Abstract
Objectives
The number of surgical procedures performed each year to treat femoroacetabular impingement (FAI) continues to rise. Although there is evidence that surgery can improve symptoms in the short-term, there is no evidence that it slows the development of osteoarthritis (OA). We performed a feasibility study to determine whether patient and surgeon opinion was permissive for a Randomised Controlled Trial (RCT) comparing operative with non-operative treatment for FAI.
Methods
Surgeon opinion was obtained using validated questionnaires at a Specialist Hip Meeting (n = 61, 30 of whom stated that they routinely performed FAI surgery) and patient opinion was obtained from clinical patients with a new diagnosis of FAI (n = 31).
Results
Clinical equipoise was demonstrated when surgeons were given clinical scenarios and asked whether they would manage a patient operatively or non-operatively. A total of 23 surgeons (77%) who routinely perform FAI surgery were willing to recruit patients into a RCT, and 28 patients (90%) were willing to participate. 75% of responding surgeons believed it was appropriate to randomise patients to non-operative treatment for ≥ 12 months. Conversely, only eight patients (26%) felt this was acceptable, although 29 (94%) were willing to continue non-operative treatment for six months. More patients were concerned about their risk of developing OA than their current symptoms, although most patients felt that the two were of equal importance.
Conclusions
We conclude that a RCT comparing operative and non-operative management of FAI is feasible and should be considered a research priority. An important finding for orthopaedic surgical trials is that patients without life-threatening pathology appear willing to trial a treatment for six months without improvement in their symptoms.
Article focus
The number of surgical procedures performed each year to treat femoroacetabular impingement (FAI) continues to rise exponentially in the absence of evidence to show long-term benefit
Funding bodies increasingly request evidence of study feasibility
Our study aim was to determine whether surgeon and patient opinion is permissive of a randomised controlled trial (RCT) to investigate whether surgery is superior to non-operative management of FAI
Key messages
RCT to compare operative and non-operative management of FAI is feasible
Patients are willing to continue non-operative management without improvement in their symptoms for no longer than six months
Individual equipoise is not required for a surgeon to recruit patients into a RCT
Strengths and limitations
This is a large feasibility study that addresses both patient and surgeon opinion
Similar studies have reliably predicted recruitment rates in subsequent clinical trials
Opinions of those surveyed may not be representative of national or international opinion
Introduction
Randomised controlled trials (RCTs) are considered to be the optimal study design for comparing the efficacy and effectiveness of treatments. However, they are particularly difficult to perform in the field of surgery due to difficulties in blinding, standardising procedures, and a lack of acceptance by patients and surgeons.1,2 Only 7% of research articles in surgical journals are RCTs,3 and treatments in surgery are half as likely to be based on evidence from RCTs than in medicine.4,5 Furthermore, as a result of the above difficulties, RCTs pertaining to surgical intervention may be poorly constructed and may not always represent the ideal study type.2
A RCT is not always possible because once a procedure has been accepted as best practice, it may be considered unethical to allocate patients to an alternative treatment.6 RCTs require a setting of equipoise, a situation where both patients and clinicians feel a genuine conscious uncertainty as to the optimal management strategy.7 Equipoise can relate to either an individual or a community. Individual equipoise refers to a setting where an individual is undecided as to the optimal treatment. Community equipoise refers to a setting where individuals may not display equipoise, but broadly equal numbers of individuals within a community are proponents of each different treatment. Individual equipoise is considered necessary for trial recruitment, whereas community equipoise is considered necessary for a trial to be ethically acceptable.8-10
Patient preference for a particular treatment is a frequent cause of trial abandonment, primarily because patients often have a preferred treatment in mind.11,12 Equipoise is therefore essential within the patient population, but is highly dependent on the information made available. Measurement of surgeon and patient preference is a crucial aspect of feasibility assessment,7 and is increasingly requested by funding bodies. Feasibility studies seek to determine whether a study is possible, and to estimate important parameters for the design of the main study.13 In this instance, the proposed study is a RCT to determine whether surgery in patients with femoroacetabular impingement (FAI) can delay the onset of osteoarthritis (OA) compared with non-operative treatment.
FAI describes abnormalities in the orientation of the acetabulum (pincer impingement) or abnormalities in the shape of the femoral head and neck (cam impingement). This results in abutment of the femoral head–neck junction against the acetabular rim and labrum, progressing to cartilage delamination and OA at this site.14,15 This pathogenesis is supported by several observations. A 20-year longitudinal study of an asymptomatic population-based cohort has shown that the presence of a cam lesion increases the risk of future THR by sixfold.16 Cam lesions have been observed in > 50% of patients undergoing hip replacement,17 and significant cartilage damage has also been seen intra-operatively at the specific sites where impingement occurs.18 Lastly, early signs of cartilage damage have been detected using delayed Gadolinium Enhanced Magnetic Resonance Imaging of Cartilage (dGEMRIC) in asymptomatic patients with cam lesions.19
The United Kingdom maintains a database (Hospital Episode Statistics) of all medical procedures performed on the National Health Service. Analysis of this database reveals that the number of surgical procedures performed each year to treat FAI is rising exponentially, with a 442% increase over the past 10 years.20 This trend has been mirrored in the United States, and in 2012 the American Academy of Orthopaedic Surgeons hosted a FAI Research Symposium to address the increasing recognition and intervention for this condition.
The options for treatment include physiotherapy and activity modification, or surgery to treat the cartilage damage with or without reshaping the impinging bony deformities. While cohort studies show that each of these treatment options can improve symptoms in the short-term,21-23 the greatest potential benefit lies in being able to arrest or delay the onset of OA, for which there is currently no evidence. There are now calls for a RCT to make a robust comparison between the above treatment modalities. We constructed this study with the goal of determining the feasibility of a RCT studying FAI surgery. Our specific aims were to ascertain whether specialist hip surgeons believe that a RCT for FAI management is required, to establish whether there is evidence of surgeon and patient equipoise, and to determine whether patients are willing to participate in such a trial.
Information gained may also help to decide the optimum trial design. We identified three areas of design as being crucial to trial feasibility: Which patients should be included in a RCT? How long are patients and surgeons prepared to continue a treatment if symptoms do not improve? What should be used as the primary outcome measure?
Materials and Methods
We designed two different questionnaires to ascertain surgeon and patient preferences.
Surgeon preference
A written questionnaire was developed to establish surgeon preferences using a combination of questions requiring discrete ‘yes’ or ‘no’ responses, and validated bidirectional linear analogue scales requiring surgeons to mark their responses on an 11-point scale from -5 to +5 (Fig. 1).7 Data were collected at the 2012 meeting of the British Hip Society during a session focusing on pathology of the hip in the young adult. This forum has a high attendance by consultant surgeons specialising in the management of FAI. Responses were analysed in numerical format and using a surgical equipoise ratio (SER).7 This scale comprises three digits, representing the number of surgeons with negative value responses, those at zero, and those with positive value responses.
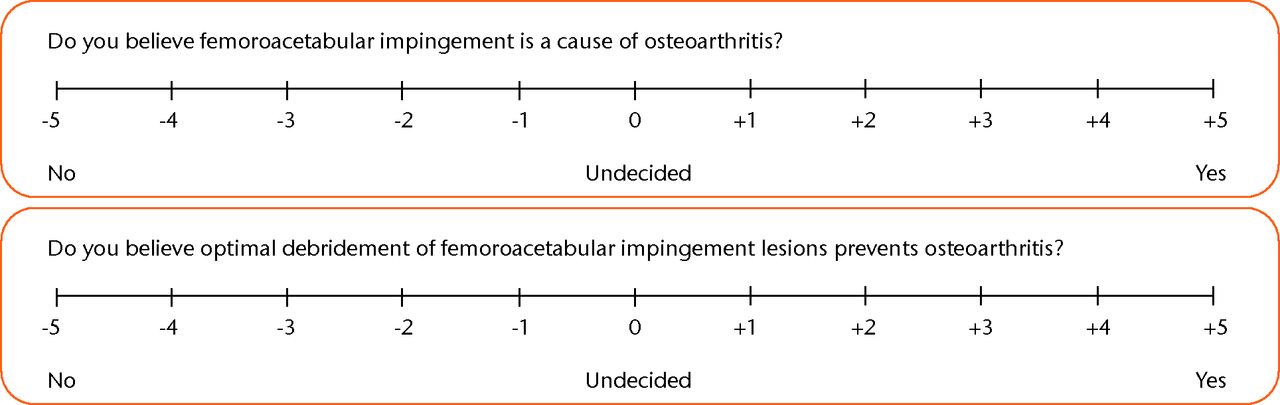
Fig. 1
Example questions using a bidirectional linear scale to determine surgeon or patient opinion.
Patient preference
A Patient Information Leaflet was developed with the aid of a Patient Advocate (PL), attached to which was a written questionnaire. This included a description of FAI and how it results in damage to the labrum and articular cartilage. It also contained an overview of conservative and surgical treatment options, along with a discussion of the potential risks and benefits of each. Conservative management was said to comprise weekly physiotherapy and activity modification, and surgical treatment was said to comprise hip arthroscopy with labral debridement or repair, and osteochondroplasty. During a three week period, all patients aged between 18 and 65 years with newly diagnosed FAI seen in clinic at the Nuffield Orthopaedic Centre, Oxford, and the Royal Berkshire Hospital, Reading, were invited to read through the Patient Information Leaflet and answer the questionnaire to identify patient preferences for the management of their hip pathology. The 11-point bidirectional scale was used to ask patients whether their principal concern was the risk of developing arthritis or their current pain, and a linear scale from 0 to 24 months (with three-month divisions) was used to ask patients how long they would be happy to trial non-operative management if their symptoms did not improve. Patients were seen again immediately after they completed the questionnaire to address any further questions.
Results
Surgeon preference
Questionnaires were completed by 61 surgeons, 30 of whom indicated that they routinely performed surgery for FAI. Of these 30 surgeons who routinely performed FAI surgery, six (20%) performed < ten procedures annually, four (13%) performed between ten and 20, six (20%) performed between 20 and 30, four (13%) performed between 30 and 50, three (10%) performed between 50 and 100, and seven (23%) performed > 100 procedures per year. The preferred surgical approach of these surgeons was arthroscopic (53%, n = 16), followed by arthroscopic and mini-open (30%, n = 9), followed by open only (17%, n = 5). The questionnaire also asked surgeons to state their preferred Patient Reported Outcome Measure (PROM). Some authors did not respond and others gave multiple measures: the Non-Arthritic Hip Score (NAHS)24 was the most common (49%), followed by the Oxford hip score (OHS)25 (20%), the Hip Disability and Osteoarthritis Outcome Score (HOOS)26 (18%), the Harris hip score (HHS)27 (9%) and the Hip Outcome Score28 (9%).
Of all surgeons surveyed, 75% stated that they would modify their practice in response to level 1 evidence demonstrating FAI surgery was superior to conservative management in delaying the onset of OA. This question was answered by 28 surgeons who routinely perform FAI surgery, of whom 21 (75%) said they would modify their practice, and by 20 surgeons who do not routinely perform FAI surgery, of whom 15 (75%) said they would modify their practice. Of the surgeons who routinely perform surgery for FAI, 23 of 28 responses (82%) indicated that they were willing to participate in a multi-centre RCT. However, if the two non-responders are assumed to be unwilling, 77% of surgeons would be willing to participate in such a trial.
Approximately 75% of surgeons found it appropriate to randomise patients to non-surgical management for ≥ 12 months, and of those surgeons who did not believe this is appropriate, the shortest specified length of time was six months. This question was answered by 28 surgeons who routinely perform FAI surgery, of whom 20 (71%) believed > 12 months was appropriate, and 20 surgeons who do not routinely perform FAI surgery, of whom 16 (80%) believed > 12 months was appropriate.
The responses to questions using the bidirectional linear scale are both tabulated (Table I) and displayed as histograms (Figs 2 to 8). Not every surgeon responded to every question, and the total responses for each question are given in Table I. The majority of surgeons believed FAI is a cause of osteoarthritis with a surgical equipoise ratio (SER) of 7:6:39 for ‘no’, ‘undecided’ and ‘yes’, respectively (Fig. 2), although the belief that optimal debridement of FAI lesions prevented the development of osteoarthritis was more controversial, with an SER of 13:19:20 for ‘no’, ‘undecided’ and ‘yes’, respectively (Fig. 3). When asked whether they would manage a patient non-operatively or operatively on first presentation, the SER was 29:6:15 and 10:6:34 for mild and severe symptoms, respectively, representing ‘non-operatively’, ‘undecided’ and ‘operatively’ (Figs 4 and 5). Considering the subgroup of 23 surgeons who routinely perform FAI surgery and who were willing to recruit patients into a RCT, the SER was 12:2:9 for mild symptoms and 3:1:19 for severe symptoms, representing ‘non-operatively’, ‘undecided’ and ‘operatively’, respectively (Figs 6 and 7). Evidence of early osteoarthritis was likely to make a surgeon less likely to perform FAI surgery, giving a SER of 35:4:8, representing ‘yes’ (less likely), ‘undecided’ and ‘no’, respectively (Fig. 8).
Table I
Surgeon responses to each of the questions asked using a bidirectional linear scale. Responses are displayed as the Surgical Equipoise Scale7 (FAI, femoroacetabular impingement; OA, osteoarthritis)
| Surgeon response | ||||
|---|---|---|---|---|
| Question | No | Undecided | Yes | |
| Do you believe FAI is a cause of osteoarthritis? | ||||
| All surgeons (n = 52) | 7 | 6 | 39 | |
| Surgeons who routinely perform FAI surgery (n = 28) | 2 | 2 | 24 | |
| Surgeons who do not routinely perform FAI surgery (n = 24) | 5 | 4 | 15 | |
| Does optimal debridement of FAI lesions prevent OA? | ||||
| All surgeons (n = 52) | 13 | 19 | 20 | |
| Surgeons who routinely perform FAI surgery (n = 28) | 3 | 8 | 17 | |
| Surgeons who do not routinely perform FAI surgery (n = 24) | 10 | 11 | 3 | |
| Non-operatively | Undecided | Operatively | ||
| How do you manage patients with mild symptoms on first presentation (limits sports)? | ||||
| All surgeons (n = 50) | 29 | 6 | 15 | |
| Surgeons who routinely perform FAI surgery (n = 28) | 13 | 3 | 12 | |
| How do you manage patients with severe symptoms on first presentation (limits daily activity)? | ||||
| All surgeons (n = 50) | 10 | 6 | 34 | |
| Surgeons who routinely perform FAI surgery (n = 28) | 5 | 1 | 22 | |
| Yes | Undecided | No | ||
| Does evidence of early osteoarthritis on imaging make you less likely to perform FAI surgery? | ||||
| All surgeons (n = 47) | 35 | 4 | 8 | |
| Surgeons who routinely perform FAI surgery (n = 28) | 20 | 0 | 8 | |
Table II
Patient responses to each of the questions asked using a linear scale
| Patient response | |||||
|---|---|---|---|---|---|
| Question | Future arthritis | Equal importance | Current pain | ||
| What is more important: Treating your current pain or reducing your future risk of arthritis? | 12 | 14 | 5 | ||
| How long would you be prepared to trial physiotherapy before wishing to have surgery if your symptoms did not improve? | Mean 7.5 months (3 to 18) | ||||
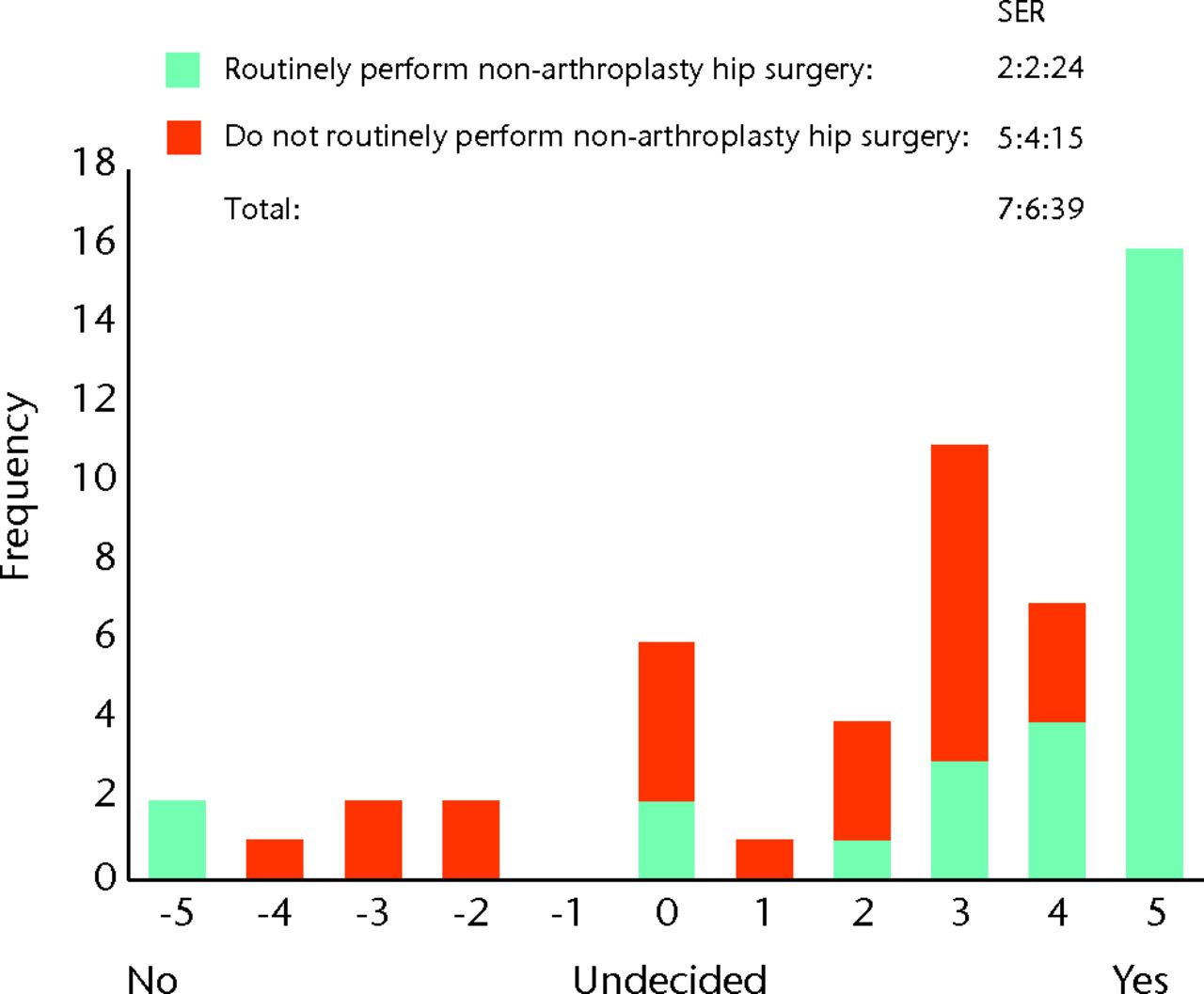
Fig. 2
Bar chart showing the surgeon responses to the question ‘Do you believe femoroacetabular impingement (FAI) is a cause of osteoarthritis?’ The responses of surgeons who routinely perform FAI surgery are represented in blue, and those who do not are in red. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
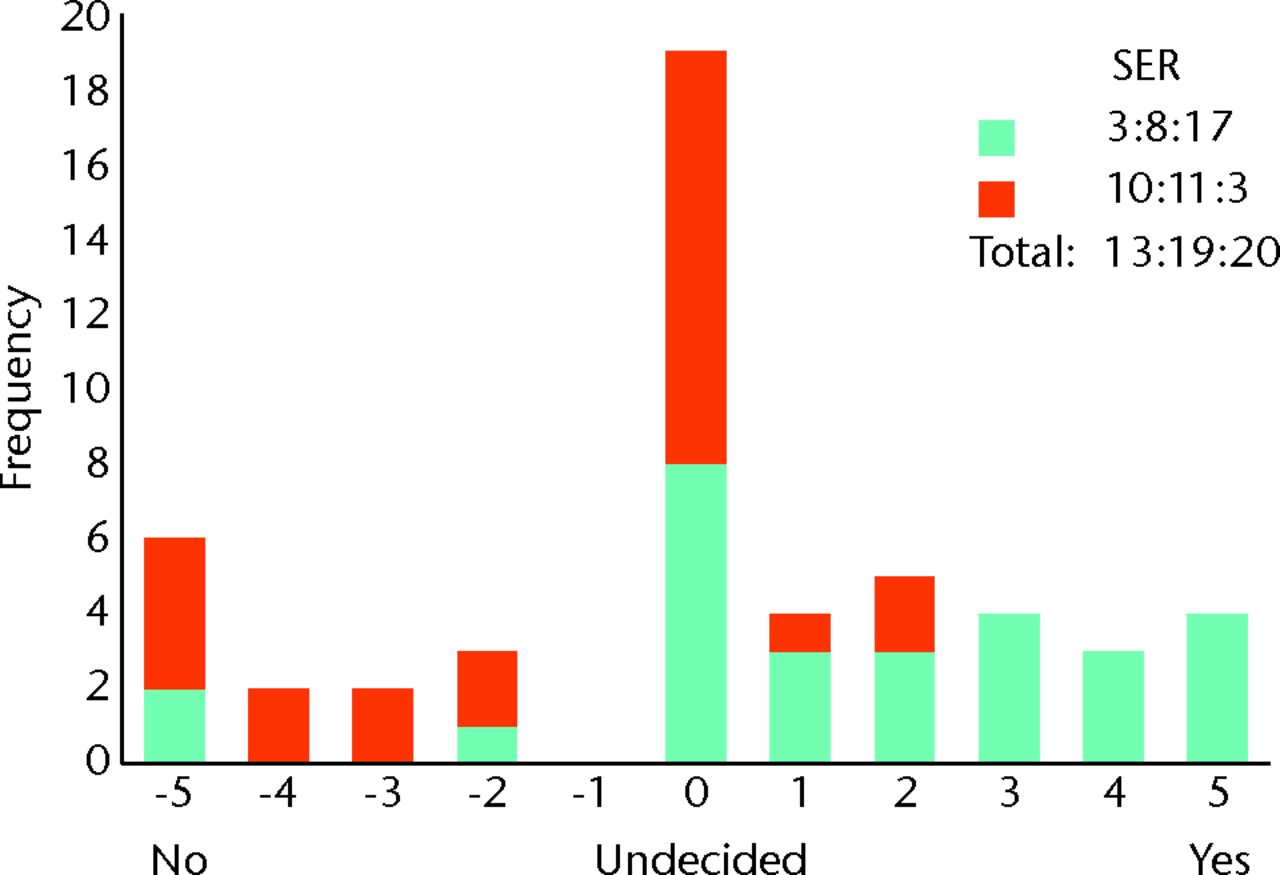
Fig. 3
Bar chart showing the surgeon responses to the question ‘Does optimal debridement of femoroacetabular impingement (FAI) lesions prevent osteoarthritis?’ The responses of surgeons who routinely perform FAI surgery are represented in blue, and those who do not are in red. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
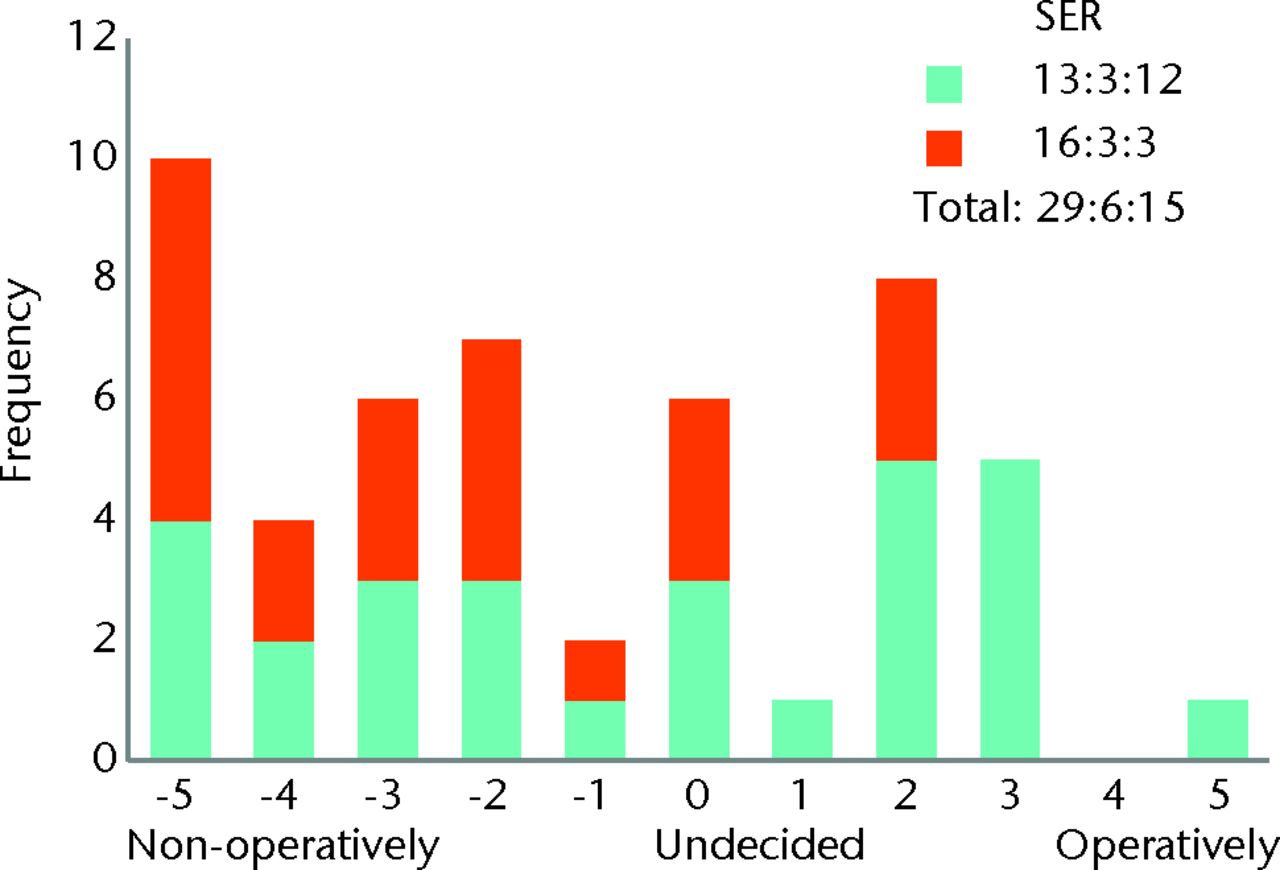
Fig. 4
Bar chart showing the surgeon responses to the question ‘How do you manage patients with mild symptoms on first presentation (limits sports)?’ The responses of surgeons who routinely perform femoroacetabular impingement (FAI) surgery are represented in blue, and those who do not are in red. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
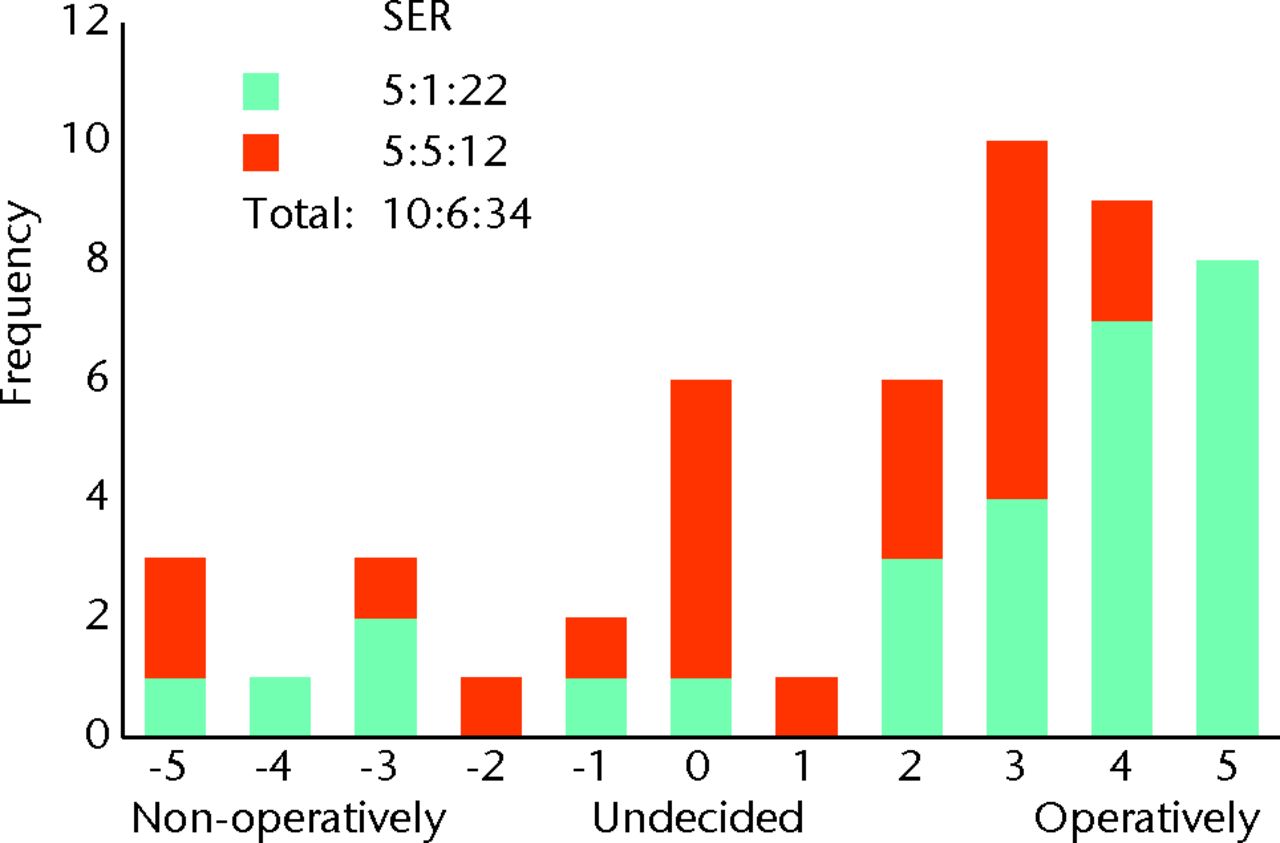
Fig. 5
Bar chart showing the surgeon responses to the question ‘How do you manage patients with severe symptoms on first presentation (limits daily activity)?’ The responses of surgeons who routinely perform femoroacetabular impingement (FAI) surgery are represented in blue, and those who do not are in red. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
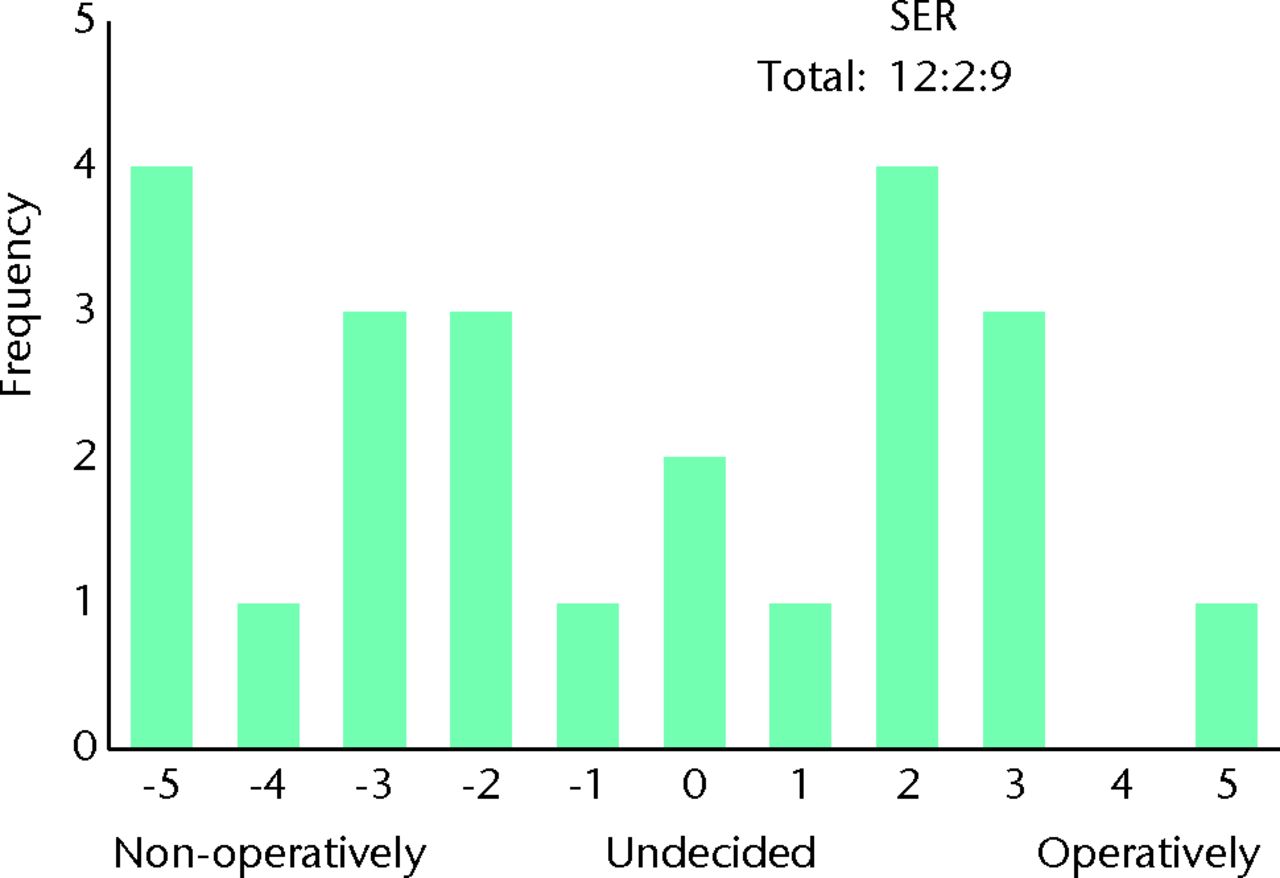
Fig. 6
Bar chart showing the responses to the question ‘How do you manage patients with mild symptoms on first presentation (limits sports)?’ for the subgroup of 23 surgeons who routinely perform femoroacetabular impingement (FAI) surgery and who would be willing to recruit patients into a randomised controlled trial. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
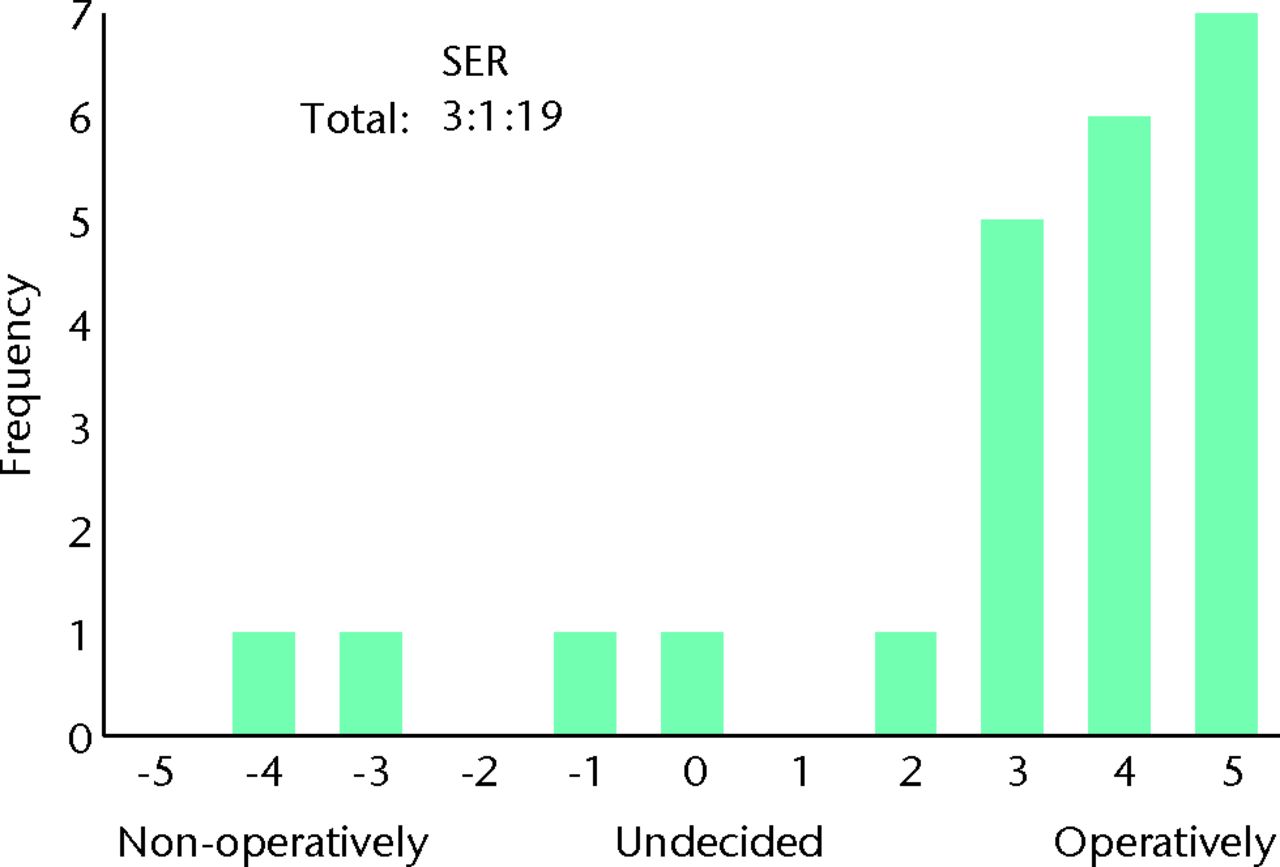
Fig. 7
Bar chart showing the responses to the question ‘How do you manage patients with severe symptoms on first presentation (limits daily activity)?’ for the subgroup of 23 surgeons who routinely perform femoroacetabular impingement (FAI) surgery and who would be willing to recruit patients into a randomised controlled trial. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
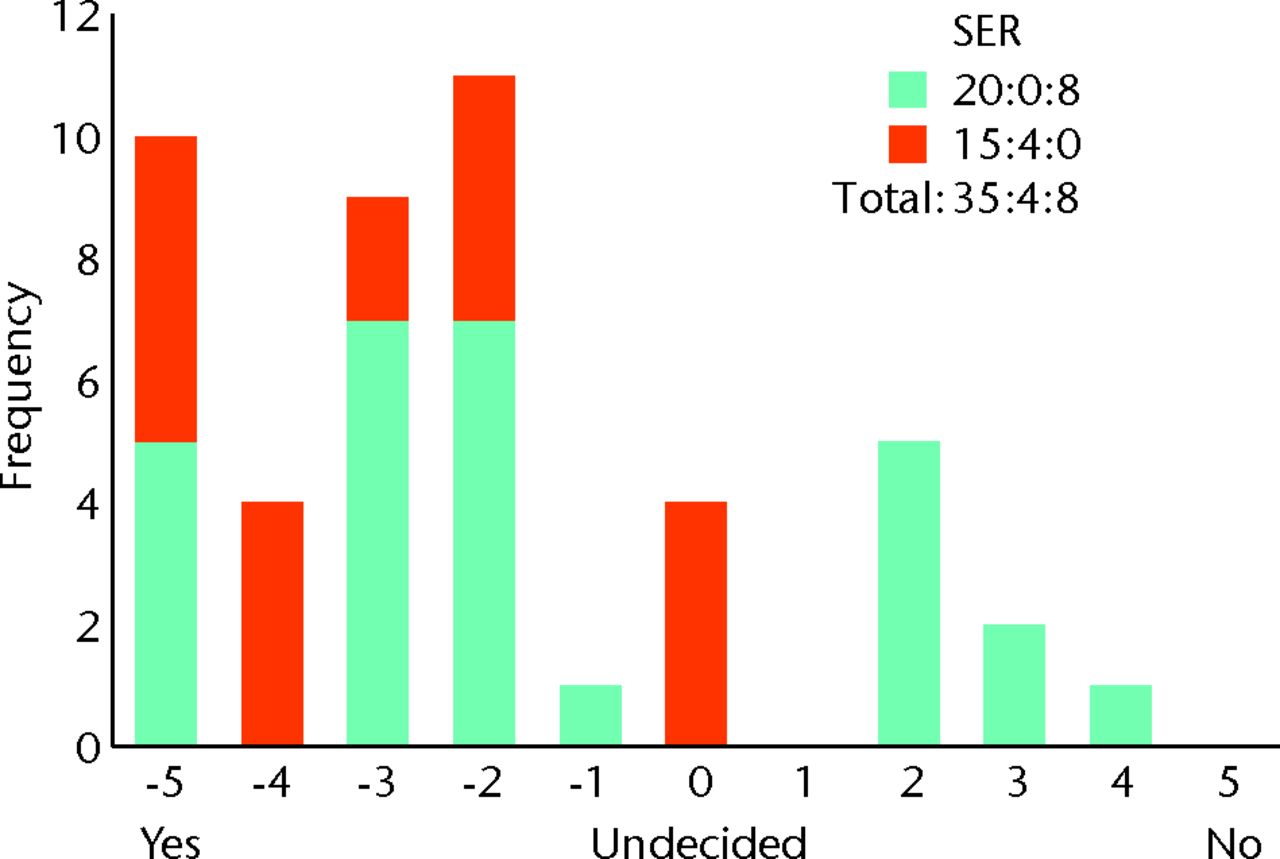
Fig. 8
Bar chart showing the surgeon responses to the question ‘Does evidence of early osteoarthritis on imaging make you less likely to perform femoroacetabular impingement (FAI) surgery?’ The responses of surgeons who routinely perform femoroacetabular impingement (FAI) surgery are represented in blue, and those who do not are in red. The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
Patient preference
Questionnaires were completed by 31 patients (19 female and 12 male) with a mean age of 37 years (21 to 63). Prior to onset of symptoms, 26 patients (84%) reported regular participation in sporting activities. A total of 28 patients (90%) stated that they would be willing to participate in a trial randomising to either operative or non-operative management. Of the three remaining patients, two indicated that they would find randomisation to the non-operative arm unacceptable, and one found randomisation to the operative arm unacceptable.
When asked whether they were more concerned about future arthritis or their current pain, 12 patients (39%) indicated arthritis, five (16%) current pain, and the remaining 14 patients (45%) felt them to be of equal importance. When asked how long they were prepared to trial physiotherapy if their symptoms did not improve, the mean response was 7.5 months (3 to 18), with the majority (94%, n = 29) indicating that they found ≥ six months to be acceptable (Table II, Figs 9 and 10).
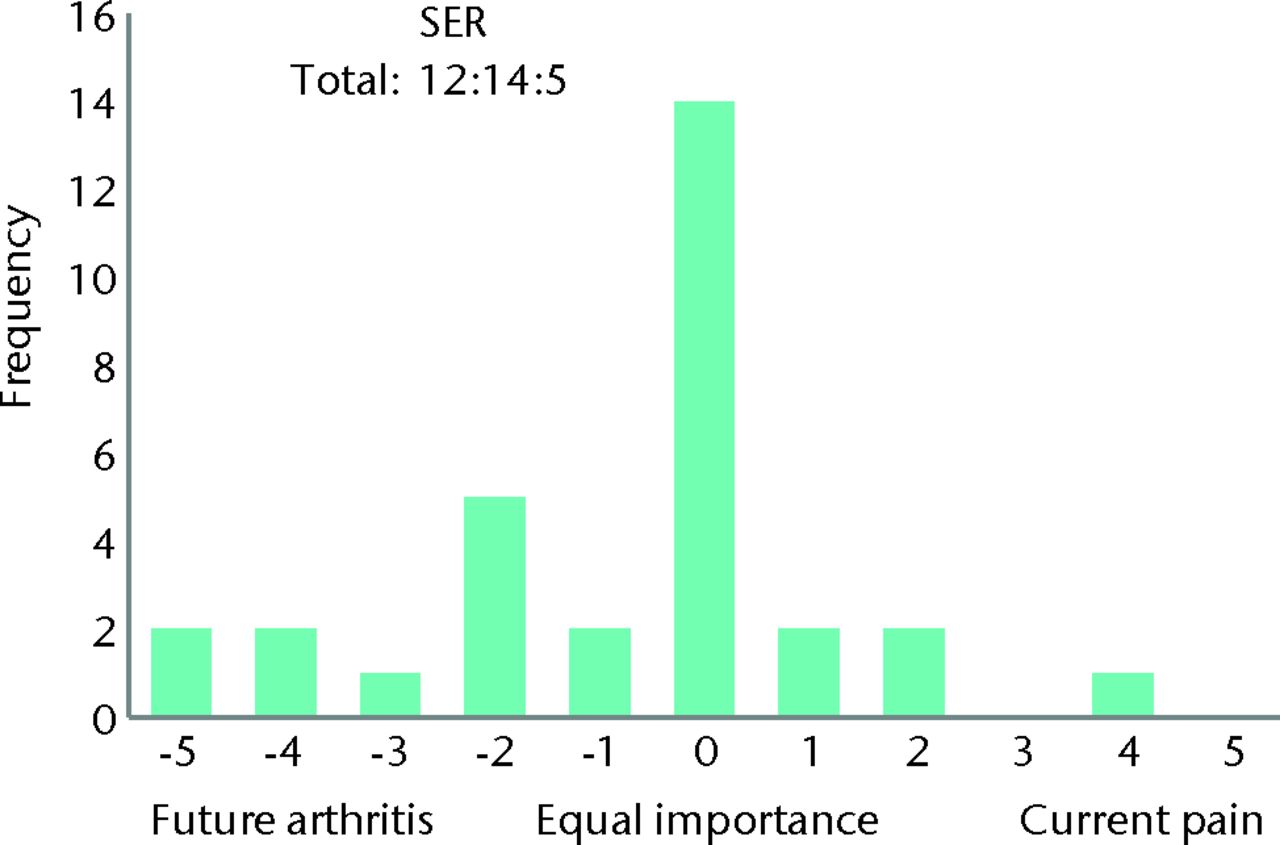
Fig. 9
Bar chart showing the patient response to the question ‘What is more important: treating your current pain or reducing you future risk of arthritis?’ The three digits of the surgical equipoise ratio (SER) represent the sum of the scores within the range ‘-5 to -1’, followed by scores of ‘0’, followed by scores within the range ‘+1 to +5’.
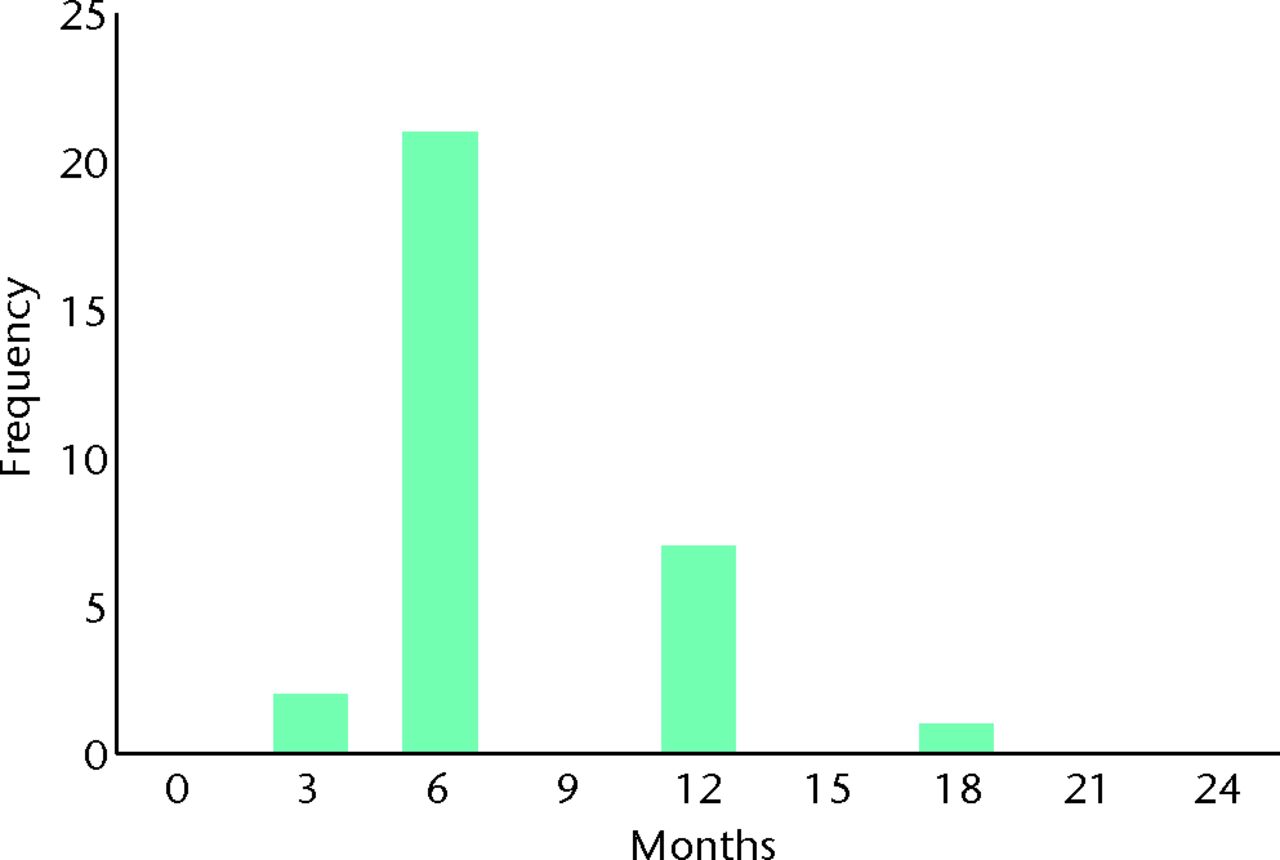
Fig. 10
Bar chart showing the patient response to the question ‘How long would you be prepared to trial physiotherapy before wishing to have surgery if your symptoms did not improve?’ Responses are plotted on a unidirectional linear scale from 0 to 24 months.
Discussion
The value of this feasibility study is threefold: it determines whether there is a requirement for a study, it establishes whether surgeon and patient opinion is permissive of the proposed trial, and it also provides guidance for trial design.
Our study demonstrates a requirement for a RCT to compare the operative and non-operative management of FAI. Although surgeons in our feasibility study displayed uncertainty as to whether osteochondroplasty for FAI could prevent OA, the number of surgical procedures performed each year to treat FAI continues to rise exponentially. This may reflect increasing evidence that surgery can improve symptoms in the short-term,21-23 however, our study shows that the primary concern of patients was more frequently the prevention of OA rather than their current pain. This supports the need for a well-designed RCT. Indeed, 75% of surgeons said they would modify their clinical practice in response to level 1 evidence showing FAI surgery slowed the development of OA over conservative management strategies.
We have also shown that surgeon and patient opinion was permissive for a RCT, and that there was evidence of equipoise. The ideal position of equipoise for a trial is when both patient and clinician have no particular preference for alternative treatment strategies. In reality, this theoretical model is rarely seen. Community equipoise describes disagreement within an expert clinical body as to the preferred treatment strategy. If the majority of clinicians strongly believe one treatment is superior to another, it would not be ethical to randomise patients to the treatment that is perceived to be inferior. When members of the public were asked to specify the degree of community equipoise they would require to cast an approving vote on an ethical committee, half believed a RCT was unethical when community equipoise is disturbed beyond 70% cent of clinicians favouring one of the treatment options.29 By this threshold our study demonstrates adequate community equipoise, as when we asked surgeons their preferred treatment for a patient with mild and severe symptoms, no more than 70% of surgeons favoured either treatment option. When presented with patients experiencing mild symptoms, 58% of surgeons favoured non-operative management, whereas with patients experiencing severe symptoms, 68% of surgeons favoured operative management (Figs 4 and 5). These findings suggest it is ethical to perform a RCT for all symptom severities.
An individual is in equipoise when they themselves do not display a preference for one treatment option over another. This is considered essential if clinicians are to willingly recruit patients into a trial, and also minimises researcher bias for either treatment arm.30 In our study, individual equipoise is illustrated when a surgeon marks ‘undecided’ as their preferred treatment option for a given clinical scenario. As the number of surgeons without a treatment preference increases, the number of surgeons willing to recruit patients would be expected to rise. Although a study may require relatively few surgeons for adequate recruitment, if no surgeon displays individual equipoise, trial recruitment is unlikely to be possible. We found that 12% of surgeons were undecided as to whether to adopt operative or non-operative management in patients with both mild and severe symptoms. Whereas for mild symptoms this represented an equal number of surgeons who did and did not perform FAI surgery, for severe symptoms, the vast majority were surgeons who did not routinely perform FAI surgery (Figs 4 and 5). This finding suggests that few surgeons would be willing to recruit patients into a RCT; however, this was not the case. We found 77% of surgeons who routinely perform FAI surgery to be willing to recruit patients into a RCT. This supports the theory that individual equipoise in clinicians is not essential.31 Surgeons who had a strong preference for one treatment arm were still willing to recruit patients into a trial (Figs 6 and 7).
A RCT for FAI management with operative and non-operative treatment arms should achieve a high rate of patient recruitment, as 90% of patients indicated that they were willing to participate. But recruitment and drop-out rates are conditional on trial design. A crucial factor is the length of time patients are randomised to non-surgical treatment. While 75% of surgeons indicated that it is appropriate to adopt a non-operative treatment strategy for one year or more, only 26% of patients found this acceptable if their symptoms did not improve. This is likely to result in low recruitment rates and high drop-out rates. Given that the majority of patients surveyed (94%) found six months to be acceptable, cross-over to a surgical treatment arm must be allowed at this time point in order to ensure success of the trial. This finding dictates the primary outcome measure and sample size of such a trial, and feasibility therefore depends on the ability to detect a clinically relevant difference within six months. This is only possible using advanced imaging techniques to detect surrogate markers of OA, such as T2 mapping or dGEMRIC.32 The finding that patients are happy to pursue a treatment strategy for six months without improvement in their symptoms may have important implications for clinical trials addressing other orthopaedic pathology.
When considering the inclusion criteria for a RCT, we demonstrated strong community opinion that surgeons are less likely to perform FAI surgery in the presence of early OA (Fig. 8). In order to avoid confusion we intentionally excluded a specific definition of early OA, as there are many classifications, and few are commonly used in a clinical setting. Nevertheless, responses suggest surgeons would find it unacceptable to perform FAI surgery on patients with established OA. We therefore believe trial inclusion would need to be limited to patients with a Tönnis grade33 or Kellgren-Lawrence score34 < 2. We also recommend that a RCT addresses arthroscopic surgery, and uses the Non-Arthritic Hip Score as the PROM, as these are preferred by the majority of surgeons and ensure applicability of study findings.
There are limitations to this feasibility study. The patients and surgeons completing our questionnaires do not necessarily represent national or international opinion, despite this being one of the largest surgical feasibility studies published, and not every surgeon answered every question. The same two surgeons who routinely perform FAI surgery did not mark their responses on the bidirectional scales, and a higher number of surgeons who do not routinely perform FAI surgery did not complete all of the questions. This may be because they did not feel sufficiently well informed to provide a response, but it could also be a reflection on the survey design.
Although we are cautious that a patient may be more likely to consent to a trial in theory than in practice when approached by the trial team, similar studies to ours have reliably predicted recruitment rates in the subsequent trial.35-37 It is also extremely difficult not to display bias towards the operative treatment arm. If symptoms do not improve with non-operative therapy, it is possible to consider surgery. However, the reverse is not possible when surgery reflects a definitive and irreversible procedure. As a result, patients may interpret this to mean surgery is the optimum treatment. This is a significant hurdle to performing a surgical RCT, and one that is extremely difficult to overcome.
Conclusions
All proposed studies should demonstrate, rather than assume, feasibility. Our study demonstrates that a RCT is required to investigate whether surgical debridement of FAI slows the development of osteoarthritis compared with a non-operative control. We also showed that this study is feasible in terms of both surgeon and patient opinion. Consensus opinion is a dynamic concept, and while surgeon and patient opinions currently appear permissive, this may not be the case in the future. We recommend that a RCT for FAI treatment be conducted, and that it is considered a research priority. Important findings of this study relevant to the design of other clinical trials are that individual surgeon equipoise is not essential, and that surgeons expressing a strong preference for a particular treatment strategy may still be happy to recruit patients into a RCT. Also, patients are happy to pursue a treatment strategy for six months, even if their symptoms do not improve.
The authors would like to thank P. Lovell for his role as Patient Advocate.
1 McLeod R . Randomized, controlled trials: is there a role for them in surgery?Ann Surg2006;244:684–685.CrossrefPubMed Google Scholar
2 McCulloch P , TaylorI, SasakoM, LovettB, GriffinD. Randomised trials in surgery: problems and possible solutions. BMJ2002;324:1448–1451.CrossrefPubMed Google Scholar
3 Solomon MJ , McLeodRS. Clinical studies in surgical journals: have we improved?Dis Colon Rectum1993;36:43–48. Google Scholar
4 Ellis J , MulliganI, RoweJ, SackettDL. Inpatient general medicine is evidence based: A-Team, Nuffield Department of Clinical Medicine. Lancet1995;346:407–410. Google Scholar
5 Howes N , ChaglaL, ThorpeM, McCullochP. Surgical practice is evidence based. Br J Surg1997;84:1220–1223. Google Scholar
6 Black N . Why we need observational studies to evaluate the effectiveness of health care. BMJ1996;312:1215–1218.CrossrefPubMed Google Scholar
7 Young JM , SolomonMJ, HarrisonJD, SalkeldG, ButowP. Measuring patient preference and surgeon choice. Surgery2008;143:582–588.CrossrefPubMed Google Scholar
8 Freedman B . Equipoise and the ethics of clinical research. N Engl J Med1987;317:141–145.CrossrefPubMed Google Scholar
9 Miller FG . Clinical equipoise and risk-benefit assessment. Clin Trials2012;9:621–627.CrossrefPubMed Google Scholar
10 Young JM , SolomonMJ, HarrisonJD, SalkeldG, ButowP. Measuring patient preference and surgeon choice. Surgery2008;143:582–588.CrossrefPubMed Google Scholar
11 Ross S , GrantA, CounsellC, et al.Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol1999;52:1143–1156.CrossrefPubMed Google Scholar
12 Solomon MJ , McLeodRS. Should we be performing more randomized controlled trials evaluating surgical operations?Surgery1995;118:459–467.CrossrefPubMed Google Scholar
13 Arain M , CampbellMJ, CooperCL, LancasterGA. What is a pilot or feasibility study?: a review of current practice and editorial policy. BMC Med Res Methodol2010;10:67. Google Scholar
14 Ganz R , ParviziJ, BeckM, et al.Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res2003;417:112–120.CrossrefPubMed Google Scholar
15 Tibor LM , LeunigM. The pathoanatomy and arthroscopic management of femoroacetabular impingement. Bone Joint Res2012;1:245–257.CrossrefPubMed Google Scholar
16 Thomas GE , KiranA, BatraRN, et al.The association between hip morphology and end-stage osteoarthritis at 12-year follow up. Osteoarthritis Cartilage2012;20(Suppl 1):S204. Google Scholar
17 Clohisy JC , DobsonMA, RobisonJF, et al.Radiographic structural abnormalities associated with premature, natural hip-joint failure. J Bone Joint Surg [Am]2011;93-A(Suppl 2):3–9.CrossrefPubMed Google Scholar
18 Reichenbach S , LeunigM, WerlenS, et al.Association between cam-type deformities and magnetic resonance imaging-detected structural hip damage: a cross-sectional study in young men. Arthritis Rheum2011;63:4023–4030.CrossrefPubMed Google Scholar
19 Pollard TC , McNallyEG, WilsonDC, et al.Localized cartilage assessment with three-dimensional dGEMRIC in asymptomatic hips with normal morphology and cam deformity. J Bone Joint Surg [Am]2010;92-A:2557–2569.CrossrefPubMed Google Scholar
20 No authors listed. Hospital Episode Statistics. http://www.hesonline.nhs.uk (date last accessed 5 December 2012). Google Scholar
21 Clohisy JC , St JohnLC, SchutzAL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop Relat Res2010;468:555–564.CrossrefPubMed Google Scholar
22 Ng VY , AroraN, BestTM, PanX, EllisTJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med2010;38:2337–2345.CrossrefPubMed Google Scholar
23 Bedi A , ChenN, RobertsonW, KellyBT. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy2008;24:1135–1145.CrossrefPubMed Google Scholar
24 Christensen CP , AlthausenPL, MittlemanMA, LeeJA, McCarthyJC. The nonarthritic hip score: reliable and validated. Clin Orthop Relat Res2003;406:75–83.CrossrefPubMed Google Scholar
25 Dawson J , FitzpatrickR, CarrA, MurrayD. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg [Br]1996;78-B:185–190.PubMed Google Scholar
26 Klässbo M , LarssonE, MannevikE. Hip disability and osteoarthritis outcome score: an extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand J Rheumatol2003;32:46–51. Google Scholar
27 Harris WH . Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Joint Surg [Am]1969;51-A:737–755. Google Scholar
28 Martin RL , KellyBT, PhilipponMJ. Evidence of validity for the hip outcome score. Arthroscopy2006;22:1304–1311.CrossrefPubMed Google Scholar
29 Johnson N , LilfordRJ, BrazierW. At what level of collective equipoise does a clinical trial become ethical?J Med Ethics1991;17:30–34.CrossrefPubMed Google Scholar
30 Markman M . Ethical difficulties with randomized clinical trials involving cancer patients: examples from the field of gynecologic oncology. J Clin Ethics1992;3:193–195.PubMed Google Scholar
31 Freedman B . Equipoise and the ethics of clinical research. N Engl J Med1987;317:141–145.CrossrefPubMed Google Scholar
32 Roos EM , DahlbergL. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum2005;52:3507–3514.CrossrefPubMed Google Scholar
33 Kellgren JH , LawrenceJS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis1957;16:494–502.CrossrefPubMed Google Scholar
34 Tönnis D . Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin Orthop Relat Res1976;119:39–47.PubMed Google Scholar
35 Abraham NS , HewettP, YoungJM, SolomonMJ. Non-entry of eligible patients into the Australasian Laparoscopic Colon Cancer Study. ANZ J Surg2006;76:825–829.CrossrefPubMed Google Scholar
36 Solomon MJ , PagerCK, KeshavaA, et al.What do patients want?: patient preferences and surrogate decision making in the treatment of colorectal cancer. Dis Colon Rectum2003;46:1351–1357. Google Scholar
37 Solomon MJ , PagerCK, YoungJM, RobertsR, ButowP. Patient entry into randomized controlled trials of colorectal cancer treatment: factors influencing participation. Surgery2003;133:608–613.CrossrefPubMed Google Scholar
Funding statement:
None declared
Author contributions:
A. J. R. Palmer: Study conception and design, Data collection and analysis, Drafting of article
G. E. R. Thomas: Drafting of article and critical revision
T. C. B. Pollard: Data collection and critical revision
I. Rombach: Data analysis and critical revision
A. Taylor: Study conception and design
N. Arden: Critical revision
D. J. Beard: Critical revision
A. J. Andrade: Data collection and critical revision
A. J. Carr: Critical revision
S. Glyn-Jones: Study conception and design, Drafting of article and critical revision
ICMJE Conflict of Interest:
None declared
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









