mL: 131%, 50 µg/mL: 125%; p < 0.001). The same effect was seen after two weeks (116%, 125%, 118%; all p < 0.001) and three weeks of enoxaparin treatment (118%, 122%, 119%; all p < 0.001) with post hoc Dunn’s multiple comparison showing the statistical significance between the two highest enoxaparin doses and the PBS-treated controls.
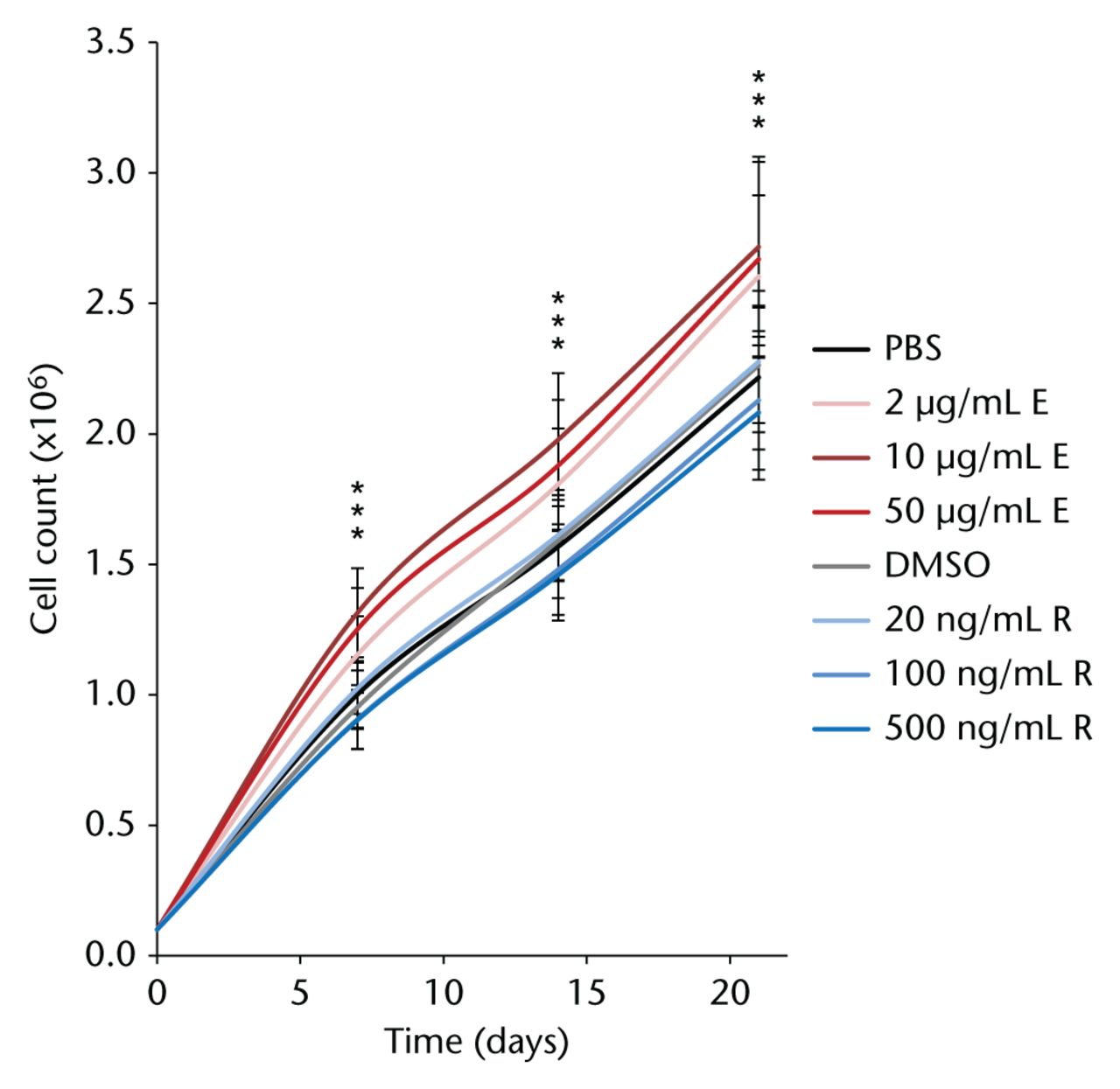
Fig. 2
Over three consecutive weeks in culture with different concentrations of enoxaparin, primary human mesenchymal stromal cells showed a significantly increased cell count compared with phosphate buffered saline (PBS)-treated control cells. Dimethyl sulfoxide (DMSO)-dissolved rivaroxaban treatment did not alter cell count at any of the time points studied. Asterisks show significance levels of Dunn’s multiple comparison post hoc tests (n = 8).
Effects on MSC mRNA expression
During 21 days of cell culture the expression level of C-X-C chemokine receptor type 4 (CXCR4) increased continually in the PBS-treated controls, while DMSO-treated controls showed the same or a slightly higher expression at all time points measured. Enoxaparin treatment of the MSCs for seven days resulted in a dose-dependent significant upregulation of CXCR4 (p < 0.001, Fig. 3a) while further culture with the drug did not cause further elevations. The same effect was observed in the expression of tumour necrosis factor alpha (TNFα) with a dose-dependent upregulation by seven days of enoxaparin treatment (p = 0.002, Fig. 3a). At all time points, there were statistically insignificant changes in CXCR4 and TNFα expression levels in the rivaroxaban-treated cells. We further found the expression level of alpha-B-crystallin (CryaB) to be consistently upregulated during enoxaparin treatment (d7: p = 0.017, d14: p = 0.002, d21: p = 0.006, Fig. 3a). Here, the effect was not only dose- but also time-dependent, as the expression level of CryaB continually increased over the course of treatment. Rivaroxaban treatment, in contrast, resulted in a significant dose-dependent downregulation of CryaB expression (d14: p = 0.012, d21: p < 0.001, Fig. 3a). Furthermore, without in vitro induction of osteogenic differentiation, we found that the expression level of insulin-like growth factor 1 (IGF1), a very early marker for osteogenic development, was dramatically downregulated by enoxaparin at all time points (d7: p < 0.001, d14: p = 0.022, d21: p < 0.001, Fig. 3b). IGF2 levels were also significantly down-regulated by enoxaparin during the first two weeks of treatment (d7: p = 0.007, d14: p = 0.048, Fig. 3b) and bone-morphogenetic protein 2 (BMP2) was significantly downregulated with enoxaparin treatment during the first and third week (d7: p = 0.017, d21: p = 0.019, Fig. 3b). Rivaroxaban treatment, in contrast, did not result in a significant change in the expression levels of these three osteogenic markers.
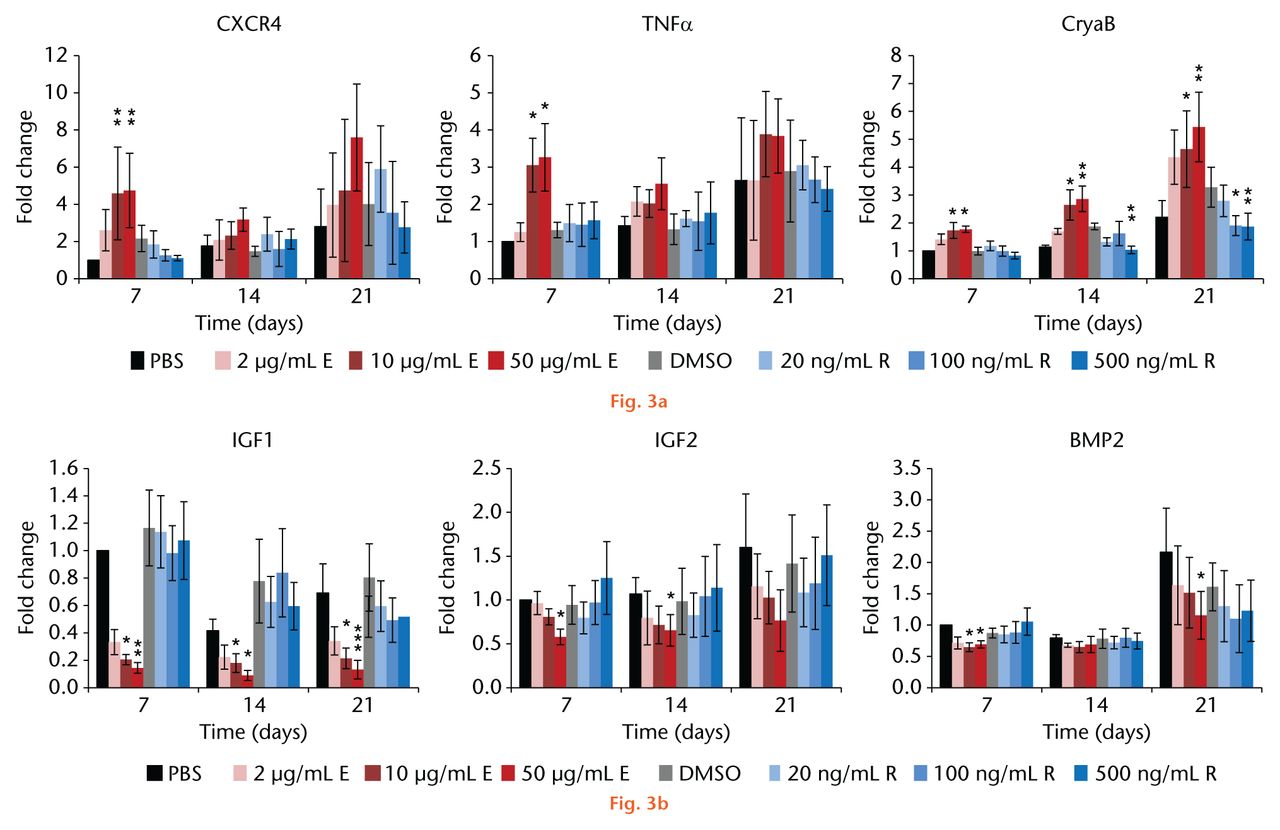
Fig.
Enoxaparin treatment of the mesenchymal stromal cells significantly: a) upregulated the expression levels of migratory (C-X-C chemokine receptor type 4 (CXCR4), tumour necrosis factor alpha (TNFα), proliferative (TNFα) and stress markers (alpha-B-crystallin, CryaB) as well as b) downregulated early markers for osteogenic development (insulin-like growth factors 1 and 2 (IGF1, IGF2) and bone morphogenetic protein (BMP2)). Following two to three weeks of rivaroxaban treatment, a significant downregulation of CryaB expression was observed while other markers were not affected by rivaroxaban treatment. Asterisks show significance levels of Dunn’s multiple comparison post hoc tests (n = 8).
Discussion
Heparin and LMWHs have been commonly used to prevent VTE following major orthopaedic surgery. The adverse effects of enoxaparin (as a commonly used LMWH) on bone and post-operative bone healing are well known.17,18 In particular, osteoporosis, a significant reduction in strength, stiffness, and energy absorbed to fracture, and an increase in osteoporotic fractures have been reported with the use of enoxaparin.5-7,9 The competitive antagonism of growth factors and heparins on osteoblast surface-binding proteins appear to inhibit the growth of osteoblasts, which plays a significant role both in the delay of fracture healing, and in secondary osteoporosis.19 Although the clinical use of the new oral factor Xa inhibitor rivaroxaban is increasing, there have only been a few reports on the modulation of bone healing in patients treated with rivaroxaban.19-22
Bone healing is a dynamic process that is composed of stages of inflammation, repair, and remodeling. Following the initial inflammatory process, MSCs are recruited to the trauma site, mediated by BMP7, SDF-1, and CXCR4.23,24 BMP2 appears to be crucial for the initiation of the healing cascade and callus formation, whereas BMP5 and 6 have been reported to induce cell proliferation.25,26 This process is followed by differentiation of pluripotent MSCs into osteoblasts, which is regulated by the Wnt family of molecules. IGF1 and IGF2, as well as BMP2, are upregulated in the osteoblastic differentiation process.23,27-29 Mature osteoblasts then carry out the remodeling process, which is initiated after three to four weeks in animal and human models.23
There have been previous reports of the effect of rivaroxaban and enoxaparin on mature osteoblasts and, therefore, in the late stages of bone healing.20 However, there have been no previous reports regarding the effect of these drugs on the early stages in the first few weeks following surgery, which may be the more relevant for clinical practice. The aim of our study was to evaluate the effect of post-operative VTE prophylaxis on these early stages.
As the process of bone healing starts with cell migration to the trauma site, we first focused on the migratory potential of MSCs under enoxaparin and rivaroxaban treatment. To our knowledge, we are the first group to demonstrate an increased migration of MSCs treated with enoxaparin, whereas there was no significant effect on migration in the rivaroxaban-treated group. Interestingly, DMSO alone (as a carrier for rivaroxaban) had a positive impact on MSC migration, but there were no additional significant effects due to the addition of rivaroxaban. In concordance with this, we found that the expression level of CXCR4 - the specific receptor for stromal-derived factor-1 (SDF-1) and a marker for target-oriented cell migration - was upregulated during the first week of treatment with enoxaparin, but not by rivaroxaban. These results were unexpected because they were not in concordance with reported delayed bone healing and osteoporosis, as one would expect better bone healing with an increased MSC migration to the trauma site.
Therefore, we focused on the second step of bone healing: the proliferation of the MSCs at the trauma site. Here, we found a significantly increased MSC cell count under enoxaparin treatment, while rivaroxaban caused a slight and non-statistically significant decrease. This was accompanied by a significant increase in the TNFα expression levels of the enoxaparin-treated cells during the first week of treatment. TNFα is the key regulator of the canonical NFκB pathway and has been shown to induce MSC proliferation and migration.30 Furthermore, we found that the small heat-shock protein CryaB, which is synthesised by cells in response to environmental stress and has positive implications for proliferation and migration,31 was upregulated in enoxaparin-treated cells during a three-week course of treatment, but downregulated over time by rivaroxaban treatment. Therefore, we have demonstrated that enoxaparin, but not rivaroxaban, triggers the migratory as well as the proliferative potential of MSCs. In general, better bone healing is achieved when cell proliferation increases. Thus at first glance, these results (increased migration and cell count under enoxaparin) seem not to be in concordance with the known negative effects of delayed fracture healing and osteoporosis under treatment with enoxaparin.
However, going one step further in bone healing, local MSCs must differentiate into mature osteoblasts to form new bone properly. At this stage, the cells lose their ability to proliferate, as proliferation and differentiation do not occur simultaneously.32,33 We therefore evaluated the effects of both drugs on the expression levels of different marker genes for osteogenic development. Enoxaparin, without the exogenous induction of osteogenic differentiation, caused a dramatic downregulation of IGF1 accompanied by significantly lowered IGF2 and BMP2 mRNA expression levels. IGF1 promotes the osteogenic differentiation of MSCs and regulates osteoblastic proliferation, differentiation, survival, and the synthesis of bone matrix in vivo. Low IGF1 levels have been found to be a risk factor for osteoporosis and fracture risk.27-29 Conditional IGF1 knockout studies in early osteoprogenitor cells also showed that osteoblasts are not able to differentiate normally and result in lower bone mass compared with wild-type controls.29,34 Similarly, differentiation of osteoprogenitor cells is impaired in IGF2 knockout mice.35 In addition, the expression levels of IGF1 and IGF2 are dramatically upregulated by MSCs as soon as day 1 of osteogenic differentiation, which indicates that these proteins are essential for very early osteogenic development.36 The results shown here indicate that enoxaparin has a positive effect on MSC migration to the trauma site and local proliferation, but inhibits differentiation of MSCs into osteoblasts, and therefore impairs normal bone healing. Rivaroxaban showed no significant effects on MSC migration, cell count or osteogenic differentiation markers, which indicates a more unaffected cell physiology with rivaroxaban than with enoxaparin.
In conclusion, we demonstrated that enoxaparin and rivaroxaban have differing effects on the early stages of human bone healing. We are the first to demonstrate that enoxaparin, as early as the first week of treatment, increases MSC potential to migrate to the trauma site and proliferate, however, enoxaparin also inhibits MSCs to differentiate into osteoblasts. Rivaroxaban has minimal to no impact on MSC migration, proliferation or mRNA expression of osteogenic markers.
Supplementary material
A table showing primer sequences is available alongside the online version of this article at www.bjr.boneandjoint.org.uk
Funding Statement
The authors declare no conflicts of interest. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
ICMJE conflict of interest
None declared.
References
1. Colwell CW Jr . Rationale for thromboprophylaxis in lower joint arthroplasty. Am J Orthop (Belle Mead NJ)2007;36(Suppl):11-13.PubMed Google Scholar
2. Turpie AG . Efficacy of a postoperative regimen of enoxaparin in deep vein thrombosis prophylaxis. Am J Surg1991;161:532-536.CrossrefPubMed Google Scholar
3. Eriksson BI , LassenMR, PENTasaccharide in HIp-FRActure Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med2003;163:1337-1342.CrossrefPubMed Google Scholar
4. Geerts WH , HeitJA, ClagettGP, et al.. Prevention of venous thromboembolism. Chest2001;119(Suppl):132S-175S.CrossrefPubMed Google Scholar
5. Street JT , McGrathM, O’ReganK, et al.. Thromboprophylaxis using a low molecular weight heparin delays fracture repair. Clin Orthop Relat Res2000;381:278-289.CrossrefPubMed Google Scholar
6. Douketis JD , GinsbergJS, BurrowsRF, et al.. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb Haemost1996;75:254-257.PubMed Google Scholar
7. Dahlman TC . Osteoporotic fractures and the recurrence of thromboembolism during pregnancy and the puerperium in 184 women undergoing thromboprophylaxis with heparin. Am J Obstet Gynecol1993;168:1265-1270.CrossrefPubMed Google Scholar
8. Squires JW , PinchLW. Heparin-induced spinal fractures. JAMA1979;241:2417-2418.PubMed Google Scholar
9. Rajgopal R , BearM, ButcherMK, ShaughnessySG. The effects of heparin and low molecular weight heparins on bone. Thromb Res2008;122:293-298.CrossrefPubMed Google Scholar
10. Wawrzy ska L , TomkowskiWZ, PrzedlackiJ, HajdukB, TorbickiA. Changes in bone density during long-term administration of low-molecular-weight heparins or acenocoumarol for secondary prophylaxis of venous thromboembolism. Pathophysiol Haemost Thromb2003;33:64-67.CrossrefPubMed Google Scholar
11. Perzborn E , StrassburgerJ, WilmenA, et al.. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939–an oral, direct Factor Xa inhibitor. J Thromb Haemost2005;3:514-521. Google Scholar
12. Eriksson BI , QuinlanDJ, WeitzJI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet2009;48:1-22.CrossrefPubMed Google Scholar
13. Eriksson BI , BorrisLC, FriedmanRJ, et al.. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med2008;358:2765-2775.CrossrefPubMed Google Scholar
14. Geyh S , OzS, CadedduRP, et al.. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia2013;27:1841-1851.CrossrefPubMed Google Scholar
15. Kubitza D , BeckaM, MueckW, ZuehlsdorfM. Rivaroxaban (BAY 59-7939)–an oral, direct Factor Xa inhibitor–has no clinically relevant interaction with naproxen. Br J Clin Pharmacol2007;63:469-476. Google Scholar
16. Ellensen VS , AbrahamsenI, LorensJ, JonungT. Effects of enoxaparin and dalteparin on proliferation and migration of patient-derived vascular smooth muscle cells. Vasa2014;43:124-131.CrossrefPubMed Google Scholar
17. Kock HJ , HandschinAE. Osteoblast growth inhibition by unfractionated heparin and by low molecular weight heparins: an in-vitro investigation. Clin Appl Thromb Hemost2002;8:251-255.CrossrefPubMed Google Scholar
18. Muir JM , AndrewM, HirshJ, et al.. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood1996;88:1314-1320.PubMed Google Scholar
19. Gigi R , SalaiM, DolkartO, et al.. The effects of direct factor Xa inhibitor (Rivaroxaban) on the human osteoblastic cell line SaOS2. Connect Tissue Res2012;53:446-450.CrossrefPubMed Google Scholar
20. Solayar GN , WalshPM, MulhallKJ. The effect of a new direct Factor Xa inhibitor on human osteoblasts: an in-vitro study comparing the effect of rivaroxaban with enoxaparin. BMC Musculoskelet Disord2011;12:247.CrossrefPubMed Google Scholar
21. Somjen D , KatzburgS, GigiR, et al.. Rivaroxaban, a direct inhibitor of the coagulation factor Xa interferes with hormonal-induced physiological modulations in human female osteoblastic cell line SaSO2. J Steroid Biochem Mol Biol2013;135:67-70.CrossrefPubMed Google Scholar
22. Pilge H , FröbelJ, MrotzekSJ, et al.. Effects of thromboprophylaxis on mesenchymal stromal cells during osteogenic differentiation: an in-vitro study comparing enoxaparin with rivaroxaban. BMC Musculoskelet Disord2016;17:108.CrossrefPubMed Google Scholar
23. Marsell R , EinhornTA. The biology of fracture healing. Injury2011;42:551-555.CrossrefPubMed Google Scholar
24. Granero-Moltó F , WeisJA, MigaMI, et al.. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells2009;27:1887-1898.CrossrefPubMed Google Scholar
25. Tsuji K , BandyopadhyayA, HarfeBD, et al.. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet2006;38:1424-1429.CrossrefPubMed Google Scholar
26. Marsell R , EinhornTA. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury2009;(Suppl 3):S4-S7.CrossrefPubMed Google Scholar
27. Xue P , WuX, ZhouL, et al.. IGF1 promotes osteogenic differentiation of mesenchymal stem cells derived from rat bone marrow by increasing TAZ expression. Biochem Biophys Res Commun2013;433:226-231.CrossrefPubMed Google Scholar
28. Wang T , WangY, MenendezA, et al.. Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired Endochondral Bone Formation During Fracture Healing. J Bone Miner Res2015;30:1572-1584.CrossrefPubMed Google Scholar
29. Crane JL , ZhaoL, FryeJS, et al.. IGF-1 Signaling is Essential for Differentiation of Mesenchymal Stem Cells for Peak Bone Mass. Bone Res2013;1:186-194.CrossrefPubMed Google Scholar
30. Böcker W , DochevaD, PrallWC, et al.. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J Mol Med (Berl)2008;86:1183-1192.CrossrefPubMed Google Scholar
31. van de , SchootbruggeC, BussinkJ, SpanPN, et al.. αB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer2013;13:128.CrossrefPubMed Google Scholar
32. Wagner ER , LutherG, ZhuG, et al.. Defective Osteogenic Differentiation in the Development of Osteosarcoma. Sarcoma2011;2011.CrossrefPubMed Google Scholar
33. Birmingham E , NieburGL, McHughPE, et al.. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater2012;23:13-27.CrossrefPubMed Google Scholar
34. Xian L , WuX, PangL, et al.. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med2012;18:1095-1101.CrossrefPubMed Google Scholar
35. Hardouin SN , GuoR, RomeoPH, NagyA, AubinJE. Impaired mesenchymal stem cell differentiation and osteoclastogenesis in mice deficient for Igf2-P2 transcripts. Development2011;138:203-213.CrossrefPubMed Google Scholar
36. Hamidouche Z , FromiguéO, RingeJ, HäuplT, MariePJ. Crosstalks between integrin alpha 5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol2010;11:44.CrossrefPubMed Google Scholar









