Abstract
Construction of a functional skeleton is accomplished through co-ordination of the developmental processes of chondrogenesis, osteogenesis, and synovial joint formation. Infants whose movement in utero is reduced or restricted and who subsequently suffer from joint dysplasia (including joint contractures) and thin hypo-mineralised bones, demonstrate that embryonic movement is crucial for appropriate skeletogenesis. This has been confirmed in mouse, chick, and zebrafish animal models, where reduced or eliminated movement consistently yields similar malformations and which provide the possibility of experimentation to uncover the precise disturbances and the mechanisms by which movement impacts molecular regulation. Molecular genetic studies have shown the important roles played by cell communication signalling pathways, namely Wnt, Hedgehog, and transforming growth factor-beta/bone morphogenetic protein. These pathways regulate cell behaviours such as proliferation and differentiation to control maturation of the skeletal elements, and are affected when movement is altered. Cell contacts to the extra-cellular matrix as well as the cytoskeleton offer a means of mechanotransduction which could integrate mechanical cues with genetic regulation. Indeed, expression of cytoskeletal genes has been shown to be affected by immobilisation. In addition to furthering our understanding of a fundamental aspect of cell control and differentiation during development, research in this area is applicable to the engineering of stable skeletal tissues from stem cells, which relies on an understanding of developmental mechanisms including genetic and physical criteria. A deeper understanding of how movement affects skeletogenesis therefore has broader implications for regenerative therapeutics for injury or disease, as well as for optimisation of physical therapy regimes for individuals affected by skeletal abnormalities.
Cite this article: Bone Joint Res 2015;4:105–116
Article focus
- Human skeletal malformations can result from reduced or absent mechanical stimulation in utero.
- Embryonic skeletal development involves temporal and spatial coordination of cartilage and bone tissue, which relies on networks of complex biochemical interactions within and between cells. Gene expression and the signalling environment are altered when mechanical stimulation is reduced.
- Research in this area has important implications for the development of new approaches, both preventive and therapeutic, to skeletal disorders.
Key messages
- Animal models and cell culture have provided evidence for the importance of appropriate mechanical stimulation during normal skeletal development.
- There is potential to harness this knowledge for regenerative therapies, recreating the required mechanical and signalling environment to guide the formation of robust cartilage and bone tissues from stem cells in culture.
- Foetal and newborn skeletal health depends on mechanical stimulation in utero, suggesting that physical therapy could correct or lessen the effects of infant skeletal malformations resulting from a range of movement-inhibiting conditions.
Introduction
The vertebrate skeleton is remarkably well constructed for support, protection, and movement. While the adaptability of the mature skeleton in response to physical stimuli has been long accepted (e.g. loss of bone mass by astronauts; increased strength of bones in the dominant limbs of athletes), the role of movement in shaping the skeleton during embryogenesis has been acknowledged only relatively recently. A number of intriguing studies have emerged which have documented the clinical significance of mechanical stimulation to normal foetal skeletal development and the consequential impact of reduced foetal movement. Importantly, they have highlighted the role of functional contractile muscle in the transduction of dynamic physical loads to form bones and joints. Developing limbs experience a relatively large range of movement with respect to other skeletal elements. As such, they are particularly subject to altered mechano-stimulatory cues and make for a striking example of the importance of movement for proper embryonic patterning.
Mechanical stimuli have historically been recognised as vital to bone repair and maintenance, dating back to Julius Wolff’s 1888 law which characterised bone strength as proportional to the physical loads placed upon the structure.1,2 An understanding of bone as an adaptable, responsive material was expanded upon through Frost’s mechanostat theory, wherein forces exerted on the skeleton by associated muscle direct a biochemical response which carries out the bone remodelling.3 This idea refocused attention on the possibility that physical cues could directly influence molecular and cellular processes within tissues. However, it was not until recently that these concepts were considered in terms of skeletal development in utero. A wealth of new evidence currently exists to support mechanical stimulation as a vital feature of healthy embryonic development.
Construction of a sturdy yet dynamic skeletal system involves the interrelated processes of bone development and joint formation. In the appendicular skeleton, failure of synchronisation between the development of diarthroses (full-movement joints) and long bones results in an embryo with limited movement. The embryo’s capacity to perform muscular movements will effectively feed back into further development and later remodelling of the skeleton. Thus, proper skeletal formation is enabled by, and in turn enables a full range of embryonic movement. Although the impact that movement has on skeletal development in utero has now been well demonstrated, our understanding of how mechanical stimuli influence genetic and molecular regulation of developmental processes is very limited. It is therefore important to determine the mechanisms of mechanotransduction involved in skeletal development, as they have a lifelong reach on form and function.
Understanding how mechanical stimuli generated by foetal movement impact skeletal development will augment our fundamental knowledge of tissue formation and has at least two important potential avenues for clinical application. First, in informing possible therapeutic interventions in utero where foetal movement is restricted and second, in improving strategies for bone and cartilage tissue regeneration from stem cells for skeletal regenerative therapies.
In this review, we synthesise current knowledge of skeletal development and the impact of mechanical stimulation in order to highlight current research aims as well as potential clinical and regenerative applications.
Foetal movement and clinical consequences of reduced movement
Muscle-controlled movement begins early and continues throughout embryonic development. In humans, the first foetal movement is recorded at nine weeks postmenstrual age (approximately Carnegie stage 184) just after innervation of the forelimbs during Carnegie stages 14 to 175 as the skeletal rudiments are forming. The temporal relationship between movement and skeletal development hints at a close functional relationship.
In the 1970s, newborns with joint contractures, pulmonary hypoplasia, facial deformities and overall growth retardation were suggested by some to suffer from specific autosomal-recessive mutations, whereas others argued that this phenotype resulted from related, though discrete, disorders.6-8 The discovery that application of a muscle relaxant to pregnant female rats could generate the same set of neonatal abnormalities led Moessinger9 to formalise a description of a foetal akinesia deformation sequence (FADS) where inhibition of foetal movement for prolonged periods could result in developmental deformations. The underlying causes of FADS are diverse, comprising disorders of the central nervous system, musculature, and connective tissue, as well as extrinsic factors such as maternal drug use or illness or foetal crowding.10 However, the common factor is reduced foetal movement leading to a similar spectrum of defects in bone and joint formation including hypomineralised and brittle bones prone to fracture, and contracture or dysplasia of the joints.11-14 Reduced movement can lead to a condition called temporary brittle bone disease (TBBD)15-17 which, as its name suggests, is a transient condition where bone fails to form properly during embryonic development and which affects newborn babies and young children, leaving them susceptible to non-accidental injury during their first year of life.18 It is distinct from osteogenesis imperfecta (OI), also called brittle bone disease (BBD), where mutated collagen proteins build malformed bones, and which is a permanent condition.19 TBBD generally regresses within the first year as bones are sufficiently strengthened by normal mechanical stimuli. Nonetheless, the transient state of heightened risk of fracture is dangerous and stressful for affected newborn babies and can lead to the added difficulty of suspected abuse from the parent or caregiver. Both the role of reduced foetal movement in causing TBBD and the subsequent recovery following post-natal movement indicate potential therapeutic interventions for affected infants.
Animal models of altered embryonic movement
The hypomineralised bones and unstable joints of infants born following reduced activity in utero indicate disturbances in skeletal development, and animal models provide a convenient means of investigating the precise steps of development altered in such an environment. Given the evolutionary conservation of musculoskeletal development among vertebrates, the two most established models of mammalian and avian development, namely mouse and chick, are well suited, with some limitations. Regular episodes of stereotypical muscular movement start at embryonic day (E) 12.5/Theiler stage (TS)20 20 in a mouse model and E 3.5/Hamburger and Hamilton (HH)21 stage 21 in a chick model, which is concurrent with the appearance of limb skeletal rudiments and innervation in both species.22-24 Muscle masses and tendons are gradually forming at these stages, so although it is not certain exactly when effective mechanical loads begin to be transmitted to cells of the cartilage anlagen,25 movement occurs from early stages of appendicular skeletal development and is an integral feature of the system.
The mechanical environment of these model systems can be manipulated genetically and physically to examine embryonic skeletal development in conditions of reduced movement. Advantages of the chick system include ease of access to the embryo for observation and manipulation. It enables multiple observations of the same embryo over the course of development, as chick eggs can be windowed for observation or cultured ex ovo.26 Movement is most commonly reduced in chicks using neuromuscular blocking agents to induce rigid or flaccid paralysis in ovo, or skeletal rudiments can be cultured and manipulated in vitro.27-35 Pharmacological agents such as the neurotransmitter-blocker curare,36 can also be used in pregnant rodents to block foetal movement. However, administration of pharmacological agents is intrusive and is more technically demanding. In contrast, the mouse offers the possibility of more sophisticated genetic manipulation. Mutant embryos where specific genes required for muscle formation and contraction have been inactivated provide more convenient and absolute models of immobilisation. Different genetic mutations result in reduced, absent, or non-contractile skeletal muscle,37-41 allowing examination of skeletal effects in a variety of environments. Reduced muscle and immobilisation models have also been developed in zebrafish,42-44 whose transparent embryos allow for easy visualisation and live imaging of development.
An overview of normal skeletal development
Limbs first appear as small buds of mesenchymal cells covered by ectoderm at species-specific positions along the anteroposterior (AP) body axis20,21,45 (Fig. 1 (1)) The first sign of skeletal development is the condensation of mesenchymal cells at the core of the bud46 in the location of future skeletal elements (Fig. 1 (2)). In the proximal forelimb bud for example, a y-shaped condensation represents the future humerus, radius, and ulna. Distal condensations are progressively added and future joint sites become apparent as areas of increased cell density called interzones (Fig. 1 (2).47,48 Mesenchymal condensations prefigure an immediately subsequent pattern of cartilage differentiation (chondrogenesis), indicated first by expression of the Sox9 transcription factor49 (Fig. 1 (3), Fig. 2 (i)). The emerging cartilage cells (chondrocytes) build up an extracellular matrix (ECM) of collagens, primarily types I and II, and structural proteoglycans in an avascular environment.50-53 This transient cartilage template is progressively replaced by bone via endochondral ossification (Fig. 2 (ii, iii)). At the joint interzones (Fig. 1 (2), Fig. 3), cells are organised into three territories which later form the permanent articular cartilage at the ends (epiphyses) of adjacent rudiments, and the intervening joint cavity, encapsulated by a synovial membrane. Thus, formation of the limb skeleton involves co-ordinated endochondral ossification, differentiation of permanent cartilage at the joint interfaces, and construction of functional cavitated joints, in addition to the development of associated structures such as tendons, ligaments, and menisci.
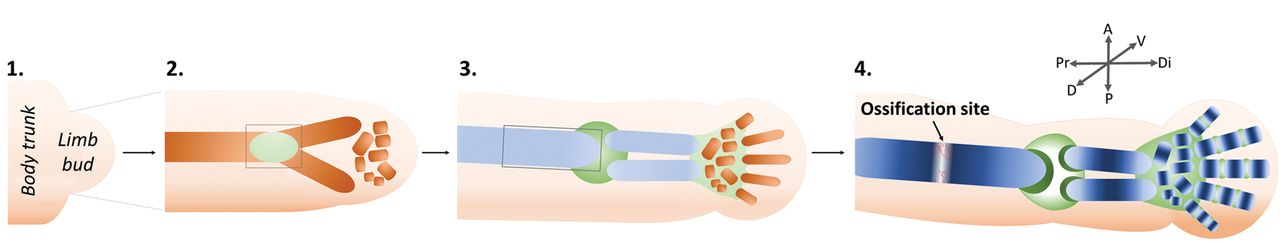
Fig. 1
Diagrams showing an overview of limb bud outgrowth and development. A generalised forelimb bud is represented, progressing through the major stages of skeletal development. Limbs first appear as buds of mesenchymal cells covered by ectoderm (1). Condensation of mesenchymal cells at the core of the bud is the earliest sign of the future skeletal elements (brown) e.g., humerus, radius, and ulna (2). Distal condensations, such as those of the carpals and digits, are progressively added. Future joint sites (green) become apparent within the mesenchymal condensations. The condensations differentiate into cartilage (light blue) (3). This cartilage matures, beginning at the mid-diaphysis of each rudiment (dark blue), proximal rudiments ahead of distal, (4) and is replaced by endochondral bone at the ossification site. Concurrently, joint interzones differentiate (3) and cavitate (4). Grey boxes in (2) and (3) indicate regions of synovial joint formation and endochondral ossification, respectively; these processes are described in detail in Figures 3 and 2, respectively.
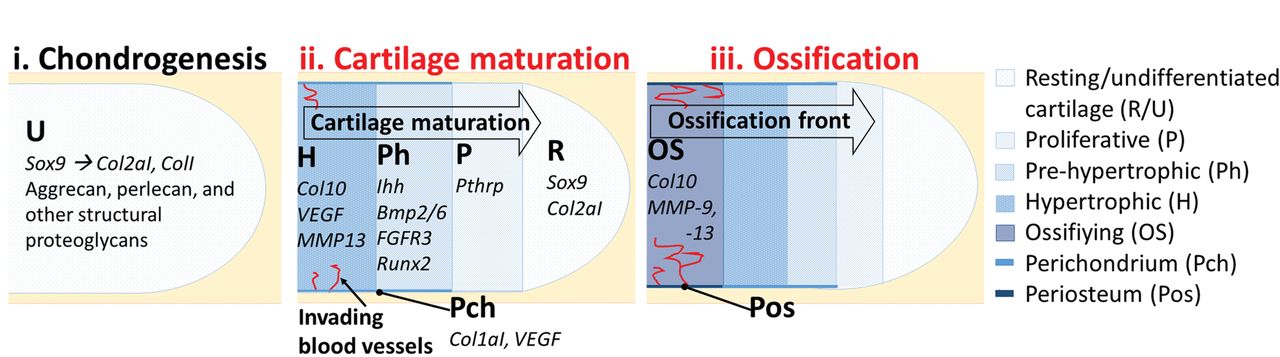
Fig. 2
Diagrams showing an overview of endochondral ossification. The initiation of cartilage formation (chondrogenesis) is marked by expression of the Sox9 transcription factor, followed by production of an extracellular matrix (ECM) of collagens I and II and structural proteoglycans (i). This transient cartilage matures progressively. Cartilage cells (chondrocytes) progress through stages of proliferation, pre-hypertrophy, and hypertrophy (ii). Maturation commences at the mid-point of the long bone shaft and proceeds toward the ends of the long bone. Each stage is marked by expression of a particular cohort of genes (examples indicated). As chondrocytes undergo hypertrophy and die, they are replaced by bone-forming cells (osteoblasts), carried in from the perichondrium via invading blood vessels (red) (iii). Osteoblasts employ matrix metalloproteinases (MMPs) to break down the cartilage ECM and replace it with collagen X-rich bone. Processes affected by immobilisation are denoted in red (explained in the text).
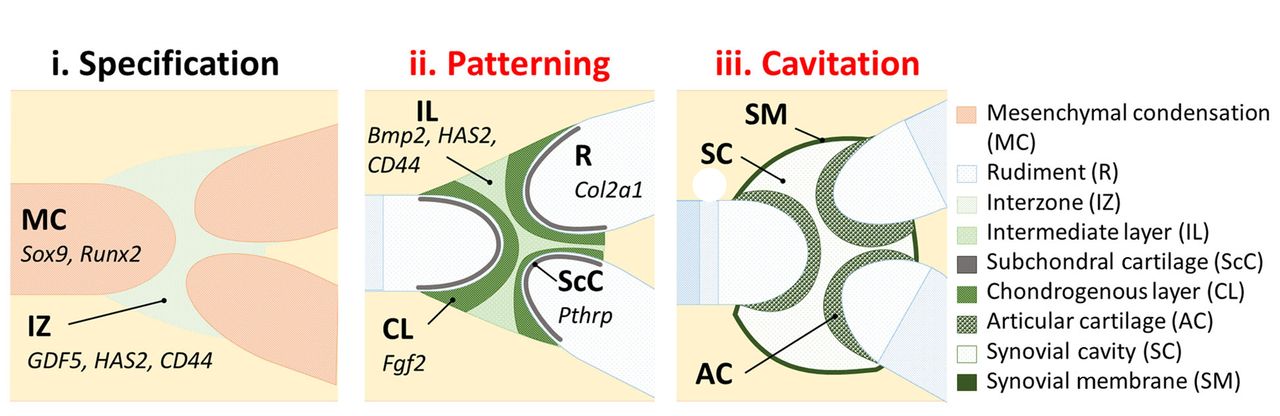
Fig. 3
Diagrams showing an overview of synovial joint formation. Future joint locations are first identifiable as regions of increased cell density, the joint interzones (i). At these interzones, cells organise into morphologically and molecularly recognisable zones. The chondrogenous layers are distinct from the intermediate layer, each territory expressing specific genes (examples shown) (ii). Permanent articular cartilage forms at each of the rudiment ends (epiphyses), and the joint cavity, encapsulated by a synovial membrane, forms at the intermediate site (iii). Processes affected by immobilisation are denoted in red (explained in the text).
The well organised spatial patterns of cell differentiation in the developing limb build over time, with initial patterning established by molecular signalling from the adjacent ectoderm and flank mesoderm, influencing patterns of chondrogenesis as the limb buds grow out.54,55 Our knowledge of such mechanisms is advancing steadily. For example, a Turing reaction-diffusion mechanism involving an integrated wingless-related integration site (Wnt) and bone morphogenetic protein (BMP) signalling and the key chondrogenic transcriptional regulator Sox9, has recently been demonstrated to generate the multiple digit pattern in the distal limb.56 Such early patterning mechanisms can set up crude morphogenically-determined spatial territories which are then developed with local cell–cell interactions and signalling, as described below.
During endochondral ossification (Fig. 2), the chondrocytes transition through proliferative, pre-hypertrophic and hypertrophic phases, apoptosis, and are replaced by bone-forming cells (osteoblasts) at the ossification site, which begins at the mid-point of the diaphysis and spreads to both epiphyses. The perichondrium encapsulates the rudiment and regulates hypertrophy of adjacent chondrocytes.57 It also contributes to ossification by generating osteoblasts which build the periosteum (bone collar) locally and are carried into the cartilage rudiment by invading blood vessels to ossify the hypertrophic zone.58,59 As the chondrocytes mature, the ECM is also remodelled. Matrix metalloproteinases assist in degradation of the collagen II- and aggrecan-rich ECM of immature chondrocytes, allowing for hypertrophic chondrocytes to build a collagen X- and matrix glycoprotein-rich environment to be mineralised by invading osteoblasts.60-63 At birth, most of the cartilage template is replaced by mineralised bone with the exception of growth plates, which will persist as a source of immature chondrocytes for elongation of the long bone until the organism reaches its adult size. Permanent articular cartilage persists at the ends of the long bones at the point where it caps the epiphyses to reduce friction at joint articulations.
Mature synovial joints of the limb allow for a wide range of movement. This type of joint consists of bones separated by a fluid-filled cavity, encapsulated by a double membrane.64 Synovial joint formation can be divided into three distinct stages:65 definition of the joint site (specification) (Fig. 3 (i) (Fig. 3 (i)) is followed by differentiation of joint cell territories (patterning) (Fig. 3 (ii) is followed by differentiation of joint cell territories (patterning) (Fig. 3 (ii) (Fig. 3 (ii)), and finally formation of the joint cavity (cavitation) (Fig. 3 (iii), and finally formation of the joint cavity (cavitation) (Fig. 3 (iii) (Fig. 3 (iii)). Morphogenesis of the rudiment ends occurs as tissue territories are defined within the joint prior to cavitation.66 The molecular programme responsible for joint specification is largely unknown, however, recent efforts have identified a target gene of the non-canonical transforming growth factor-beta (TGF-β) pathway, c-Jun, as an early determiner of synovial joint position,67 influencing expression of the well-established early molecular marker of the joint interzone, Gdf5.68
Three distinct cellular layers can be distinguished within the interzone based on cell density, orientation, and gene expression. The joint cavity itself forms at the midline (intermediate layer), while the two outer chondrogenous layers contribute to articular cartilage.69 Genetic lineage tracking experiments have proved useful for determining interzonal cell origin. Articular and epiphyseal plate chondrocytes were shown to have a common Sox9-positive identity during early chondrogenesis,70 however, these cells diverge into distinct populations of interzone and transient cartilage cells from the earliest discernible stage of joint development. Gdf5-positive interzone cells give rise to articular cartilage, ligaments, and the lining of the synovium,69 while matrilin-1-positive non-articular chondrocytes never contribute to the joint.71 The conditions which lead interzonal cells to differentiate into persistent cartilage, rather than proceed through hypertrophy and ossification, are not fully understood; yet, some molecular clues exist. For example, cells in permanent cartilage never express pre-hypertrophic markers such as Indian hedgehog (Ihh) and BMP6.72
There is also a contribution of cells migrating into the intermediate zone of the joint at later stages (E14.5) from surrounding non-cartilaginous tissues,65,73 and cells lying posterior to the nascent elbow joint have been shown to migrate and contribute to joint regeneration following surgical removal in the chick embryo.74 TGF-β type II receptor expressing cells reside at the periphery of the joint until later in development (E16.5) when they contribute to joint structures such as the synovial lining, ligaments, and menisci.75 Peripheral joint cells such as these could be slow-cycling stem/progenitor cells. It is unknown how these cells might relate to stem cells isolated from mature articular cartilage.76
Joint specification and tissue patterning are followed by cavitation, where the midline of the interzone transitions from a cell-dense region to a cell-free fluid space. Key to this transition is localised production of hyaluronan, a diffuse ligand found at the interzone of developing joints and in the lubricating synovial fluid of mature joints.77,78 A transition from cell cohesion to dissociation caused by the increased production of hyaluronan may be responsible for joint cavitation.79,80
The effects of reduced embryo movement on endochondral ossification, rudiment morphology and joint formation
The thin, hypomineralised bones of infants subject to reduced foetal movement indicate that immobilisation affects the process of bone formation.11 Animal models of embryonic immobilisation confirm that endochondral ossification and joint formation are profoundly affected by altered movement (indicated in Figures 2 and 3, respectively; also described in supplementary Table i). In both chick and mouse models, pharmacologically- or genetically-induced immobilisation causes abnormal ossification81,82 and mechanically substandard bones.83 Reduced ossification of tibiotarsi in neuromuscular-blocked chick embryos was accompanied by altered expression of chondrocyte maturation markers Ihh and type X, alpha 1 collagen (Col10a1) in pre-hypertrophic and hypertrophic zones, respectively, suggesting reduced proliferation at the expense of maturation.31 A similar effect was seen in muscleless mouse embryos with reduced and misshapen ossification fronts in some rudiments, in particular the humerus and scapula. Interestingly, forelimbs and proximal rudiments were more affected than hind limbs and distal rudiments, respectively.82 Although different molecular cues present in fore- and hind limbs may contribute to this phenomenon,84 fore- and hind limbs could in fact experience different sources and combinations of mechanical stimulation. Computational modelling predicted that hind limbs experience more stimulation through passive movement (displacement caused by maternal and littermate activity), which might partially compensate for the lack of intrinsic movement.85 This finding, combined with the transient nature of TBBD in infants, suggests that controlled physical therapy has the potential to alleviate the clinical consequences of reduced embryonic movement.
Growth and maturation of embryonic skeletal rudiments are intrinsically linked processes and immobilisation has been shown to affect size and shape by altering the balance of these cellular events. Immobilised mouse and chick embryos demonstrate cartilaginous rudiments which are shorter and have poorly-defined morphological features such as condyles or tuberosities,30-33,82,86-88 corresponding to reduced chondrocyte proliferation in the growth plate,89 terminal condyles,90 and bony eminence of the humeral tuberosity91 under conditions of immobilisation or altered mechanical input. In vitro, proliferation of immature chondrocytes is stimulated by cycles of mechanical stress,92 suggesting that immobilisation during embryonic development could inhibit chondrocyte proliferation in diverse regions of skeletal growth, thereby altering the size and morphology of rudiments and their ultimate functionality. Another aspect of cartilage morphogenesis shown to be disrupted in immobilised zebrafish and mildly affected in mice is the intercalation of chondrocytes into columns organised along the elongating rudiment.43
The occurrence of hip dysplasia following reduced movement in human infants indicates an effect on joint formation14 and early studies on immobilised chick embryos showed dramatic fusion of joints in some cases.93 Now a variety of studies in animal models have shown joint reduction or fusion in limbs as well as vertebrae and head joints.27-30,32,82,86,87,94-99 Although most chick studies have used rigid paralysis, flaccid paralysis also causes joint reduction,28 and hypermobilisation results in joint enlargement.100 These findings collectively demonstrate that appropriate dynamic stimulation is necessary for synovial joint formation.
As outlined earlier, joint development involves a number of phases (Fig. 3) and immobilisation studies have begun to reveal which of these are influenced by movement. Specification is not affected since joint sites, marked by cells expressing Gdf5, appear correctly positioned in immobilised embryos. However, specific joint tissues fail to differentiate appropriately at the joint sites.32,82,99,101 In chicks, immobilisation causes notable joint tissue disorganisation before cavitation: tissue-specific expression of fibroblast growth factor 2 (Fgf2) in the chondrogenous layer and BMP2 in the intermediate layer is lost, while rudiment-specific type II, alpha 1 collagen (Col2aI) and parathyroid hormone-related protein (Pthrp) are expressed across the joint territory,32 which is similar to findings in muscleless mouse embryos.82 These findings show that signalling events which delineate between tissues at developing joints are seriously affected by altered mechanical input, as specified interzone cells lose their joint identity and instead adopt the chondrogenic fate of their neighbours. Joint morphogenesis precedes cavitation102 and, as previously mentioned, is also impacted by immobilisation with alterations to the shape of condyles in immobilised chick embryos.32 Movement therefore contributes to a mechanically-stimulated gene regulatory programme responsible for appropriate rudiment morphogenesis and joint differentiation before cavitation in order to determine synovial joint functionality.
A mechanical basis for the impact of movement: the integration of molecular and biophysical signalling
The mechanical environment
As cells proliferate and differentiate into dedicated tissues, they respond to spatially- and temporally-organised signals including mechanical forces. Skeletal movement delivers mechanical loading in the form of several types of specific physical stimuli such as stress, strain, hydrostatic pressure and fluid flow.81,90 The stimuli generated in developing tissues cannot currently be directly measured, however, finite element (FE) analysis, informed by the morphology of emerging tissues in the limb and direct measurement of mechanical properties of the developing interzone, can predict patterns of stimuli generated across space and time by limb flexion/extension. This modelling shows that dynamic patterns of predicted stimuli correspond spatially and temporally to those tissues and processes most affected by reduced movement.66,81,85,90 For example, peak stimuli at the mid-diaphysis before the onset of ossification spread proximally and distally ahead of the ossification front.81 In the knee joint, FE models predict peak stimuli at the future patella and condyles of the distal femur, as well as dynamic patterns of hydrostatic pressure among the emerging territories of the joint interzone. All of these elements are affected by immobilisation.32 These findings confirm that mechanical forces in the developing skeleton are dynamic and linked to the processes of tissue patterning and maturation.
Efforts to recapitulate developing joint or bone tissue formation in vitro have used mesenchymal stem cells (MSCs) as multi-potent progenitor cells, as they are capable of forming fat, cartilage and bone under appropriate culture conditions.103 As in embryonic tissues, mechanical stimuli have profound effects on the differentiation of stem cells in culture; cyclic pressures at varying magnitudes can induce chondrogenesis or osteogenesis when supplemented with appropriate biochemical signals.104,105 Efforts to recapitulate cartilage and bone tissue formation also make use of adult-derived, induced pluripotency stem (iPS) cells.106,107 These cells are self-renewing and respond to biochemical signals for cartilage and bone formation, indicating that they may be useful for tissue engineering regimes which incorporate mechanical stimulation.
The molecular environment
During endochondral ossification and joint development, a balance of factors affecting multiple signalling pathways, including transforming growth factor beta (TFG-β)/BMP, FGF and Wnt, calibrates an appropriate rate of cartilage maturation.108-111 As a result, distinct regions of the developing endochondral skeleton have characteristic gene expression profiles. Parathyroid hormone-related protein (Pthrp) marks proliferating chondrocytes, while Ihh signalling in pre-hypertrophic chondrocytes spurs on maturation. A feedback loop of these factors guarantees a steady rate of maturation.112-114 Additional signals are also exchanged between the perichondrium and the enclosed cartilage, indicative of a mutually-dependent relationship between these two structures.115,116 Furthermore, hedgehog signalling is also implicated in multiple steps of joint development,117,118 and co-ordinated signalling between the two processes is clear.
Wnt signalling
In both bone and joint development, Wnt signalling is particularly complex. Spatial and temporal control of canonical (β-catenin-based) and non-canonical Wnt signalling is crucial to the correct differentiation of cartilage and bone in both the rudiment and joint. While chondrogenic differentiation in the rudiments requires suppression of the canonical Wnt pathway, canonical Wnt signalling is implicated in chondrocyte hypertrophy and osteoblastogenesis and inhibits proliferation.110,119-121 Canonical Wnt signalling is also active at sites of joint formation, where it inhibits cartilage formation.122-125 In contrast, the non-canonical Wnt planar cell polarity (PCP) and calcium (Ca2+) pathways promote chondrogenesis.120 Of note, activated PCP signalling is necessary for convergent extension of proliferative chondrocytes, which contributes to rudiment morphology.126,127 Later, non-canonical pathways become important for osteoblastogenesis, and may be involved in crosstalk with the canonical Wnt and BMP pathways during bone formation.128-130
TGF-β/BMP signalling
In the cartilage rudiment, canonical TGF-β signalling expands mesenchymal cell populations, primes cells for chondrogenic signals,131-133 encourages chondrocyte proliferation and acts against maturation and differentiation.134-138 As noted earlier, TGF-β type II receptor is required at the joint and might support a joint-specific source of stem cells.75 The BMP subfamily is present throughout the cartilage rudiment and in addition to influencing cartilage formation, it stimulates proliferation and alternately slows or accelerates hypertrophy in maturing chondrocytes both in vivo and in vitro.58,139-145 BMP ligands, including Gdf5, and antagonists are expressed at developing synovial joints, suggesting that a balance of BMP signalling here is necessary to prevent overgrowth of chondrogenous tissues.68,146,147 In addition to the canonical pathways involving Smad transcription factors, BMPs and other TGF-β superfamily ligands have also been shown to induce non-canonical mitogen-activated protein kinase (MAPK) signalling cascades both in vivo and in vitro.148 Crosstalk between the canonical and non-canonical pathways can modulate chondrocyte differentiation and cartilage matrix synthesis.149
FGF signalling
FGF ligands and receptors (FGFRs) are present throughout limb bud development and across different zones of chondrocyte maturation, and take part in chondrogenesis, regulation of cell proliferation, and osteoblastogenesis.150-154 FGF signalling is also found at the perichondrium and the presumptive joint.101,152
Interpretation and integration of mechanical signals by the developing skeletal system
As evidence builds regarding the importance of mechanical stimulation from movement for specific aspects of skeletal development, it is necessary that we clarify the mechanisms that integrate mechanical and molecular signals. We currently cannot explain how the cells of the developing skeletal system can sense and transduce such mechanical stimuli into cell differentiation and tissue patterning outcomes, however, exciting clues are starting to appear on two fronts: first, how the size, shape, and stiffness of the substrate influences how a cell behaves and second, how developmentally important signalling pathways are primed or modulated by mechanical cues.
Wide interest was generated in the influence of mechanical cues on cell differentiation following the demonstration that stem cells are primed for differentiation by the stiffness of their substrate, with rigid, stiff, or soft substrates supporting osteogenic, myogenic, or neurogenic differentiation, respectively.155 This is sensed through integrin receptors coupled to the cytoskeleton and controlled by the Ras-homolog gene family, member A/Rho-associated, coiled-coil containing protein kinase (RhoA/ROCK) pathway which moderates intracellular signalling and is implicated in both chondrogenesis and osteogenesis.156-159 Elegant experiments have shown that cell shape alone, reflective of substrate stiffness, can influence cell differentiation.160,161 Through the physical framework of the cell, the extracellular environment is tethered to the nucleus, meaning that any change in the stiffness of the ECM or deformation of the cell can influence cell behaviour.
It has also been established that a cell can sense its micro-enviroment through primary cilia, present on most cell types.162 These cilia extend into the ECM and can sense mechanical forces such as the bending induced by fluid flow.163 Stimulation of the primary cilium can influence how the cell responds to Ihh, Wnt, and TGFβ signalling164-166 and may have wider effects on other signalling pathways.165 Primary cilia have been detected in the developing skeleton, and their function is necessary for endochondral ossification.167,168 They can also influence the fate of cultured MSCs,169 which suggests a prominent role for sensory cilia in mechanotransduction during tissue patterning and cell fate determination in the developing skeleton.
As described here, the physical state and environment of the cell can influence its transcriptional activity and differentiation, but so too can exposure to signalling pathway ligands, together with known agonists and antagonists. The cytoskeleton is a highly dynamic system which is interlinked with cell signalling processes and could offer a link between mechanical environmental cues and a chemical cellular response within the developing skeletal system.170 For example, the Wnt and TGF-β pathways regulate the actin cytoskeleton and, in turn, these pathways and others are affected by cytoskeletal dynamics.171-174 In immobilised limbs, numerous genes related to cytoskeletal support and architecture are regulated differentially.175 Changes in cytoskeletal architecture in muscleless mice, for example, could affect the transport of intracellular components, which could in turn mediate or amplify alterations in cell signalling to affect cell differentiation.
The ECM of cartilage consists of collagen fibres and glycoproteins, and provides another means to facilitate the mechanosensory reactions of chondrocytes. Collagens provide structure to the cartilage, as do glycoproteins, which protect the tissue against compression and mechanical stress and can differentially regulate the diffusion and binding of signalling.176 Maturation of chondrocytes is accompanied and, perhaps, regulated by changes in ECM composition during endochondral ossification.62 Focal adhesions mediate cell–matrix interactions via integrins, which link extracellular matrix components to intracellular actin filaments. In addition to physical linkers, the complex contains the signalling molecules which control focal adhesion turnover and breakdown of the cell–matrix connection. Proper cell–matrix junctions are necessary for chondrocyte differentiation.177
Integrins and ion channels are other potentially mechanosensitive components which could transduce physical pressures to biochemical intracellular signals to regulate growth and development of chondrocytes.178-180 Blocking integrins and stretch-activated ion channels inhibits downstream signalling and cellular proliferation in chondrocytes.92,181 Additionally, mechanical loading can alter integrin expression or matrix binding through the release of soluble signalling molecules.182
Wnt signalling and mechanical stimulation
We recently highlighted the potential role of the Wnt signalling pathway in mechanotransduction in the developing skeletal rudiment.175 Comparison of the transcriptomes in muscleless, compared with control, mouse humeri at TS23, when ossification commences and the skeletal phenotype is first recorded, shows enrichment for genes associated with cytoskeletal architecture, cell signalling, and development and differentiation. Alteration of these biological processes in muscleless mouse skeletal rudiments hints at a relationship between cytoskeletal function and cell signalling during patterning of skeletal tissues. Furthermore, among the multiple cell signalling pathways affected in muscleless embryos, the greatest number of differentially regulated genes are associated with the Wnt pathway, and alteration in the spatial expression of several key pathway components was also documented, further suggesting that disruption of Wnt signalling could be responsible for abnormal skeletal patterning during mechanical inhibition.175
As described earlier, the Wnt pathway is well documented as an important driver of embryonic patterning and development. However, its integration with mechanostimulation in vivo99,175,183-185 and in vitro184-187 via both direct and downstream regulation of other signalling cascades, opens a new avenue of exploration for this important regulatory system.
Potential therapies for skeletal developmental defects and skeletal regeneration
The vertebrate musculoskeletal system has evolved to construct functional and adaptable components, using available molecular and biophysical cues. Unravelling the impact of mechanical stimulation generated by embryonic movement will advance our fundamental understanding of skeletal development which will reveal in particular how the system integrates multiple types of information at the cellular level. Furthermore, discoveries in this area have evident biomedical implications and could lead to the development of novel therapeutics to inform treatments for restricted foetal movement in utero and also for age- or injury-related degeneration of skeletal tissues.
In the case of congenital problems caused by reduced movement such as low bone mineral density or joint dysplasia, a better understanding of the type and magnitude of stimulation required for normal skeletogenesis could lead to the development of appropriate physical therapy regimes in utero to substitute for autogenerated movement.85 Post-natal supplemented diets do not seem to be effective enough to reverse low BMD that is already present,188 suggesting a need for movement-based therapies. This is encouraged by the success of gentle yet effective exercise programmes to treat reduced BMD in premature babies.189-191 This represents a simple but meaningful method for caregivers and parents to ensure the physical fitness of affected newborns, with the potential for lifelong results.192 Another possibility for therapeutic intervention is treatment with ultrasound, which is suggested to mimic the effect of mechanical loading on bone formation.193 Although treatment for joint fusion can be quite invasive, a combination of surgical treatment and physical therapy, started during infancy, can reduce lifelong impediments to movement in individuals with severe contractures.194,195
Mechanical forces are not just important during development. Strong bones and smoothly-articulating joints are interrelated features of a healthy skeleton and facilitate movement and an active life. Biophysical cues generated by movement in turn feed back to maintain and remodel skeletal tissues throughout life.196,197 With age, we not only become less mobile, but also our skeletal tissues become less responsive to loading.198 Although changes in the hormonal environment are certainly important in the reduced response and onset of osteoporosis,199-201 the current emphasis on hormonal replacement treatment could be augmented by uncovering the correct stimulation required to shift the balance between resorption and deposition of bone, and thus enhance skeletal integrity.202
Appropriate and effective administration of therapeutic regimes to affected individuals will require a precise understanding of both molecular and mechanical controls of skeletogenesis, as well as the complex interplay between these types of cues during the development and maintenance of healthy bones and joints. As mice and chicks are particularly useful for investigating the effect of mechanical stimulation on skeletal patterning, signalling and cellular events in these models are currently being explored with the aspiration of applying such findings to therapeutics in humans. The discovery that appropriate mechanical signals are instrumental in guiding correct differentiation of permanent articular cartilage is of particular importance in informing regimes for the generation of articular cartilage from stem cells for joint replacement therapies. Currently, adult-derived stem cells cannot be induced to form permanent cartilage in vitro, but instead proceed to hypertrophy, and therefore cannot be used for replacement of articular cartilage, necessitating prosthetic replacement of the joint in the case of osteoarthritis and joint injury. Understanding how dynamic mechanical stimuli guide the formation of stable articular cartilage in the embryo would have an enormous potential benefit for the establishment of protocols to guide stem cell differentiation and in developing less invasive and more sustainable therapies in such cases. We still have much to learn about how cells integrate molecular and mechanical signals to form particular cell types stably, and how this knowledge can be translated clinically. Nevertheless, research in this area holds great promise for future practical applications.
1 Frost HM . Wolff's Law and bone's structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod1994;64:175–188.CrossrefPubMed Google Scholar
2 Chen JH , LiuC, YouL, SimmonsCA. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. J Biomech2010;43:108–118.CrossrefPubMed Google Scholar
3 Frost HM . From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec2001;262:398–419.CrossrefPubMed Google Scholar
4 de Vries JI , FongBF. Normal fetal motility: an overview. Ultrasound Obstet Gynecol2006;27:701–711.CrossrefPubMed Google Scholar
5 Shinohara H , NaoraH, HashimotoR, HattaT, TanakaO. Development of the innervation pattern in the upper limb of staged human embryos. Acta Anat (Basel)1990;138:265–269.CrossrefPubMed Google Scholar
6 Lazjuk G , CherstvoyED, LurieIW, NedzvedMK. Pulmonary hypoplasia, multiple ankyloses, and camptodactyly: one syndrome or some related forms?Helv Paediatr Acta1978;33:73–79. Google Scholar
7 Pena SD , ShokeirMH. Syndrome of camptodactyly, multiple ankyloses, facial anomalies and pulmonary hypoplasia--further delineation and evidence for autosomal recessive inheritance. Birth Defects Orig Artic Ser1976;12:201–208.PubMed Google Scholar
8 Hageman G , WillemseJ, van KetelBA, BarthPG, LindhoutD. The heterogeneity of the Pena-Shokeir syndrome. Neuropediatrics1987;18:45–50.CrossrefPubMed Google Scholar
9 Moessinger AC . Fetal akinesia deformation sequence: an animal model. Pediatrics1983;72:857–863.PubMed Google Scholar
10 Hall JG . Pena-Shokeir phenotype (fetal akinesia deformation sequence) revisited. Birth Defects Res A Clin Mol Teratol2009;85:677–694.CrossrefPubMed Google Scholar
11 Rodríguez JI , Garcia-AlixA, PalaciosJ, PaniaguaR. Changes in the long bones due to fetal immobility caused by neuromuscular disease. A radiographic and histological study. J Bone Joint Surg [Am]1988;70-A:1052–1060.PubMed Google Scholar
12 Rodríguez JI , PalaciosJ, García-AlixA, PastorI, PaniaguaR. Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int1988;43:335–339.CrossrefPubMed Google Scholar
13 Miller ME , HangartnerTN. Temporary brittle bone disease: association with decreased fetal movement and osteopenia. Calcif Tissue Int1999;64:137–143.CrossrefPubMed Google Scholar
14 Aronsson DD , GoldbergMJ, KlingTF Jr, RoyDR. Developmental dysplasia of the hip. Pediatrics1994;94:201–208.CrossrefPubMed Google Scholar
15 Paterson CR , BurnsJ, McAllionSJ. Osteogenesis imperfecta: the distinction from child abuse and the recognition of a variant form. Am J Med Genet1993;45:187–192.CrossrefPubMed Google Scholar
16 Paterson CR , MonkEA. Temporary brittle bone disease: relationship between clinical findings and judicial outcome. Pediatr Rep2011;3:24.CrossrefPubMed Google Scholar
17 Miller ME . The lesson of temporary brittle bone disease: all bones are not created equal. Bone2003;33:466–474. Google Scholar
18 Marini JC , BlissettAR. New genes in bone development - what's new in osteogenesis imperfecta. J Clin Endocrinol Metab2013;98:3095–3103. Google Scholar
19 Forlino A , CabralWA, BarnesAM, MariniJC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol2011;7:540–557.CrossrefPubMed Google Scholar
20 Theiler K The House Mouse: Atlas of Embryonic Development. New York: Springer-Verlag, 1989. Google Scholar
21 Hamburger V , HamiltonHL. A series of normal stages in the development of the chick embryo. J Morphol1951;88:49–92.CrossrefPubMed Google Scholar
22 Hamburger V , BalabanM. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Developmental Biology1963;7:533–545.CrossrefPubMed Google Scholar
23 Martin P . Tissue patterning in the developing mouse limb. International Journal of Developmental Biology1990;34:323–336.PubMed Google Scholar
24 Suzue T , ShinodaY. Highly reproducible spatiotemporal patterns of mammalian embryonic movements at the developmental stage of the earliest spontaneous motility. Eur J Neurosci1999;11:2697–2710.CrossrefPubMed Google Scholar
25 Marturano JE , ArenaJD, SchillerZA, GeorgakoudiI, KuoCK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci USA2013;110:6370–6375.CrossrefPubMed Google Scholar
26 Schomann T , QunneisF, WideraD, KaltschmidtC, KaltschmidtB. Improved method for ex ovo-cultivation of developing chicken embryos for human stem cell xenografts. Stem Cells Int2013;2013:960958.CrossrefPubMed Google Scholar
27 Drachman DB , SokoloffL. The role of movement in embryonic joint development. Developmental Biology1966;14:401–420. Google Scholar
28 Osborne AC , LambKJ, LewthwaiteJC, DowthwaiteGP, PitsillidesAA. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J Musculoskelet Neuronal Interact2002;2:448–456.PubMed Google Scholar
29 Lelkes G . Experiments in vitro on the role of movement in the development of joints. J Embryol Exp Morphol1958;6:183–186.PubMed Google Scholar
30 Mitrovic D . Development of the articular cavity in paralyzed chick embryos and in chick embryo limb buds cultured on chorioallantoic membranes. Acta Anat (Basel)1982;113:313–324.CrossrefPubMed Google Scholar
31 Nowlan NC , PrendergastPJ, MurphyP. Identification of mechanosensitive genes during embryonic bone formation. PLoS Comput Biol2008;4:1000250.CrossrefPubMed Google Scholar
32 Roddy KA , PrendergastPJ, MurphyP. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS One2011;6:17526.CrossrefPubMed Google Scholar
33 Hall BK , HerringSW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol1990;206:45–56.CrossrefPubMed Google Scholar
34 Nolte PA , Klein-NulendJ, AlbersGH, et al.Low-intensity ultrasound stimulates endochondral ossification in vitro. J Orthop Res2001;19:301–307.CrossrefPubMed Google Scholar
35 Henstock JR , RotherhamM, RoseJB, El HajAJ. Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone2013;53:468–477.CrossrefPubMed Google Scholar
36 Rodríguez JI , PalaciosJ, RuizA, et al.Morphological changes in long bone development in fetal akinesia deformation sequence: an experimental study in curarized rat fetuses. Teratology1992;45:213–221.CrossrefPubMed Google Scholar
37 Rudnicki MA , SchnegelsbergPN, SteadRH, et al.MyoD or Myf-5 is required for the formation of skeletal muscle. Cell1993;75:1351–1359. Google Scholar
38 Franz T , KotharyR, SuraniMA, HalataZ, GrimM. The Splotch mutation interferes with muscle development in the limbs. Anat Embryol (Berl)1993;187:153–160.CrossrefPubMed Google Scholar
39 Laurin M , FradetN, BlangyA, et al.The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci USA2008;105:15446–15451.CrossrefPubMed Google Scholar
40 Pai AC . Developmental genetics of a lethal mutation, muscular dysgenesis (mdg), in the mouse: II. Developmental analysis. Dev Biol1965;11:93–109. Google Scholar
41 Nowlan NC , SharpeJ, RoddyKA, PrendergastPJ, MurphyP. Mechanobiology of embryonic skeletal development: Insights from animal models. Birth Defects Res C Embryo Today2010;90:203–213.CrossrefPubMed Google Scholar
42 Hinits Y , WilliamsVC, SweetmanD, et al.Defective cranial skeletal development, larval lethality and haploinsufficiency in Myod mutant zebrafish. Dev Biol2011;358:102–112.CrossrefPubMed Google Scholar
43 Shwartz Y , FarkasZ, SternT, AszódiA, ZelzerE. Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev Biol2012;370:154–163.CrossrefPubMed Google Scholar
44 van der Meulen T , SchipperH, van LeeuwenJL, KranenbargS. Effects of decreased muscle activity on developing axial musculature in nicb107 mutant zebrafish (Danio rerio). J Exp Biol2005;208:3675–3687.CrossrefPubMed Google Scholar
45 O’Rahilly R , MullerF. Developmental Stages in Human Embryos. Carnegie Institution of Washington1987:Pub 637.CrossrefPubMed Google Scholar
46 Hall BK , MiyakeT. All for one and one for all -- condensations and the initiation of skeletal development. Bioessays2000;22:138–147. Google Scholar
47 Holder N . An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol1977;39:115–127.PubMed Google Scholar
48 Mitrovic D . Development of the diarthrodial joints in the rat embryo. Am J Anat1978;151:475–485.CrossrefPubMed Google Scholar
49 Bi W , DengJM, ZhangZ, BehringerRR, de CrombruggheB. Sox9 is required for cartilage formation. Nat Genet1999;22:85–89.CrossrefPubMed Google Scholar
50 Kosher RA , KulykWM, GaySW. Collagen gene expression during limb cartilage differentiation. J Cell Biol1986;102:1151–1156.CrossrefPubMed Google Scholar
51 Watanabe H , YamadaY, KimataK. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem1998;124:687–693.CrossrefPubMed Google Scholar
52 Handler M , YurchencoPD, IozzoRV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn1997;210:130–145.CrossrefPubMed Google Scholar
53 Yin M , PacificiM. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev Dyn2001;222:522–533.CrossrefPubMed Google Scholar
54 Tickle C . Vertebrate limb development. Curr Opin Genet Dev1995;5:478–484.CrossrefPubMed Google Scholar
55 Zeller R , López-RíosJ, ZunigaA. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet2009;10:845–858.CrossrefPubMed Google Scholar
56 Raspopovic J , MarconL, RussoL, SharpeJ. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science2014;345:566–570. Google Scholar
57 Long F , LinsenmayerTF. Regulation of growth region cartilage proliferation and differentiation by perichondrium. Development1998;125:1067–1073.CrossrefPubMed Google Scholar
58 Minina E , WenzelHM, KreschelC, et al.BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development2001;128:4523–4534.CrossrefPubMed Google Scholar
59 Colnot C , LuC, HuD, HelmsJA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol2004;269:55–69.CrossrefPubMed Google Scholar
60 Schmid TM , LinsenmayerTF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol1985;100:598–605.CrossrefPubMed Google Scholar
61 Vu TH , ShipleyJM, BergersG, et al.MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell1998;93:411–422.CrossrefPubMed Google Scholar
62 Ortega N , BehonickDJ, WerbZ. Matrix remodeling during endochondral ossification. Trends Cell Biol2004;14:86–93.CrossrefPubMed Google Scholar
63 Paiva KB , GranjeiroJM. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch Biochem Biophys2014;561:74–87.CrossrefPubMed Google Scholar
64 Pitsillides AA , AshhurstDE. A critical evaluation of specific aspects of joint development. Dev Dyn2008;237:2284–2294.CrossrefPubMed Google Scholar
65 Pacifici M , KoyamaE, ShibukawaY, et al.Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci2006;1068:74–86.CrossrefPubMed Google Scholar
66 Roddy KA , NowlanNC, PrendergastPJ, MurphyP. 3D representation of the developing chick knee joint: a novel approach integrating multiple components. J Anat2009;214:374–387.CrossrefPubMed Google Scholar
67 Kan A , TabinCJ. c-Jun is required for the specification of joint cell fates. Genes Dev2013;27:514–524.CrossrefPubMed Google Scholar
68 Storm EE , KingsleyDM. GDF5 coordinates bone and joint formation during digit development. Dev Biol1999;209:11–27.CrossrefPubMed Google Scholar
69 Koyama E , ShibukawaY, NagayamaM, et al.A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol2008;316:62–73.CrossrefPubMed Google Scholar
70 Soeda T , DengJM, de CrombruggheB, et al.Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis2010;48:635–644.CrossrefPubMed Google Scholar
71 Hyde G , DoverS, AszodiA, et al.Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol2007;304:825–833.CrossrefPubMed Google Scholar
72 Eames BF , de la FuenteL, HelmsJA. Molecular ontogeny of the skeleton. Birth Defects Res C Embryo Today2003;69:93–101.CrossrefPubMed Google Scholar
73 Hyde G , Boot-HandfordRP, WallisGA. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J Anat2008;213:531–538.CrossrefPubMed Google Scholar
74 Özpolat BD , ZapataM, Daniel FrugéJ, et al.Regeneration of the elbow joint in the developing chick embryo recapitulates development. Dev Biol2012;372:229–238.CrossrefPubMed Google Scholar
75 Li T , LongobardiL, MyersTJ, et al.Joint TGF-beta type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev2013;22:1342–1359. Google Scholar
76 Archer CW , WilliamsR, NelsonL, KhanIM. Articular Cartilage-Derived Stem Cells: Identification, Characterisation and their Role in Spontaneous Repair. Rheumatol Curr Res2012;S3:005. Google Scholar
77 Toole BP , JacksonG, GrossJ. Hyaluronate in morphogenesis: inhibition of chondrogenesis in vitro. Proc Natl Acad Sci USA1972;69:1384–1386.CrossrefPubMed Google Scholar
78 Edwards JC , WilkinsonLS, JonesHM, et al.The formation of human synovial joint cavities: a possible role for hyaluronan and CD44 in altered interzone cohesion. J Anat1994;185(Pt2):355–367.PubMed Google Scholar
79 Underhill C , DorfmanA. The role of hyaluronic acid in intercellular adhesion of cultured mouse cells. Exp Cell Res1978;117:155–164.CrossrefPubMed Google Scholar
80 Dowthwaite GP , FlanneryCR, FlannellyJ, et al.A mechanism underlying the movement requirement for synovial joint cavitation. Matrix Biol2003;22:311–322.CrossrefPubMed Google Scholar
81 Nowlan NC , MurphyP, PrendergastPJ. A dynamic pattern of mechanical stimulation promotes ossification in avian embryonic long bones. J Biomech2008;41:249–258.CrossrefPubMed Google Scholar
82 Nowlan NC , BourdonC, DumasG, et al.Developing bones are differentially affected by compromised skeletal muscle formation. Bone2010;46:1275–1285.CrossrefPubMed Google Scholar
83 Sharir A , SternT, RotC, ShaharR, ZelzerE. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development2011;138:3247–3259.CrossrefPubMed Google Scholar
84 Rodriguez-Esteban C , TsukuiT, YoneiS, et al.The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature1999;398:814–818.CrossrefPubMed Google Scholar
85 Nowlan NC , DumasG, TajbakhshS, PrendergastPJ, MurphyP. Biophysical stimuli induced by passive movements compensate for lack of skeletal muscle during embryonic skeletogenesis. Biomech Model Mechanobiol2012;11:207–219.CrossrefPubMed Google Scholar
86 Hosseini A , HoggDA. The effects of paralysis on skeletal development in the chick embryo. I. General effects. J Anat1991;177:159–168.PubMed Google Scholar
87 Rot-Nikcevic I , ReddyT, DowningKJ, et al.Myf5-/- MyoD-/- amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev Genes Evol2006;216:1–9. Google Scholar
88 Gomez C , DavidV, PeetNM, et al.Absence of mechanical loading in utero influences bone mass and architecture but not innervation in Myod-Myf5-deficient mice. J Anat2007;210:259–271. Google Scholar
89 Germiller JA , GoldsteinSA. Structure and Function of Embryonic Growth Plate in the Absence of Functioning Skeletal Muscle. J Orthop Res1997;15:362–370.CrossrefPubMed Google Scholar
90 Roddy KA , KellyGM, van EsMH, MurphyP, PrendergastPJ. Dynamic patterns of mechanical stimulation co-localise with growth and cell proliferation during morphogenesis in the avian embryonic knee joint. J Biomech2011;44:143–149.CrossrefPubMed Google Scholar
91 Blitz E , ViukovS, SharirA, et al.Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell2009;17:861–873.CrossrefPubMed Google Scholar
92 Wu QQ , ChenQ. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res2000;256:383–391.CrossrefPubMed Google Scholar
93 Persson M . The role of movements in the development of sutural and diarthrodial joints tested by long-term paralysis of chick embryos. J Anat1983;137(Pt3):591–599.PubMed Google Scholar
94 Fell HB , CantiRG. Experiments on the development in vitro of the avian knee joint. Proc R Soc B1934;116:316–351. Google Scholar
95 Hamburger V , WaughM. The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Physiological Zoology1940;13:367–380. Google Scholar
96 Murray PD , DrachmanDB. The role of movement in the development of joints and related structures: the head and neck in the chick embryo. J Embryol Exp Morphol1969;22:349–371.PubMed Google Scholar
97 Ruano-Gil D , Nardi-VilardagaJ, Tejedo-MateuA. Influence of extrinsic factors on the development of the articular system. Acta Anat (Basel)1978;101:36–44.CrossrefPubMed Google Scholar
98 Mikic B , WongM, ChiquetM, HunzikerEB. Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. J Orthop Res2000;18:406–415.CrossrefPubMed Google Scholar
99 Kahn J , ShwartzY, BlitzE, et al.Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell2009;16:734–743.CrossrefPubMed Google Scholar
100 Ruano-Gil D , Nardi-VilardagaJ, Teixidor-JohéA. Embryonal hypermobility and articular development. Acta Anat (Basel)1985;123:90–92.CrossrefPubMed Google Scholar
101 Kavanagh E , ChurchVL, OsborneAC, et al.Differential regulation of GDF-5 and FGF-2/4 by immobilisation in ovo exposes distinct roles in joint formation. Dev Dyn2006;235:826–834.CrossrefPubMed Google Scholar
102 Nowlan NC , SharpeJ. Joint shape morphogenesis precedes cavitation of the developing hip joint. J Anat2014;224:482–489.CrossrefPubMed Google Scholar
103 Pittenger MF , MackayAM, BeckSC, et al.Multilineage potential of adult human mesenchymal stem cells. Science1999;284:143–147.CrossrefPubMed Google Scholar
104 Kelly DJ , JacobsCR. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today2010;90:75–85.CrossrefPubMed Google Scholar
105 Delaine-Smith RM , ReillyGC. Mesenchymal stem cell responses to mechanical stimuli. Muscles Ligaments Tendons J2012;2:169–180.PubMed Google Scholar
106 Ko JY , KimKI, ParkS, ImGI. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials2014;35:3571–3581.CrossrefPubMed Google Scholar
107 Tsumaki N , OkadaM, YamashitaA. iPS cell technologies and cartilage regeneration. Bone2015;70:48–54.CrossrefPubMed Google Scholar
108 Ornitz DM . FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev2005;16:205–213.CrossrefPubMed Google Scholar
109 Pogue R , LyonsK. BMP signaling in the cartilage growth plate. Curr Top Dev Biol2006;76:1–48.CrossrefPubMed Google Scholar
110 Guo X , MakKK, TaketoMM, YangY. The Wnt/beta-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS One2009;4:6067.CrossrefPubMed Google Scholar
111 Minina E , KreschelC, NaskiMC, OrnitzDM, VortkampA. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell2002;3:439–449.CrossrefPubMed Google Scholar
112 Karaplis AC , LuzA, GlowackiJ, et al.Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev1994;8:277–289.CrossrefPubMed Google Scholar
113 Lanske B , KaraplisAC, LeeK, et al.PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science1996;273:663–666.CrossrefPubMed Google Scholar
114 Vortkamp A , LeeK, LanskeB, et al.Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science1996;273:613–622.CrossrefPubMed Google Scholar
115 St-Jacques B , HammerschmidtM, McMahonAP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev1999;13:2072–2086.CrossrefPubMed Google Scholar
116 Chung UI , SchipaniE, McMahonAP, KronenbergHM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest2001;107:295–304.CrossrefPubMed Google Scholar
117 Koyama E , OchiaiT, RountreeRB, et al.Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Ann N Y Acad Sci2007;1116:100–112.CrossrefPubMed Google Scholar
118 Liu J , LiQ, KuehnMR, et al.Sonic hedgehog signaling directly targets Hyaluronic Acid Synthase 2, an essential regulator of phalangeal joint patterning. Dev Biol2013;375:160–171.CrossrefPubMed Google Scholar
119 Rudnicki JA , BrownAM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol1997;185:104–118.CrossrefPubMed Google Scholar
120 Hartmann C , TabinCJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development2000;127:3141–3159.CrossrefPubMed Google Scholar
121 Enomoto-Iwamoto M , KitagakiJ, KoyamaE, et al.The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol2002;251:142–156.CrossrefPubMed Google Scholar
122 Guo X , DayTF, JiangX, et al.Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev2004;18:2404–2417.CrossrefPubMed Google Scholar
123 Ryu JH , KimSJ, KimSH, et al.Regulation of the chondrocyte phenotype by beta-catenin. Development2002;129:5541–5550.CrossrefPubMed Google Scholar
124 Später D , HillTP, O'sullivanRJ, et al.Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development2006;133:3039–3049.CrossrefPubMed Google Scholar
125 Day TF , GuoX, Garrett-BealL, YangY. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell2005;8:739–750.CrossrefPubMed Google Scholar
126 Li Y , DudleyAT. Noncanonical frizzled signaling regulates cell polarity of growth plate chondrocytes. Development2009;136:1083–1092.CrossrefPubMed Google Scholar
127 Wang B , SinhaT, JiaoK, SerraR, WangJ. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet2011;20:271–285.CrossrefPubMed Google Scholar
128 Liu Y , BhatRA, Seestaller-WehrLM, et al.The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol2007;21:376–387.CrossrefPubMed Google Scholar
129 Okamoto M , UdagawaN, UeharaS, et al.Noncanonical Wnt5a enhances Wnt/[bgr]-catenin signaling during osteoblastogenesis. Sci Rep2014;4:4493. Google Scholar
130 Nemoto E , EbeY, KanayaS, et al.Wnt5a signaling is a substantial constituent in bone morphogenetic protein-2-mediated osteoblastogenesis. Biochem Biophys Res Commun2012;422:627–632.CrossrefPubMed Google Scholar
131 Derynck R , AkhurstRJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol2007;9:1000–1004.CrossrefPubMed Google Scholar
132 Karamboulas K , DranseHJ, UnderhillTM. Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFbeta signals. J Cell Sci2010;123:2068–2076.CrossrefPubMed Google Scholar
133 Keller B , YangT, ChenY, et al.Interaction of TGFbeta and BMP signaling pathways during chondrogenesis. PLoS One2011;6:16421. Google Scholar
134 Serra R , JohnsonM, FilvaroffEH, et al.Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol1997;139:541–552.CrossrefPubMed Google Scholar
135 Yang X , ChenL, XuX, et al.TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol2001;153:35–46.CrossrefPubMed Google Scholar
136 Pelton RW , SaxenaB, JonesM, MosesHL, GoldLI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol1991;115:1091–1105.CrossrefPubMed Google Scholar
137 Matsunaga S , YamamotoT, FukumuraK. Temporal and spatial expressions of transforming growth factor-betas and their receptors in epiphyseal growth plate. Int J Oncol1999;14:1063–1067.CrossrefPubMed Google Scholar
138 Sakou T , OnishiT, YamamotoT, et al.Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res1999;14:1145–1152.CrossrefPubMed Google Scholar
139 Duprez D , BellEJ, RichardsonMK, et al.Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev1996;57:145–157.CrossrefPubMed Google Scholar
140 Cheng H , JiangW, PhillipsFM, et al.Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg [Am]2003;85-A:1544–1552.CrossrefPubMed Google Scholar
141 Yoon BS , LyonsKM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem2004;93:93–103.CrossrefPubMed Google Scholar
142 Kobayashi T , LyonsKM, McMahonAP, KronenbergHM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci USA2005;102:18023–18027.CrossrefPubMed Google Scholar
143 Lavery K , SwainP, FalbD, Alaoui-IsmailiMH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem2008;283:20948–20958.CrossrefPubMed Google Scholar
144 Mailhot G , YangM, Mason-SavasA, et al.BMP-5 expression increases during chondrocyte differentiation in vivo and in vitro and promotes proliferation and cartilage matrix synthesis in primary chondrocyte cultures. J Cell Physiol2008;214:56–64.CrossrefPubMed Google Scholar
145 Caron MM , EmansPJ, CremersA, et al.Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis Cartilage2013;21:604–613.CrossrefPubMed Google Scholar
146 Brunet LJ , McMahonJA, McMahonAP, HarlandRM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science1998;280:1455–1457.CrossrefPubMed Google Scholar
147 Edwards CJ , Francis-WestPH. Bone morphogenetic proteins in the development and healing of synovial joints. Semin Arthritis Rheum2001;31:33–42.CrossrefPubMed Google Scholar
148 Chang L , KarinM. Mammalian MAP kinase signalling cascades. Nature2001;410:37–40.CrossrefPubMed Google Scholar
149 Watanabe H , de CaesteckerMP, YamadaY. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem2001;276:14466–14473.CrossrefPubMed Google Scholar
150 Ohbayashi N , ShibayamaM, KurotakiY, et al.FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev2002;16:870–879.CrossrefPubMed Google Scholar
151 Liu Z , XuJ, ColvinJS, OrnitzDM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev2002;16;859-869.:.CrossrefPubMed Google Scholar
152 Davidson D , BlancA, FilionD, et al.Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem2005;280:20509–20515.CrossrefPubMed Google Scholar
153 Peters KG , WernerS, ChenG, WilliamsLT. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development1992;114:233–243.CrossrefPubMed Google Scholar
154 Iwata T , ChenL, LiC, et al.A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet2000;9:1603–1613.CrossrefPubMed Google Scholar
155 Engler AJ , SenS, SweeneyHL, DischerDE. Matrix elasticity directs stem cell lineage specification. Cell2006;126:677–689.CrossrefPubMed Google Scholar
156 Mammoto A , MammotoT, IngberDE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci2012;125:3061–3073.CrossrefPubMed Google Scholar
157 Provenzano PP , KeelyPJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci2011;124:1195–1205.CrossrefPubMed Google Scholar
158 Woods A , WangG, BeierF. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem2005;280:11626–11634.CrossrefPubMed Google Scholar
159 Arnsdorf EJ , TummalaP, KwonRY, JacobsCR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci2009;122:546–553.CrossrefPubMed Google Scholar
160 McBeath R , PironeDM, NelsonCM, BhadrirajuK, ChenCS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell2004;6:483–495.CrossrefPubMed Google Scholar
161 Mammoto T , MammotoA, TorisawaYS, et al.Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell2011;21:758–769.CrossrefPubMed Google Scholar
162 Singla V , ReiterJF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science2006;313:629–633.CrossrefPubMed Google Scholar
163 Malone AM , AndersonCT, TummalaP, et al.Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA2007;104:13325–13330.CrossrefPubMed Google Scholar
164 Wong SY , ReiterJF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol2008;85:225–260.CrossrefPubMed Google Scholar
165 He X . Cilia put a brake on Wnt signalling. Nat Cell Biol2008;10:11–13.CrossrefPubMed Google Scholar
166 Clement CA , AjbroKD, KoefoedK, et al.TGF-beta signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep2013;3:1806–1814. Google Scholar
167 Anderson CT , CastilloAB, BrugmannSA, et al.Primary cilia: cellular sensors for the skeleton. Anat Rec (Hoboken)2008;291:1074–1078.CrossrefPubMed Google Scholar
168 Haycraft CJ , SerraR. Cilia involvement in patterning and maintenance of the skeleton. Curr Top Dev Biol2008;85:303–332.CrossrefPubMed Google Scholar
169 Hoey DA , TormeyS, RamcharanS, O'BrienFJ, JacobsCR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells2012;30:2561–2570.CrossrefPubMed Google Scholar
170 Alenghat FJ , IngberDE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE2002;2002:6.CrossrefPubMed Google Scholar
171 Akiyama T , KawasakiY. Wnt signalling and the actin cytoskeleton. Oncogene2006;25:7538–7544.CrossrefPubMed Google Scholar
172 Matsumoto S , FumotoK, OkamotoT, KaibuchiK, KikuchiA. Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J2010;29:1192–1204.CrossrefPubMed Google Scholar
173 Edlund S , LandströmM, HeldinCH, AspenströmP. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell2002;13:902–914.CrossrefPubMed Google Scholar
174 Wang YK , YuX, CohenDM, et al.Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev2012;21;1176-1186.:.CrossrefPubMed Google Scholar
175 Rolfe RA , NowlanNC, KennyEM, et al.Identification of mechanosensitive genes during skeletal development: alteration of genes associated with cytoskeletal rearrangement and cell signalling pathways. BMC Genomics2014;15:48.CrossrefPubMed Google Scholar
176 Perrimon N , HäckerU. Wingless, hedgehog and heparan sulfate proteoglycans. Development2004;131:2509–2511.CrossrefPubMed Google Scholar
177 Koshimizu T , KawaiM, KondouH, et al.Vinculin functions as regulator of chondrogenesis. J Biol Chem2012;287:15760–15775.CrossrefPubMed Google Scholar
178 Martinac B . Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci2004;117:2449–2460.CrossrefPubMed Google Scholar
179 Ross TD , CoonBG, YunS, et al.Integrins in mechanotransduction. Curr Opin Cell Biol2013;25:613–618.CrossrefPubMed Google Scholar
180 Ramage L , NukiG, SalterDM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports2009;19:457–469.CrossrefPubMed Google Scholar
181 Chowdhury TT , SalterDM, Bader DL LeeDA. Integrin-mediated mechanotransduction processes in TGFbeta-stimulated monolayer-expanded chondrocytes. Biochem Biophys Res Commun2004;318:873–881.CrossrefPubMed Google Scholar
182 Streuli CH , AkhtarN. Signal co-operation between integrins and other receptor systems. Biochem J2009;418:491–506.CrossrefPubMed Google Scholar
183 Robinson JA , Chatterjee-KishoreM, YaworskyPJ, et al.Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem2006;281:31720–31728.CrossrefPubMed Google Scholar
184 Hens JR , WilsonKM, DannP, et al.TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res2005;20:1103–1113.CrossrefPubMed Google Scholar
185 Case N , MaM, SenB, et al.Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem2008;283:29196–29205.CrossrefPubMed Google Scholar
186 Caverzasio J , ManenD. Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology2007;148:5323–5330.CrossrefPubMed Google Scholar
187 Bikkavilli RK , FeiginME, MalbonCC. G alpha o mediates WNT-JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J Cell Sci2008;121:234–245.CrossrefPubMed Google Scholar
188 Weiler HA , Fitzpatrick-WongSC, SchellenbergJM, et al.Minimal enteral feeding within 3 d of birth in prematurely born infants with birth weight < or = 1200 g improves bone mass by term age. Am J Clin Nutr2006;83:155–162. Google Scholar
189 Moyer-Mileur L , LuetkemeierM, BoomerL, ChanG. Effect of physical activity on bone mineralization in premature infants. J Pediatr1995;127:620–625.CrossrefPubMed Google Scholar
190 Eliakim A , DolfinT, WeissE, et al.The effects of exercise on body weight and circulating leptin in premature infants. J Perinatol2002;22:550–554.CrossrefPubMed Google Scholar
191 Nemet D , DolfinT, LitmanowitzI, et al.Evidence for exercise-induced bone formation in premature infants. Int J Sports Med2002;23:82–85.CrossrefPubMed Google Scholar
192 McQueen D , LakesK, RichJ, et al.Feasibility of a caregiver-assisted exercise program for preterm infants. J Perinat Neonatal Nurs2013;27:184–192.CrossrefPubMed Google Scholar
193 Perry MJ , ParryLK, BurtonVJ, et al.Ultrasound mimics the effect of mechanical loading on bone formation in vivo on rat ulnae. Med Eng Phys2009;31:42–47.CrossrefPubMed Google Scholar
194 Lampasi M , AntonioliD, DonzelliO. Management of knee deformities in children with arthrogryposis. Musculoskelet Surg2012;96:161–169.CrossrefPubMed Google Scholar
195 Binkiewicz-Glinska A , Sobierajska-RekA, BakulaS, et al.Arthrogryposis in infancy, multidisciplinary approach: case report. BMC Pediatr2013;13:184.CrossrefPubMed Google Scholar
196 Vanwanseele B , LucchinettiE, StussiE. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage2002;10:408–419.CrossrefPubMed Google Scholar
197 Spencer HT , BowenRE, CaputoK, GreenTA, LawrenceJF. Bone mineral density and functional measures in patients with arthrogryposis. J Pediatr Orthop2010;30:514–518.CrossrefPubMed Google Scholar
198 Frost HM . On our age-related bone loss: insights from a new paradigm. J Bone Miner Res1997;12:1539–1546.CrossrefPubMed Google Scholar
199 Callewaert F , BoonenS, VanderschuerenD. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol Metab2010;21:89–95.CrossrefPubMed Google Scholar
200 Zaman G , SaxonLK, SuntersA, et al.Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone2010;46:628–642.CrossrefPubMed Google Scholar
201 Khosla S . Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci2013;68:1226–1235.CrossrefPubMed Google Scholar
202 Rubin C , JudexS, QinYX. Low-level mechanical signals and their potential as a non-pharmacological intervention for osteoporosis. Age Ageing2006(35Suppl2)32–36.CrossrefPubMed Google Scholar
Funding statement:
CAS is supported by a studentship from the Irish Research Council. R. A. Rolfe was supported by a studentship from Trinity College Dublin (Innovation Bursary). P. Murphy reports receipt of a grant from the Wellcome Trust to complete this study. All three authors have received financial assistance from the Trinity College Travel grant award scheme.
Author contributions:
C. A. Shea: Writing the paper
R. A. Rolfe: Writing the paper
P. Murphy: Writing the paper
ICMJE Conflict of Interest:
None declared
©2015 Murphy et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
Supplementary material. A table showing the observed effects of reduced or absent movement on skeletal development is available alongside the online version of this article at www.bjr.boneandjoint.org.uk









