Abstract
Objectives
The need for bone tissue supplementation exists in a wide range of clinical conditions involving surgical reconstruction in limbs, the spine and skull. The bone supplementation materials currently used include autografts, allografts and inorganic matrix components; but these pose potentially serious side-effects. In particular the availability of the autografts is usually limited and their harvesting causes surgical morbidity. Therefore for the purpose of supplementation of autologous bone graft, we have developed a method for autologous extracorporeal bone generation.
Methods
Human osteoblast-like cells were seeded on porous granules of tricalcium phosphate and incubated in osteogenic media while exposed to mechanical stimulation by vibration in the infrasonic range of frequencies. The generated tissue was examined microscopically following haematoxylin eosin, trichrome and immunohistochemical staining.
Results
Following 14 days of incubation the generated tissue showed histological characteristics of bone-like material due to the characteristic eosinophilic staining, a positive staining for collagen trichrome and a positive specific staining for osteocalcin and collagen 1. Macroscopically, this tissue appeared in aggregates of between 0.5 cm and 2 cm.
Conclusions
We present evidence that the interaction of the cellular, inorganic and mechanical components in vitro can rapidly generate three-dimensional bone-like tissue that might be used as an autologous bone graft.
Article focus
We hypothesised that human bone could be generated in vitro.
Key message
The key message of the study is that we have presented a method for generation of a sufficient amount of autologous bone that might be used as autologous bone graft, without need for additional surgery.
Strengths and limitations of this study
The strength of this study is that we found clear evidence of bone-like tissue generation in vitro. The limitation of this study is that in vivo evidence of the generated tissue incorporation into bone gap is lacking, but this will be addressed in future studies.
Introduction
The need for bone tissue supplementation exists in a wide range of clinical conditions, including surgical reconstruction following trauma or other pathological conditions in limbs, the spine and skull.1 The amount of bone supplementation required and its intended purpose dictates the source of the tissue: for example, for bone inductive purposes, a supplementation of fresh autologous cancellous bone containing cellular, mineral and humeral components is required, and is usually taken from a non-involved body site. The material for bone conductive support purposes might require autografts, allografts or inorganic components of the bone matrix. All of these options for bone supplementation (autografts, allografts or inorganic matrix components) pose potentially serious side-effects and complications and have different levels of efficiency. The availability of autografts, which have the highest osteoinductive ability, is usually limited to their anatomical site and their harvesting can cause considerable surgical morbidity.2 The successful use of allografts is also limited because of the high risk of ‘docking site’ nonunion (or rejection of whole graft) and infection. The osteoconductive properties of inorganic material such as tricalcium phosphate, calcium phosphate and calcium sulphate are effective, predominantly in the filling of small gaps in bone.3 Therefore, the possibility for in vitro generation of a sufficient amount of autologous bone for inductive and conductive purposes might resolve these difficulties and complications.
For the purpose of supplementation of autologous bone graft sufficient for the requirements of an individual patient, we developed a method for the generation of extracorporeal autologous bone. By this method, bone matrix generating cells – osteoblasts – were seeded on an inorganic supporting matrix of porous tricalcium phosphate, in a specially designed bioreactor, allowing exposure of cells to osteogenic medium and their stimulation by biomechanical activation by mechanical vibration in an infrasonic range of frequencies.4 We hypothesised that by using this method we could generate live tissue with biochemical characteristics identical or similar to human bone.
Materials and Methods
Cells
The source of the osteoblasts were mesenchymal precursor cells that originated from disposable human cancellous bone samples, each between 2 g and 3 g in total, collected during elective hip replacements in four patients. These patients were two men and two women who had no systemic illnesses and who were aged between 60 and 65 years. The patients gave signed informed consent and the use of these cells was approved by the Institutional Ethical Committee. The site of the collection of bone samples was at least 5 cm distant from the subchondral bone area.
Culture technique
The osteoblasts were initially grown as explant primary cultures in a special bone inductive medium containing Dulbecco’s Modified Eagles Medium (DMEM) with heat-inactivated fetal calf serum (10%), 20 mM HEPES buffer, 2 mM L-Glutamine, 100 µM ascorbate-2-phosphate, 10 nM dexamethasone, 50 U/ml penicillin, and 150 µg/ml streptomycin at 37°C in a humidified atmospheric environment of 95% air with 5% CO2 (v:v), for between 20 and 30 days. The human bone cell cultures obtained by this standard method have been shown to express osteoblast-like characteristics,5,6 such as polygonal multipolar morphology, expression of the enzyme alkaline phosphatase, synthesis of a collagen-rich extracellular matrix with predominantly type I collagen and small amounts of collagen types III and V, and non-collagenous proteins, such as sialoprotein (BSP) and osteocalcin. Additionally, these cells demonstrate matrix mineralisation in vitro and bone formation in vivo. We have previously shown that these cells have osteoblastic characteristics such as multipolar morphology, adhesion to plastic surfaces, cellular alkaline phosphatase activity, positive Von Kossa staining, and osteopontin and osteocalcin expression.7
Following monolayer confluency, 106 cells were passaged onto three-dimensional granules of tricalcium phosphate (diameter 0.5 mm, pores 300 µ to 500 µ, total volume 5 cc) and cultured in the same type of osteogenic media in the same environmental conditions.
Mechanical stimulation
These cultures were also exposed to mechanical stimulation by horizontal vibration at an infrasonic range of frequencies. The reason for application of mechanical stimulation to the osteoblasts is the ability of these cells to increase bone matrix elaboration according to the vector (direction and magnitude of the mechanical forces to which they are exposed).8
Plates containing the cultured tissue were connected to a horizontally orientated shaker. The amplitude, wave of movement shape, and frequency of the vibration provided by the shaker were controlled by an amplifier and pulse generator. Vibration peak-to-peak acceleration was measured with a piezoelectric accelerometer and displayed on a vibration measuring amplifier. The displacement of vibration movement was calculated from the acceleration values. A sine-shaped vibration at 20 Hz frequency, (25 to 30) × 10-6 m of displacement amplitude and peak-to-peak acceleration of 0.5 m/sec2 (± 0.1 m/sec2) was applied to the well plates (Fig. 1). These vibration parameters have been found to be optimal for the induction of human osteoblast-like cells proliferation and metabolic activity.4 The rationale to use these parameters of vibration, i.e. frequency of 20 Hz, is based on the normal vibromiogram pattern, which reflects the basic skeletal muscle contraction at rest with subsequent mechanical effect on the adjacent bone and essentially osteoblast stimulation in vivo.9
The samples were exposed to the vibration protocol for four minutes once every 24 hours, a protocol previously found to be effective in stimulating the metabolic activity of human osteoblasts.4,10
Staining methods
On three, seven and 14 days following the start of the experiment, samples of the generated material were decalcified, embedded in paraffin, sectioned and stained by haematoxylin and eosin (HE) according to standard protocols, and inspected microscopically in order to evaluate the tissue morphology. After 14 days the samples were stained by the trichrome method for the general detection of collagen and by immunohistochemical assays for collagen 1 (mouse anti IgG collagen type 1, cat. SC-59772), and by osteocalcin (rabbit anti osteocalcin, cat. SC-30044) in order to determine the characteristics of the bone in the generated material. The microscopic HE-stained images of generated tissue were compared with microscopic images of stained samples of normal control bone tissue. All samples used as controls were from biopsies taken for non-related clinical reasons from patients who were not involved in the study. Similarly, the microscopic images of the generated tissue stained immunohistochemically for collagen 1 and osteocalcin were compared with the immunohistochemically stained normal bone samples as a positive control. They were also compared with cartilage samples as a negative control for collagen 1 staining, and to a sample of kidney tissue as a negative control for osteocalcin staining. Images of the generated and normal bone tissue stained without the addition of the antibodies to collagen 1 and osteocalcin were inspected as double negative controls.
Results
Three days after treatment of the cells in the bioreactor, there was histological evidence by HE-staining of islets of bone-like matrices with strong eosinophilic staining (Fig. 2) which became abundant after two weeks of culture (Fig. 3). There was also evidence of collagen deposition by the osteoblasts after two weeks of treatment in the bioreactor (trichrome staining; Fig. 4). Immunohistochemical staining showed that the deposed collagen was mostly of type 1 (Fig. 5) and that the tissue contained osteocalcin (Fig. 6). On examination of the generated tissue following seven days of incubation, islets of cartilage were seen in HE-stained samples (Fig. 7). These cartilage islets disappeared after 14 days of incubation. Macroscopically, the generated tissue had a three-dimensional granular shape, between 5 mm and 20 mm in diameter (Fig. 8).

Fig. 2
Micrograph of the generated tissue after three days of culture of the generated tissue. Areas of bone matrix-like material are evident (haematoxylin and eosin staining).

Fig. 3
Micrographs of a) the generated tissue after two weeks of culture, showing more organised bone-like areas, similar in appearance to b) a sample of a normal human bone (both haematoxylin and eosin staining).

Fig. 4
Micrograph of the generated tissue after two weeks of culture (trichrome staining). Generated collagen is evident (blue colour).

Fig. 5
Micrographs showing immunohistochemical stainings for collagen 1 (brown colour) in a) experimentally generated tissue, b) normal cancellous bone (positive control), c) cartilage (stained negative control) and d) experimentally generated tissue without antibody staining (not stained negative control). Similar positive staining is evident in the generated tissue and in normal bone sample. Negative controls show no staining to collagen 1.
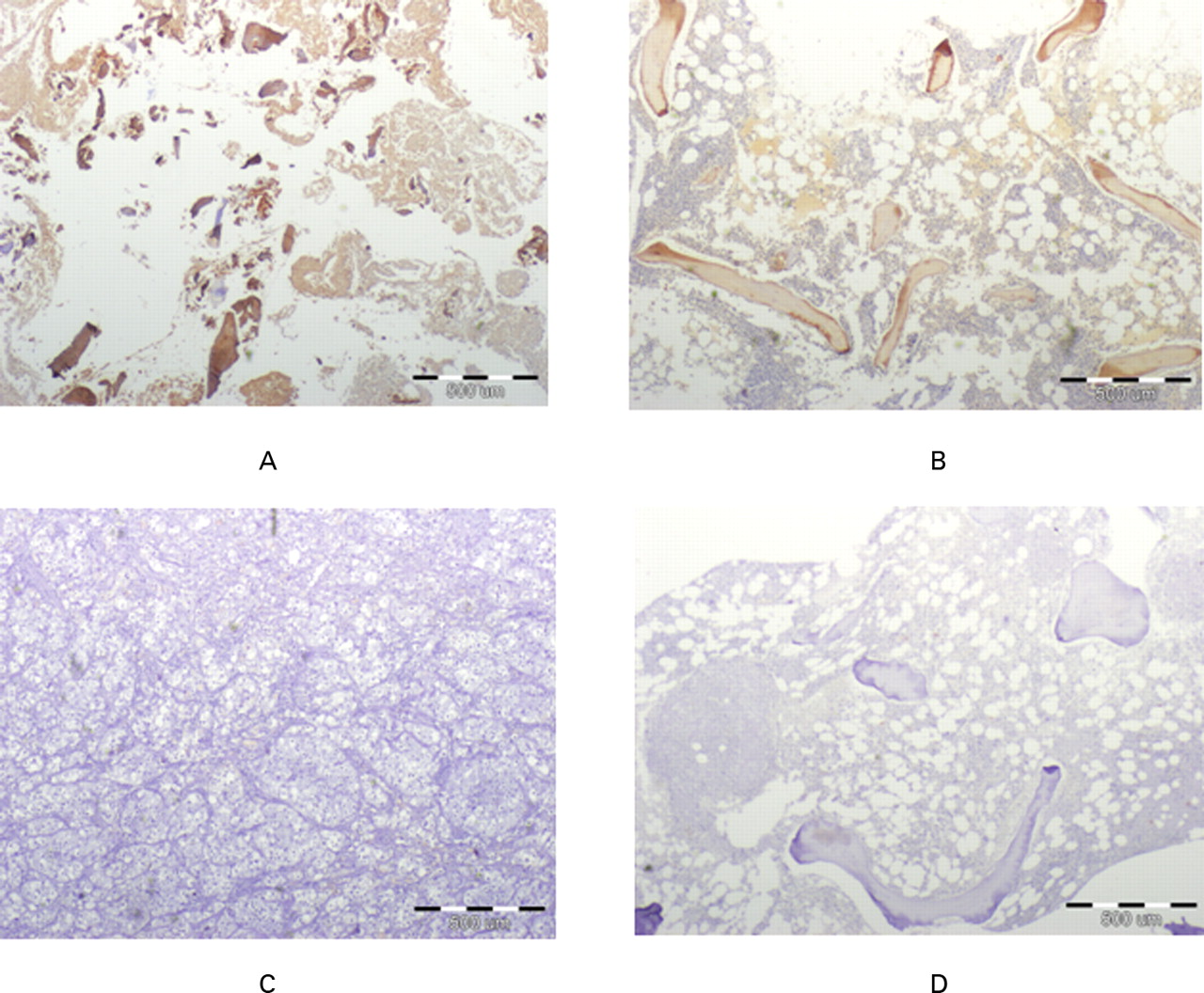
Fig. 6
Micrographs showing immunohistochemical staining for osteocalcin (brown colour) in a) experimentally generated tissue, b) normal cancellous bone (positive control), c) kidney (negative control) and d) normal cancellous bone without antibody staining (not stained negative control). Similar positive staining is evident in the generated tissue and in normal bone sample. Negative controls show no staining to osteocalcin.

Fig. 7
Micrograph showing cartilogenous tissue in the generated tissue following seven days of incubation (haematoxylin and eosin staining).

Fig. 8
Photograph showing the macroscopic appearance of the samples of generated tissue: solid pieces with maximum diameter of 20 mm.
Discussion
We used human osteoblasts in osteogenic media seeded on an inorganic scaffold and exposed to infrasonic mechanical stimulation in order to generate bone tissue in vitro. All of these components were planned to mimic the optimal biomechanical conditions for bone formation in vivo.11 The unique use of mechanical stimulation in the infrasonic range of movements should be similar the physiological mechanical stimulation of bone by resting muscles according to a normal resting vibromiogram, which contract in this range of mechanical parameters.9
There are other methods for generating a stock of osteoblast-like cells from the progenitor cells without using the osteogenic humoral factors, by implementation of suspension of microspheres in 2% agarose,12 TiO2 nanotubules13 and poly-caprolactone (PCL) nanotubules.14 Currently it is unknown which of these methods for osteoblast maturation from the progenitor cells, either by explant cultures in osteogenic media or by the other techniques mentioned above, is more efficient and safe for the generation of bone tissue intended for clinical use. Future studies of cytotoxicity and tumorigenicity of the generated bone-like material should solve this technical uncertainty, when a clinical method of implementing this tissue will be developed.
In this report we showed that the interaction of the cellular, inorganic and mechanical components in the described in vitro bioreactor can rapidly generate three-dimensional bone-like tissue. The bone characteristics of the generated tissue are supported by its microscopic appearance, which is similar to normal bone on HE staining and by its abundance of collagen 1 and osteocalcin, the main and essential components of human bone matrix, as demonstrated by immunohistochemical staining. The pieces of the generated tissue were measured in diameters between 10 and 20 times longer than the tricalcium phosphate granules which were seeded by the osteoblasts. This finding indicates the aggregation of generated tissue in larger pieces, or possibly its local expansion by the generated matrix.
The results also indicate that the generation of bone in this experimental set-up probably follows an ‘endochondral-like pattern’ since transient cartilage generation was evident in the first week of incubation. Therefore, according to the histological and immunohistochemical evidence presented here, we can cautiously claim that the tissue generated by the described method has clear characteristics of human bone.
There is evidence that cultured osteoblasts may generate calcified bodies in a monolayer culture in in vitro perfusion chambers on different scaffolds.15-17 In these studies, it was suggested that metabolically active osteoblasts that generate calcified bodies can be considered as an efficient source for inducing the enhancement of bone generation following their implantation.18 Obviously, in these circumstances, the intended clinical use would be an uncontrolled cell implantation, rather than the use of biologically active bone graft material. The clinical efficiency of these methods has not been proven. The biologically active bone-like tissue presented here might be more effective in its osteoinductive and osteoconductive characteristics. This hypothesis should be investigated in future clinical studies investigating fracture union. In this report we show that such viable bone-like material can be generated in vitro.
1 Crenshaw AHJ. Surgical techniques and approaches. In: Canale ST, Beaty JH, eds. Campbell's operative orthopaedics. Philadelphia: Mosby Elsevier, 2008:14–23. Google Scholar
2 Pollock R , AlcelikI, BhatiaC, et al.Donor site morbidity following iliac crest bone harvesting for cervical fusion: a comparison between minimally invasive and open techniques. Eur Spine J2008;17:845–852.CrossrefPubMed Google Scholar
3 Hak DJ . The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg2007;15:525–536.CrossrefPubMed Google Scholar
4 Rosenberg N . The role of the cytoskeleton in mechanotransduction in human osteoblast-like cells. Hum Exp Toxicol2003;22:271–274.CrossrefPubMed Google Scholar
5 Gundle K, Stewart J, Screen JN, Beresford N. Isolation and culture of human bone-derived cells. In: Beresford N, Owen ME, eds. Marrow stromal cell culture. Cambridge: Cambridge University Press, 2008:43–66. Google Scholar
6 Yamanouchi K , SatomuraK, GotohY, et al.Bone formation by transplanted human osteoblasts cultured within collagen sponge with dexamethasone in vitro. J Bone Miner Res2001;16:857–867.CrossrefPubMed Google Scholar
7 Rosenberg N , SoudryM, RosenbergO, BlumenfeldI, BlumenfeldZ. The role of activin A in the human osteoblast cell cycle: a preliminary experimental in vitro study. Exp Clin Endocrinol Diabetes2010;118:708–712.CrossrefPubMed Google Scholar
8 Rubin CT Response of bone to mechanical stimulation. In: Dee R, Mango E, Hurst L, eds. Principles of orthopaedic practice. New York: McGraw-Hill Book Company, 1988:79–87. Google Scholar
9 Nigg BM Acceleration. In: Nigg BM, Herzog W, eds. Biomechanics of the musculo-skeletal system. Second ed. Chichester: John Wiley & Sons, 1999:300–301. Google Scholar
10 Rosenberg N , LevyM, FrancisM. Experimental model for stimulation of cultured human osteoblast-like cells by high frequency vibration. Cytotechnology2002;39:125–130.CrossrefPubMed Google Scholar
11 Grayson WL , FröhlichM, YeagerK, et al.Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A2010;107:3299–3304.CrossrefPubMed Google Scholar
12 Handschel J, Naujoks C, Depprich R, et al Embryonic stem cells in scaffold-free three-dimensional cell culture: osteogenic differentiation and bone generation. Head Face Med 2011;7:12. Google Scholar
13 Oh S , BrammerKS, LiYS, et al.Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A2009;106:2130–2135.CrossrefPubMed Google Scholar
14 Polini A , PisignanoD, ParodiM, QuartoR, ScaglioneS. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS One2011;6:1–8.CrossrefPubMed Google Scholar
15 Wiesmann HP , NazerN, KlattC, SzuwartT, MeyerU. Bone tissue engineering by primary osteoblast-like cells in a monolayer system and 3-dimensional collagen gel. J Oral Maxillofac Surg2003;61:1455–1462.CrossrefPubMed Google Scholar
16 Buttery LD , BourneS, XynosJD, et al.Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng2001;7:89–99.CrossrefPubMed Google Scholar
17 Kose GT , KorkuszF, KorkuszP, et al.Bone generation on PHBV matrices: an in vitro study. Biomaterials2003;24:4999–5007.CrossrefPubMed Google Scholar
18 Kim SJ , ShinYW, YangKH, et al.A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast (Ossron) injection to treat fractures. BMC Musculoskelet Disord2009;10:1–9. Google Scholar
Funding statement:
None declared
Author contributions:
N. Rosenberg: Writing the paper, Design of the experiments
O. Rosenberg: Design of the experiments, Performing the experiments, Writing the paper
ICMJE Conflict of Interest:
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









