Abstract
Aims
In the repair of condylar cartilage injury, synovium-derived mesenchymal stem cells (SMSCs) migrate to an injured site and differentiate into cartilage. This study aimed to confirm that histone deacetylase (HDAC) inhibitors, which alleviate arthritis, can improve chondrogenesis inhibited by IL-1β, and to explore its mechanism.
Methods
SMSCs were isolated from synovium specimens of patients undergoing temporomandibular joint (TMJ) surgery. Chondrogenic differentiation potential of SMSCs was evaluated in vitro in the control, IL-1β stimulation, and IL-1β stimulation with HDAC inhibitors groups. The effect of HDAC inhibitors on the synovium and condylar cartilage in a rat TMJ arthritis model was evaluated.
Results
Interleukin (IL)-1β inhibited the chondrogenic differentiation potential of SMSCs, while the HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA) and panobinostat (LBH589), attenuated inhibition of IL-1β-induced SMSC chondrogenesis. Additionally, SAHA attenuated the destruction of condylar cartilage in rat TMJ arthritis model. IL-6 (p < 0.001) and matrix metalloproteinase 13 (MMP13) (p = 0.006) were significantly upregulated after IL-1β stimulation, while SAHA and LBH589 attenuated IL-6 and MMP13 expression, which was upregulated by IL-1β in vitro. Silencing of IL-6 significantly downregulated MMP13 expression and attenuated IL-1β-induced chondrogenesis inhibition of SMSCs.
Conclusion
HDAC inhibitors SAHA and LBH589 attenuated chondrogenesis inhibition of SMSC induced by IL-1β in TMJ, and inhibition of IL-6/MMP13 pathway activation contributes to this biological progress. This study provides a theoretical basis for the application of HDAC inhibitors in the treatment of TMJ arthritis.
Cite this article: Bone Joint Res 2022;11(1):40–48.
Article focus
-
Whether histone deacetylase (HDAC) inhibitors improve the chondrogenic differentiation of synovium-derived mesenchymal stem cells (SMSCs) inhibited by interleukin (IL)-1β.
-
The relationship between HDAC inhibitors, IL-6, matrix metalloproteinase (MMP)13, and chondrogenic differentiation of SMSCs after IL-1β stimulation.
Key messages
-
Suberoykanillde hydroxamic acid (SAHA) and panobinostat (LBH589) attenuated chondrogenesis inhibition of SMSC induced by IL-1β. IL-6/MMP13 pathway inhibition contributed to this biological progress.
-
Silencing of IL-6 could directly downregulate MMP13 and attenuate inhibition of IL-1β-induced SMSC chondrogenesis.
Strengths and limitations
-
The strength of this study is that we showed that the HDAC inhibitors, SAHA and LBH589, attenuate chondrogenesis inhibition of SMSCs induced by IL-1β, and that the attenuated IL-6/MMP13 pathway activation contributed to this biological progress.
-
The main limitation of this study is lack of further exploration of the mechanism underlying attenuation of IL-6 expression by HDAC inhibitors.
Introduction
Destruction of the condylar cartilage of the temporomandibular joint (TMJ) is an important pathological process in temporomandibular arthritis.1 Because of their chondrogenic differentiation and immune regulation abilities, mesenchymal stem cells (MSCs) have been used to treat patients with cartilage injuries and osteoarthritis (OA).2-5 MSCs are isolated from both synovial fluid and synovium of the TMJ.6,7 Compared to MSCs from different sources, synovium-derived MSCs (SMSCs) show optimal chondrogenic ability.8 However, the chondrogenic ability of MSCs is inhibited by proinflammatory cytokines in OA joints, such as interleukin-1β (IL-1β).9
The expression level of IL-1β is elevated in the TMJ of patients with temporomandibular disorder (TMD) compared to that in the control group.10,11 IL-1β inhibits the synthesis of collagen in articular chondrocytes and increases the expression of articular cartilage matrix degrading enzymes.12,13 IL-1β impedes the chondrogenic differentiation of synovial fluid MSCs in the human TMJ.14 Therefore, IL-1β is also an important inflammatory cytokine that hinders the repair of condylar cartilage damage in TMD.
Histone deacetylases (HDACs) promote the deacetylation of histones, leading to increased chromatin condensation and inhibition of gene transcription.15 Recently, HDAC inhibitors have been shown to have therapeutic potential in OA;16-19 the underlying mechanism, however, remains poorly understood. HDAC inhibitors attenuate IL-6/MMP13 pathway activation in chondrocytes.20,21 In addition, a previous study found that upregulation of IL-6 may contribute to the biological process by which IL-1β inhibits the chondrogenic differentiation of synovial fluid MSCs.14 Therefore, we hypothesized that HDAC inhibitors attenuate the IL-1β-induced inhibition of SMSC chondrogenesis through inhibition of the IL-6/MMP13 pathway in the TMJ.
Here, we aimed to evaluate whether HDAC inhibitors can improve chondrogenesis inhibited by IL-1β, and to explore its mechanism.
Methods
Reagent preparation and grouping
IL-1β was dissolved using 5% trehalose buffer (Peprotech, USA), and then the solution was diluted to 10 ng/ml. IL-6 (Peprotech) and IL-6 soluble receptor (IL-6R; Novoprotein, China) was diluted to 10 ng/ml or 100 ng/m. The cells cultured in the plate were stimulated with 10 ng/ml IL-1β for 24 hours. Suberoylanilide hydroxamic acid (SAHA; Santa Cruz Biotechnology, USA) and panobinostat (LBH589; Santa Cruz Biotechnology) were dissolved in DMSO (MP Biomedicals, USA) and were diluted to 3 μM and 5 μM, respectively; the plated cells were treated with SAHA and LBH589 for two hours.
SMSC cell culture
Synovial tissues were obtained from patients with temporomandibular joint osteoarthritis (TMJOA) during open surgery of the TMJ. This study was approved by our Institutional Ethics Board. Informed consent was obtained from all patients before surgery. The synovial tissues were cut into small pieces and digested in 4 mg/ml type I collagenase (Sigma-Aldrich, USA). Then, the primary cells were suspended in complete culture medium comprising low-glucose Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific). The cells were passaged at a density of 500 cells/cm2. Cells from passages 3–5 were used in the following experiments.
Characterization of SMSCs
SMSCs were incubated with antibodies against the surface markers and isotype controls. Surface markers, including CD34, CD11b, CD19, CD44, CD45, CD90, CD105, human leucocyte antigen (HLA)-DR, and CD146 were obtained from BD Biosciences (Germany). CD73 was provided by Miltenyi Biotechnology (Germany). Flow cytometer (BD Biosciences) was used to detect the positive cells.
To confirm whether the cells after in vitro passage met the minimal definition criteria of human MSCs proposed by the Mesenchymal Tissue Stem Cell Committee of the International Society for Cellular Therapy,22 osteogenic, adipogenic, and chondrogenic differentiation was performed using the methods described previously.23
Chondrogenic induction of SMSCs
A total of 3 × 105 SMSCs were harvested and centrifuged in each 15 ml tube at 450 × g for ten minutes. The precipitate was incubated with 500 μl chondrogenetic induction medium (Stem Chondro Diff Kit, Thermo Fisher Scientific) at 37°C with the caps loosened. The medium was replaced with fresh chondrogenetic induction medium every three to four days. After one week incubation, the cartilage pellets could be observed in the bottom of the tube. The cartilage pellets were then treated with chondrogenetic induction medium containing 10 ng/ml IL-1β and/or with 3 μM SAHA or 5 μM LBH589 separately according to the grouping. The cartilage pellets or the supernatants were collected for the subsequent examination after treatment above for two weeks.
Histological analysis and immunohistochemical staining
Cartilage pellets were fixed in 4% paraformaldehyde. Sections of cartilage pellets were stained with Alcian blue (Cyagen, USA) following the manufacturer’s instructions. The sections for immunohistochemical staining were achieved after incubation with type II collagen antibody (1:100; CST) overnight. The stained sections were observed under a microscope (Axioskop40; Carl Zeiss AG, Germany).
Construction and transduction of lentiviral vectors
The lentiviral vectors Lv-green fluorescent protein (GFP, negative control) and Lv-siIL-6 (silencing IL-6) were constructed by Genechem (China). The sequence of Lv-siIL-6 was TCTCATTCTGCGCAGCTTT. The vectors were diluted with enhanced transfection solution at a multiplicity of infection (MOI) of 1:10. Afterwards, cells were treated according to grouping or dislodged by gentle pipetting and used as a supply for chondrogenic differentiation.
Enzyme-linked immunosorbent assay for soluble sulphated glycosaminoglycan in the supernatant
Cell culture supernatant was centrifuged at 3,000 × g for ten minutes. Microtiter plates coated with purified human soluble sulphated glycosaminoglycan (sGAG) antibody were provided in the enzyme-linked immunosorbent assay (ELISA) kit (Blue gene, China). The concentration of sGAG secreted in the supernatant was detected according to the manufacturer’s instructions.
RNA extraction and reverse transcription polymerase chain reaction analysis
Total RNA was extracted from SMSCs using TRIzol (Thermo Fisher Scientific); the cartilage pellets were powdered in liquid nitrogen before adding TRIzol. RNA (1 µg) was reverse-transcribed using the Superscript reverse-transcription system (Takara Biotechnology, China). The relative messenger RNA (mRNA) expression level of specific genes was measured using a SYBR Green qPCR kit (Roche, Switzerland) and calculated using the 2-ΔΔCT method. The primer sequences of the target genes are shown in Supplementary Table i.
Measurement of IL-6 in culture supernatants using a cytometric bead array assay
The culture supernatants of cartilage pellets were collected after treatment, according to the experimental grouping. The supernatants were centrifuged at 3,000 × g for ten minutes. The concentration of IL-6 was measured using a human IL-6 CBA kit (BD Biosciences, USA). Samples were prepared according to the manufacturer’s instructions. Data were analyzed using the Cytoflex software (Beckman Coulter, USA).
Western blotting
A total of four to six cartilage pellets were powdered in liquid nitrogen before protein extraction. Proteins were separated via sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) (Beyotime, China) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The membranes were incubated with primary antibodies including type II collagen, SRY-Box Transcription Factor 9 (SOX9), and actin (Cell Signaling Technology, USA) and then with a secondary antibody (Cell Signaling Technology). Proteins were detected using an enhanced chemiluminescence kit (Millipore).
Animal experiments
A total of 40 male Sprague-Dawley rats (300 g to 400 g, eight to ten weeks old) were randomly divided into four groups. No treatment was administered to the control group. The bilateral TMJs of the rest of the rats were intra-articularly injected with 200 μl of 10 mg/ml collagenase (Sigma-Aldrich) every two weeks. Four weeks after injection, the other 20 rats were injected with 200 μl of 10 μM or 20 μM SAHA once. After treatment with SAHA for two weeks, all the rats were killed. The technical procedure of injection into the TMJ of rats has been previously described.24 As TMJOA developed, the destruction of the surface of the condyles was evaluated using a stereoscopic microscope. Five synovium samples from each group were collected, mixed, and stored in TRIzol for RNA extraction. The expression of IL-1β, IL-6, IL-8, tissue inhibitor of matrix metalloproteinase-1 (TIMP1), TIMP2, A Disintegrin and Metalloproteinase with Thrombospondin motifs 4 (ADAMTS4), and ADAMTS5 was determined using reverse transcription quantitative polymerase chain reaction (RT-qPCR). The other five specimens were then fixed, decalcified, and embedded in paraffin. Sections of condyle were stained with safranin O-fast green, following the manufacturer’s instructions. This study was approved by the Animal Ethical and Welfare Committee. An ARRIVE checklist has been provided as Supplementary Material.
Statistical analysis
Data are presented as the mean and standard deviation (SD). Analysis of variance or independent-samples t-test were used to determine the statistical significance between two or more than two groups. A p-value < 0.05 was defined as statistically significant. The experiments in this study were repeated at least three times.
Results
Characterization of SMSCs derived from the TMJ
After homogeneous spindle-shaped cells were subjected to osteogenic induction for four weeks, calcium deposits could be observed after being stained with Alizarin red (Supplementary Figure aa). The upregulation of the expression of two osteogenic differentiation markers, RUNX2 (Supplementary Figure ad) and OCN (Supplementary Figure ae), was detected by RT-PCR. Lipid vesicles were observed by Oil red staining in the cells after adipogenic induction for 28 days (Supplementary Figure ab). The expression of two adipocyte-specific genes, LPL (Supplementary Figure af) and PPARG2 (Supplementary Figure ag), was significantly upregulated. After culture in chondrogenic induction medium for 28 days, histological sections of the cartilage nodules showed a cartilage matrix that was stained with Alcian blue (Supplementary Figure ac). Expression levels of the chondrogenic markers COL2 (Supplementary Figure ah) and SOX9 (Supplementary Figure ai) were significantly higher than those in the control group. Moreover, these cells expressed CD90, CD73, CD105, and CD44 at high rates (> 95%), and rarely expressed CD45, CD34, CD11b, HLA-DR, CD19, or CD146 (< 2%), as detected by flow cytometry (Supplementary Figure aj).
Chondrogenic differentiation of SMSCs was inhibited by IL-1β
After chondrogenic induction for two weeks, the expression of COL2, SOX9, aggrecan (ACAN), and MMP13 was significantly higher than that in the control group, in which the SMSCs were cultured in medium without chondrogenic induction. The concomitant addition of 10 ng/ml IL-1β to the chondrogenic induction medium inhibited the expression of COL2, SOX9, and ACAN, but increased the expression of MMP13, as shown in Supplementary Figures aa to ad. The size of cartilage nodules after chondrogenic induction with co-treatment of IL-1β was smaller than that of the induction group without IL-1β treatment (Figure 1a). Immunohistochemical results showed that type II collagen expression in the IL-1β-induced group was lower than that in the induced group (Figure 1a). The level of sGAG in the supernatant of the culture medium increased after chondrogenic induction, but decreased in the IL-1β-treated group (Figure 1b).
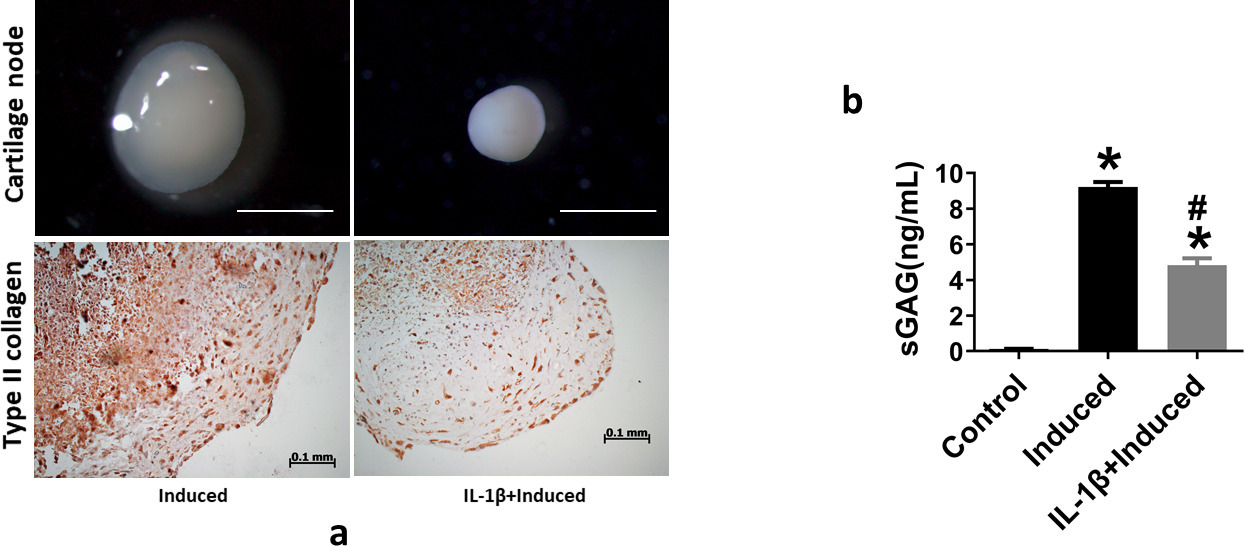
Fig. 1
a) Interleukin (IL)-1β inhibits chondrogenic differentiation of synovium-derived mesenchymal stem cells (SMSCs). Stereoscopic microscopy reveals the morphology and size of cartilage pellets after SMSCs underwent chondrogenic differentiation with or without 10 ng/ml IL-1β treatment. Scale bar = 1 mm. Immunohistochemical staining of type II collagen in cartilage pellet sections. Scale bar = 100 μm. b) Soluble sulphated glycosaminoglycan (sGAG) in the culture supernatant, as determined by enzyme-linked immunosorbent assay. *p < 0.05 compared to the control group. #p < 0.05 compared to the chondrogenic-induced group. One-way analysis of variance was used to determine the p-values. Least-Significant Difference (LSD) test was used to analyze data with homogeneous variances, and Games-Howell test was used for data with non-homogeneous variances.
HDAC inhibitor reversed IL-1β-induced inhibition of SMSC chondrogenesis
The effect of HDAC inhibitors on IL-1β-induced inhibition of SMSC chondrogenesis was evaluated by adding IL-1β and HDAC inhibitors (10 μM SAHA and 3 μM LBH589), concomitantly with the chondrogenic induction cultures. The expression of the chondrocyte marker genes COL2, SOX9, and ACAN increased after co-treatment with SAHA or LBH589 compared to that in the IL-1β-induced group (Figure 2a). A similar trend was found for type II collagen and SOX9 expression, as detected by western blotting (Supplementary Figures ca and cb). The cartilage matrix-degrading enzyme, MMP13, was downregulated by adding SAHA or LBH589 (Figure 2a). After co-treatment with SAHA or LBH589, the levels of sGAG in the culture medium increased (Figure 2b). After adding SAHA or LBH589, the size of cartilage nodules was larger than that of the induction group with IL-1β treatment (Figure 2c). The increased size of the cartilage matrix was detected by immunostaining with a type II collagen antibody (Figure 2c).
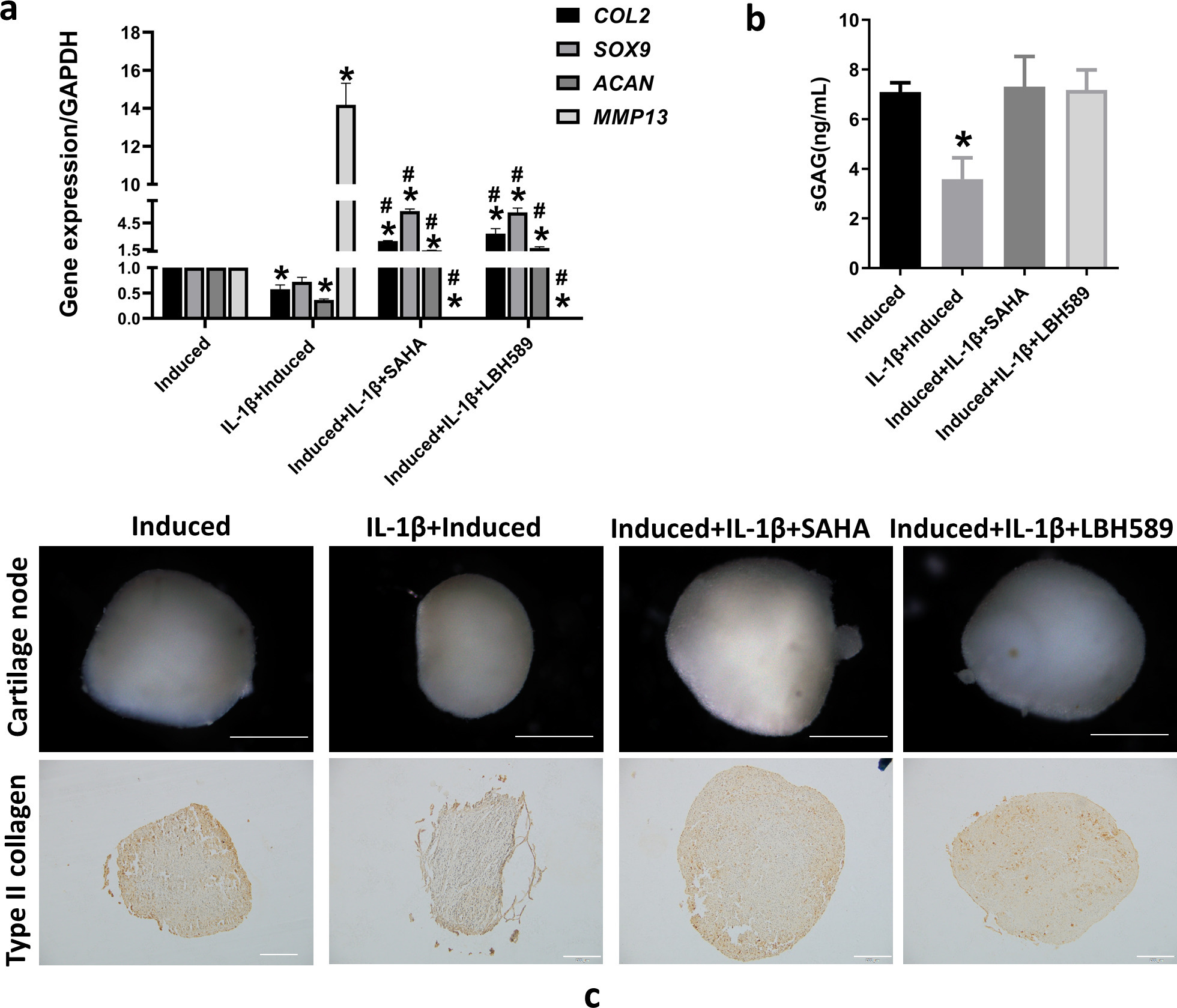
Fig. 2
Histone deacetylase (HDAC) inhibitor reverses interleukin (IL)-1β-induced inhibition of synovium-derived mesenchymal stem cell (SMSC) chondrogenesis. a) Messenger RNA expression of type II collagen (COL2), SRY-box transcription factor 9 (SOX9), aggrecan (ACAN), and matrix metalloproteinase 13 (MMP13) using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) after SMSCs were co-treated with 10 ng/ml IL-1β and 10 μM suberoylanilide hydroxamic acid (SAHA) or 3 μM LBH589 during chondrogenic induction. b) Soluble sulphated glycosaminoglycan (sGAG) in culture supernatant, as determined by enzyme-linked immunosorbent assay. c) The size of cartilage pellets by stereoscopic microscope observation. Scale bar = 0.5 mm. Type II collagen immunochemical staining of cartilage pellet sections. Scale bar = 100 μm. *p < 0.05 compared to the chondrogenic induced group. #p < 0.05 compared to the chondrogenic induced group with IL-1β treatment. One-way analysis of variance was used to determine the p-values. Least-Significant Difference (LSD) test was used to analyze data with homogeneous variances, and Games-Howell test was used for data with non-homogeneous variances. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
HDAC inhibitors decreased cartilage destruction factors in the synovium and protected TMJ cartilage in vivo
To further verify the cartilage protection effect of HDAC inhibitors in vivo, a TMJOA rat model was constructed by injecting collagenase into the TMJ cavity. Then, the damage to the condylar cartilage was evaluated after injecting 10 μM or 20 μM SAHA into the TMJ cavity of the TMJOA model group for two weeks. After the rats were killed, condyles were removed and observed under a stereomicroscope. Obvious cartilage destruction and a congestion reaction on the condylar surface were observed in the TMJOA group. Cartilage destruction and hyperemia decreased after injecting SAHA (Figure 3a). This result was further confirmed by safranin O-fast green staining of the condyle sections. The cartilage matrix that was red-stained in the section decreased significantly in the TMJOA group, but it was partially alleviated after co-treatment with SAHA in a concentration-dependent manner (Figure 3a). RT-qPCR analysis showed that gene expression of inflammatory factors (IL-1β, IL-6, IL-8, and tumour necrosis factor (TNF)-α) and cartilage matrix metabolism-related enzymes (TIMP1, TIMP2, ADAMTS4, and ADAMTS5) in the synovial tissue from the TMJ were both decreased in the SAHA treatment group compared to that in the TMJOA group (Figure 3b).
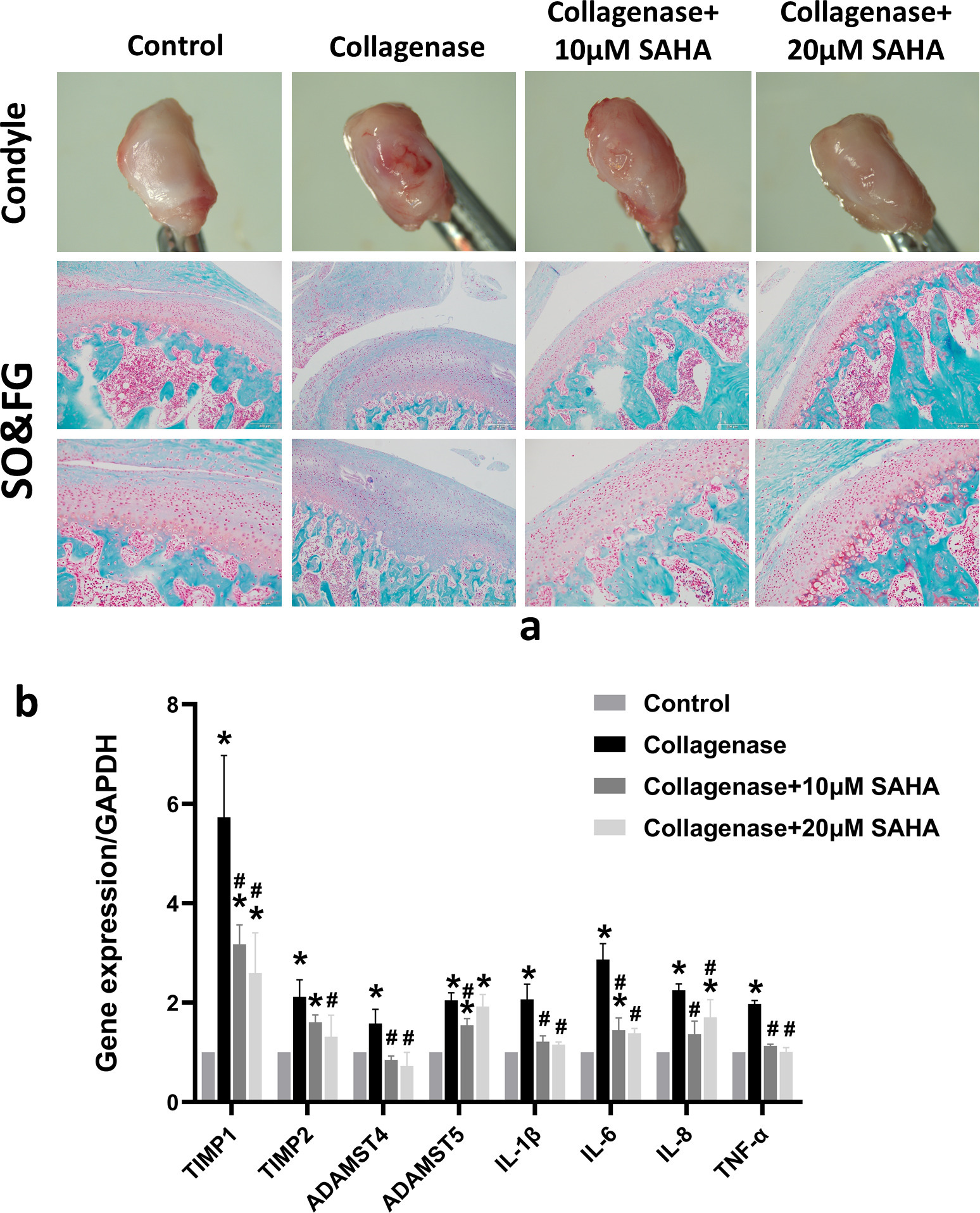
Fig. 3
Histone deacetylase (HDAC) inhibitor decreases cartilage destruction factors in the synovium and protects temporomandibular joint (TMJ) cartilage in vivo. a) Evaluation of the rat condyle articular surface using a stereoscopic microscope. Safranin O-fast green (SO&FG) staining of condyle sections. The TMJ osteoarthritis (OA) rat model was established by injecting 200 μl 10 mg/ml collagenase into the TMJ cavity for two weeks. A total of 200 μl 10 μM or 20 μM suberoylanilide hydroxamic acid (SAHA) was injected into the TMJ cavity of TMJOA rats for two weeks, which was considered the collagenase + SAHA-treated group. Red staining indicates the cartilage matrix. Scale bar = 100 μm. b) Expression of interleukin (IL)-1β, IL-6, IL-8, tumour necrosis factor (TNF-α), TIMP metallopeptidase inhibitor 1 (TIMP1), TIMP2, ADAM metallopeptidase with thrombospondin type 1 motif 4 (ADAMTS4), and ADAMTS5 in the synovial tissue from the TMJ of rats, as determined by reverse transcription-quantitative polymerase chain reaction. *p < 0.05 compared to the control group. #p < 0.05 compared to the collagenase group. One-way analysis of variance was used to determine the p-values. Least-Significant Difference (LSD) test was used to analyze data with homogeneous variances, and Games-Howell test was used for data with non-homogeneous variances.
HDAC inhibitor attenuated IL-6 contributing to the improvement of IL-1β-induced inhibition of chondrogenesis
The levels of IL-6 in the supernatant of chondrogenic induction medium and gene expression were both significantly increased after IL-1β stimulation, but decreased after co-treatment with the HDAC inhibitors (Figures 4a and 4b). To further analyze the role of IL-6 in chondrogenic differentiation of SMSC induced by IL-1β, IL-6 was silenced using lentiviral transfection. Knockdown efficiency was confirmed by measuring gene expression (Supplementary Figure da). Silencing IL-6 suppressed the induced gene expression of IL-6 and in the supernatant of chondrogenic induction medium (Supplementary Figures db and dc) and MMP13 induced by IL-1β (Figure 4c). After chondrogenic induction for 21 days, silencing of IL-6 increased the expression of chondrocyte marker genes (COL2, SOX9, and ACAN), while decreasing the cartilage destruction factors MMP13 and IL-6 in the cartilage pellets (Figure 4d). Stereomicroscopy showed that silencing of IL-6 expression restored the size of cartilage nodules, which was affected by IL-1β. The accumulation of type II collagen in the cartilage nodules also increased, as revealed by immunohistochemistry (Figure 4e). In order to further confirm the role of IL-6 in this system, 10 ng/ml or 100 ng/ml IL-6 with or without the same concentration of IL-6R were added to the chondrogenic induction medium for two weeks. After chondrogenic induction with IL-6, the expression of COL2, SOX9, and ACAN were decreased (Supplementary Figures ea to ec), while the expression of MMP13 was increased (Supplementary Figure ed). The levels of sGAG in the culture medium were also decreased after being treated with IL-6 (Supplementary Figure ee).
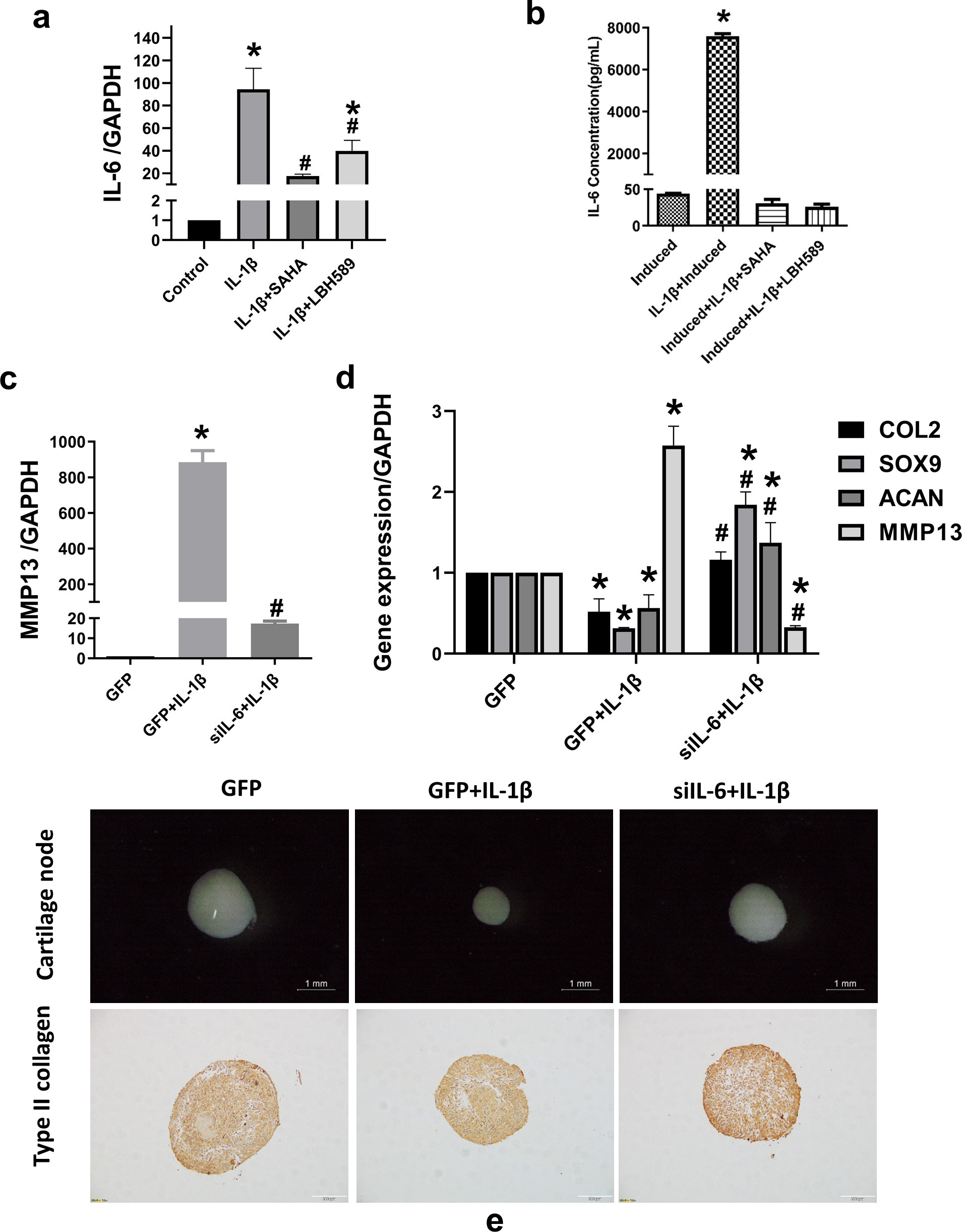
Fig. 4
Histone deacetylase (HDAC) inhibitors attenuate interleukin (IL)-6 contributing to the improvement of IL-1β-induced inhibition of chondrogenesis. a) After synovium-derived mesenchymal stem cells (SMSCs) were pre-treated with 10 μM suberoylanilide hydroxamic acid (SAHA) or 3 μM LBH589 for two hours and then treated with 10 ng/ml IL-1β for 24 hours, RNA was extracted. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to evaluate IL-6 messenger RNA (mRNA) expression; *p < 0.05 compared to the control group. #p < 0.05 compared to the IL-1β-treated group, n = 3. b) Secretion of IL-6 in the supernatant of chondrogenic induction medium, as detected by a cytometric bead array (CBA). c) The expression levels of matrix metalloproteinase 13 (MMP13) were detected after SMSCs were pre-transfected with the empty GFP vector or the knock-down IL-6 GFP vector for 48 hours and then co-treated with 10 ng/ml IL-1β for 24 hours, as detected by RT-qPCR and CBA. d) mRNA expression of type II collagen (COL2), SRY-box transcription factor 9 (SOX9), aggrecan (ACAN), MMP13, and IL-6, as estimated by RT-qPCR after chondrogenic induction; *p < 0.05 compared to the GFP group. #p < 0.05 compared to the GFP+IL-1β group. e) The size of the cartilage pellets, as estimated by stereoscopic microscopy. Scale bar = 1 mm. Immunohistochemical staining of type II collagen in cartilage pellet sections. Scale bar = 100 μm. One-way analysis of variance was used to determine the p-values. Least-Significant Difference (LSD) test was used to analyze data with homogeneous variances, and Games-Howell test was used for data with non-homogeneous variances. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
MSCs are widely distributed in various tissues of the TMJ, such as the intima of the synovium, subintima of the synovium, and synovial fluid.23 High-purity and uniform SMSCs could be obtained from primary cells cultured from synovial specimens in vitro by screening antigen markers of MSCs. While in this study, the primary cells cultured from synovial specimens were expanded in vitro at a low density, and after in vitro passage, the cells also met the minimal definition criteria of human MSCs proposed by the Mesenchymal Tissue Stem Cell Committee of the International Society for Cellular Therapy.22 Studies have revealed that SMSCs show decreased proliferation and chondrogenic capacity, but with enhanced migration, proinflammatory, and matrix degradation activities in patients with OA.25 However, the exact mechanism of action of how the inflammatory microenvironment impairs SMSC function is not fully understood.
IL-1β is generally considered to be a vital pathogenic factor in the TMJ. Expression of IL-1β is greatly increased in the synovial fluid of patients with TMJ disorders.26,27 IL-1β is the first inflammatory response factor when the TMJ is overloaded.28 Many studies have examined the pathogenic mechanism of IL-1β in joints; one reports that 10 ng/ml IL-1β inhibits collagen synthesis in rabbit articular chondrocytes and reduces expression levels of COL2 mRNA in vitro.12 Early degenerative changes and decreased synthesis of type II collagen are observed after human articular chondrocytes are pretreated with 10 ng/ml IL-1β.28 In this study, 10 ng/ml IL-1β was also used to evaluate its effect on SMSCs. In general, the current understanding of IL-1β pathogenesis in arthritis mainly has the following two points. First, IL-1β inhibits the synthesis of collagen and increases the secretion of cartilage matrix-degrading enzymes in articular chondrocytes, participating in cartilage matrix destruction.12,29 Second, IL-1β increases the secretion of cartilage matrix-degrading enzymes, such as MMP13, disrupts immunomodulatory function, and impedes chondrogenic differentiation of MSCs in the joint.14,30 Here, the proinflammatory cytokine IL-1β also exhibited a negative effect on the chondrogenic differentiation of SMSCs. Further, elevated IL-6 expression induced by IL-1β in SMSCs contributed to this biological process, indicating that blocking IL-1β-induced IL-6 upregulation in SMSCs is likely a target for improved MSC-based therapy in the TMJ.
Studies have demonstrated that changes in HDAC activity and expression lead to the initiation and progression of OA.31,32 Currently, type I and type II HDAC members are considered to be closely related to OA.33 Many studies have confirmed that HDAC inhibitors mainly protect joints by reducing the secretion of inflammatory factors that damage cartilage, such as IL-1 and IL-6, via chondrocytes secreting proteases that destroy cartilage matrix, such as MMP13, and inhibiting chondrocyte hypertrophy.34-37 Therefore, the effects of HDAC inhibitors (most of them are type I and type II HDAC member inhibitors) on OA have been well explored.
SAHA and LBH589 used in this study are the most common broad-spectrum HDAC inhibitors. They are currently approved by the Food and Drug Administration for use as anticancer drugs. Studies have confirmed that they might benefit arthritis treatment, as SAHA selectively inhibits the activation of human chondrocyte p38 and extracellular signal-regulated protein kinase 1/2 to block the production of MMPs and nitric oxide induced by IL-1β.19 SAHA induces the production of the IL-6 negative feedback regulator monocyte chemotactic protein-1-induced protein-1 in human chondrocytes, thereby inhibiting the expression of IL-6 caused by IL-1β stimulation.21 According to the current animal study, it was also confirmed that SAHA attenuated joint cartilage damage caused by collagenase, and additionally attenuated inflammatory cytokine and protease expression, which were detrimental to cartilage in the synovium. As well as protecting chondrocytes and attenuating synovial inflammation, the HDAC inhibitors SAHA and LBH589 blocked IL-1β-induced IL-6 upregulation and improved the potential of MSCs that differentiated into cartilage, which was inhibited by IL-1β.
The IL-6 concentration in synovial fluid from patients with OA is higher than that in healthy individuals.38 IL-6 expression was also significantly increased after IL-1β stimulation in SMSCs in this study. The pathogenesis of IL-6 in arthritis is similar to that of IL-1β; IL-6 plays an important role in cartilage destruction in a mouse model of OA induced by mechanical stress or hypoxia inducible factor 2a.39 IL-6 also stimulates chondrocytes to produce aggrecanase, which leads to cartilage matrix degradation.40 Additionally, IL-6 affects the behaviour of MSCs, as IL-6 inhibits the early chondrogenic differentiation of ATDC5 cells.41 In this study, IL-6 also inhibited the chondrogenic differentiation of SMSCs in the TMJ. Blocking IL-6 using knockdown technology, or the HDAC inhibitors SAHA and LBH589, significantly improved the potential of MSCs that differentiated into cartilage. Furthermore, MMP13 is one of the most important collagenases involved in the degradation of articular cartilage in OA.42 MMP13 expression is significantly upregulated in chondrocytes after IL-1β treatment. In this study, MMP13 expression was also significantly upregulated in SMSCs after treatment with IL-1β. The link between IL-6 and MMP13 remains controversial; in this study, silencing of IL-6 significantly blocked MMP13 expression.
In conclusion, the HDAC inhibitors SAHA and LBH589 attenuated the IL-1β-induced inhibition of SMSC chondrogenesis in the TMJ, and attenuated IL-6/MMP13 pathway activation that contributed to this biological progress.
References
1. Jiao K , Niu L-N , Wang M-Q , et al. Subchondral bone loss following orthodontically induced cartilage degradation in the mandibular condyles of rats . Bone . 2011 ; 48 ( 2 ): 362 – 371 . Crossref PubMed Google Scholar
2. Harrell CR , Markovic BS , Fellabaum C , Arsenijevic A , Volarevic V . Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives . Biomed Pharmacother . 2019 ; 109 : 2318 – 2326 . Crossref PubMed Google Scholar
3. Chen Chou AC , Tjoen Lie DT . Clinical outcomes of an all-arthroscopic technique for single-stage autologous matrix-induced chondrogenesis in the treatment of articular cartilage lesions of the knee . Arthrosc Sports Med Rehabil . 2020 ; 2 ( 4 ): e353 – e359 . Crossref PubMed Google Scholar
4. Gobbi A , Whyte GP . Long-term clinical outcomes of one-stage cartilage repair in the knee with hyaluronic acid-based scaffold embedded with mesenchymal stem cells sourced from bone marrow aspirate concentrate . Am J Sports Med . 2019 ; 47 ( 7 ): 1621 – 1628 . Crossref PubMed Google Scholar
5. Wang J , Zhou L , Zhang Y , Huang L , Shi Q . Mesenchymal stem cells - a promising strategy for treating knee osteoarthritis . Bone Joint Res . 2020 ; 9 ( 10 ): 719 – 728 . Crossref PubMed Google Scholar
6. Koyama N , Okubo Y , Nakao K , Osawa K , Fujimura K , Bessho K . Pluripotency of mesenchymal cells derived from synovial fluid in patients with temporomandibular joint disorder . Life Sci . 2011 ; 89 ( 19–20 ): 741 – 747 . Crossref PubMed Google Scholar
7. Sun Y , Zheng Y , Liu W , Zheng Y , Zhang Z . Synovium fragment-derived cells exhibit characteristics similar to those of dissociated multipotent cells in synovial fluid of the temporomandibular joint . PLoS One . 2014 ; 9 ( 7 ): e101896 . Crossref PubMed Google Scholar
8. Yoshimura H , Muneta T , Nimura A , Yokoyama A , Koga H , Sekiya I . Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle . Cell Tissue Res . 2007 ; 327 ( 3 ): 449 – 462 . Crossref PubMed Google Scholar
9. Ousema PH , Moutos FT , Estes BT , et al. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds . Biomaterials . 2012 ; 33 ( 35 ): 8967 – 8974 . Crossref PubMed Google Scholar
10. Kellesarian SV , Al-Kheraif AA , Vohra F , et al. Cytokine profile in the synovial fluid of patients with temporomandibular joint disorders: a systematic review . Cytokine . 2016 ; 77 : 98 – 106 . Crossref PubMed Google Scholar
11. Almeida LE , Pierce S , Zacharias J , et al. Immunohistochemical analysis of IL-1 beta in the discs of patients with temporomandibular joint dysfunction . Cranio . 2017 ; 35 ( 4 ): 233 – 237 . Crossref PubMed Google Scholar
12. Pujol J-P , Chadjichristos C , Legendre F , et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism . Connect Tissue Res . 2008 ; 49 ( 3 ): 293 – 297 . Crossref PubMed Google Scholar
13. Chadjichristos C , Ghayor C , Kypriotou M , et al. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes . J Biol Chem . 2003 ; 278 ( 41 ): 39762 – 39772 . Crossref PubMed Google Scholar
14. Liu W , Sun Y , He Y , et al. IL-1β impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint . Int J Mol Med . 2017 ; 39 ( 2 ): 317 – 326 . Crossref PubMed Google Scholar
15. Wolffe AP . Histone deacetylase: a regulator of transcription . Science . 1996 ; 272 ( 5260 ): 371 – 372 . Crossref PubMed Google Scholar
16. Chen WP , Bao JP , Hu PF , Feng J , Wu LD . Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis . Mol Biol Rep . 2010 ; 37 ( 8 ): 3967 – 3972 . Crossref PubMed Google Scholar
17. Nakamura C , Matsushita I , Kosaka E , Kondo T , Kimura T . Anti-arthritic effects of combined treatment with histone deacetylase inhibitor and low-intensity ultrasound in the presence of microbubbles in human rheumatoid synovial cells . Rheumatology . 2008 ; 47 ( 4 ): 418 – 424 . Crossref PubMed Google Scholar
18. Qu H , Li J , Wu LD , Chen WP . Trichostatin A increases the TIMP-1/MMP ratio to protect against osteoarthritis in an animal model of the disease . Mol Med Rep . 2016 ; 14 ( 3 ): 2423 – 2430 . Crossref PubMed Google Scholar
19. Zhong H , Ding Q , Chen W , Luo R . Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-κB nuclear translocation . Int Immunopharmacol . 2013 ; 17 ( 2 ): 329 – 335 . Crossref PubMed Google Scholar
20. Makki MS , Haqqi TM . Histone deacetylase inhibitor vorinostat (SAHA) suppresses IL-1β-induced matrix metallopeptidase-13 expression by inhibiting IL-6 in osteoarthritis chondrocyte . Am J Pathol . 2016 ; 186 ( 10 ): 2701 – 2708 . Crossref PubMed Google Scholar
21. Makki MS , Haqqi TM . Histone deacetylase inhibitor vorinostat (SAHA, MK0683) perturb miR-9-MCPIP1 axis to block IL-1β-induced IL-6 expression in human OA chondrocytes . Connect Tissue Res . 2017 ; 58 ( 1 ): 64 – 75 . Crossref PubMed Google Scholar
22. Dominici M , Le Blanc K , Mueller I , et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement . Cytotherapy . 2006 ; 8 ( 4 ): 315 – 317 . Crossref PubMed Google Scholar
23. Yao Y , Li ZY , Zhang H , et al. Synovial fluid‑derived synovial fragments represent an improved source of synovial mesenchymal stem cells in the temporomandibular joint . Int J Mol Med . 2018 ; 41 ( 1 ): 173 – 183 . Crossref PubMed Google Scholar
24. Fuentes R , Veuthey C , Arias A , Saravia D , Ottone NE . Injection in temporomandibular joint of rats. Description of technical protocol . Pol J Vet Sci . 2017 ; 20 ( 2 ): 207 – 211 . Crossref PubMed Google Scholar
25. Huang J , Chen C , Liang C , et al. Dysregulation of the wnt signaling pathway and synovial stem cell dysfunction in osteoarthritis development . Stem Cells Dev . 2020 ; 29 ( 7 ): 401 – 413 . Crossref PubMed Google Scholar
26. Sorenson A , Hresko K , Butcher S , et al. Expression of interleukin-1 and temporomandibular disorder: contemporary review of the literature . Cranio . 2018 ; 36 ( 4 ): 268 – 272 . Crossref PubMed Google Scholar
27. Kim Y-K , Kim S-G , Kim B-S , et al. Analysis of the cytokine profiles of the synovial fluid in a normal temporomandibular joint: preliminary study . J Craniomaxillofac Surg . 2012 ; 40 ( 8 ): e337 - 41 . Crossref PubMed Google Scholar
28. Shakibaei M , Schulze-Tanzil G , John T , Mobasheri A . Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study . Ann Anat . 2005 ; 187 ( 5–6 ): 487 – 497 . Crossref PubMed Google Scholar
29. Li X , He P , Hou Y , et al. Berberine inhibits the interleukin-1 beta-induced inflammatory response via MAPK downregulation in rat articular chondrocytes . Drug Dev Res . 2019 ; 80 ( 5 ): 637 – 645 . Crossref PubMed Google Scholar
30. Mohanraj B , Huang AH , Yeger-McKeever MJ , Schmidt MJ , Dodge GR , Mauck RL . Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro-inflammatory cytokines . J Orthop Res . 2018 ; 36 ( 11 ): 2901 – 2910 . Crossref PubMed Google Scholar
31. Vojinovic J , Damjanov N . HDAC inhibition in rheumatoid arthritis and juvenile idiopathic arthritis . Mol Med . 2011 ; 17 ( 5–6 ): 397 – 403 . Crossref PubMed Google Scholar
32. Carpio LR , Westendorf JJ . Histone deacetylases in cartilage homeostasis and osteoarthritis . Curr Rheumatol Rep . 2016 ; 18 ( 8 ): 52 . Crossref PubMed Google Scholar
33. Chen Z , Zhang Z , Guo L , et al. The role of histone deacetylase 4 during chondrocyte hypertrophy and endochondral bone development . Bone Joint Res . 2020 ; 9 ( 2 ): 82 – 89 . Crossref PubMed Google Scholar
34. Hsieh IN , Liou JP , Lee HY , Lai MJ , Li YH , Yang CR . Preclinical anti-arthritic study and pharmacokinetic properties of a potent histone deacetylase inhibitor MPT0G009 . Cell Death Dis . 2014 ; 5 : e1166 . Crossref PubMed Google Scholar
35. Oh BR , Suh DH , Bae D , et al. Therapeutic effect of a novel histone deacetylase 6 inhibitor, CKD-L, on collagen-induced arthritis in vivo and regulatory T cells in rheumatoid arthritis in vitro . Arthritis Res Ther . 2017 ; 19 ( 1 ): 154 . Crossref PubMed Google Scholar
36. Joosten LAB , Leoni F , Meghji S , Mascagni P . Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis . Mol Med . 2011 ; 17 ( 5–6 ): 391 – 396 . Crossref PubMed Google Scholar
37. Young DA , Lakey RL , Pennington CJ , et al. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption . Arthritis Res Ther . 2005 ; 7 ( 3 ): R503 - 12 . Crossref PubMed Google Scholar
38. Tsuchida AI , Beekhuizen M , Rutgers M , et al. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model . Arthritis Res Ther . 2012 ; 14 ( 6 ): R262 . Crossref PubMed Google Scholar
39. Ryu JH , Yang S , Shin Y , Rhee J , Chun CH , Chun JS . Interleukin-6 plays an essential role in hypoxia-inducible factor 2α-induced experimental osteoarthritic cartilage destruction in mice . Arthritis Rheum . 2011 ; 63 ( 9 ): 2732 – 2743 . Crossref PubMed Google Scholar
40. Flannery CR , Little CB , Hughes CE , Curtis CL , Caterson B , Jones SA . IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage . Matrix Biol . 2000 ; 19 ( 6 ): 549 – 553 . Crossref PubMed Google Scholar
41. Nakajima S , Naruto T , Miyamae T , et al. Interleukin-6 inhibits early differentiation of ATDC5 chondrogenic progenitor cells . Cytokine . 2009 ; 47 ( 2 ): 91 – 97 . Crossref PubMed Google Scholar
42. Dahlberg L , Billinghurst RC , Manner P , et al. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1) . Arthritis Rheum . 2000 ; 43 ( 3 ): 673 – 682 . Crossref PubMed Google Scholar
Author contributions
W-T. Liao: Investigation, Formal analysis, Writing – original draft.
J-D. Sun: Investigation, Formal analysis, Writing – original draft.
Y. Wang: Investigation, Formal analysis, Writing – original draft.
Y-Q. He: Investigation.
K. Su: Investigation.
Y-Y. Lu: Investigation.
G. Liao: Conceptualization.
Y-P. Sun: Conceptualization, Formal analysis, Investigation, Writing – original draft.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: grants from the National Natural Science Foundation of China (grant no. 81800996), Natural Science Foundation of Guangdong Province (grant no. 2018A030310329), the Science and Technology Planning Project of Guangdong Province (grant no. 2015A030302019), GuangDong Basic and Applied Basic Research Foundation (2020A1515110302), and Science and Technology Program of Guangzhou (202102020072).
Acknowledgements
We thank the patients who participated in our study for their commitment and all the study nurses for their invaluable work.
Open access funding
The authors confirm that the open access fee for this study was provided by the grants mentioned above.
Supplementary material
Table showing primer sequences for polymerase chain reaction, and figures showing the characterization of synovium-derived mesenchymal stem cells (SMSCs) from the temporomandibular joint, interleukin (IL)-1β inhibition of chondrogenic differentiation of SMSCs, histone deacetylase inhibitor reversal of IL-1β-induced inhibition of SMSC chondrogenesis, and IL-6 inhibition of chondrogenic differentiation of SMSCs.









