Introduction
The orthopaedic surgeon practising general trauma will invariably encounter fractures in children. Mercer Rang points out in the opening chapter of his textbook, “children are not just small adults”.1 The paediatric skeleton is more forgiving, demonstrates greater rapidity of healing and has an unrivalled capacity to remodel. Greater angulation, translation, and shortening can be accepted and reliably expected to remodel without clinical, functional or radiological shortcomings. Despite the fact that the outcomes following paediatric fractures are predictable and the healing potential offers considerable latitude for restoration of normality, there has been a global paradoxical shift from conservative treatment to early fracture fixation. This has perhaps been driven by the desire for immediate correction of clinical deformities or by financial incentives to minimise hospital costs accrued with prolonged admissions during periods of immobilisation. This review examines the evidence and indications for conservative treatment, and describes our preferences for the management of some commonly encountered paediatric fractures.
Remodeling
The ability of the paediatric skeleton to remodel lies at the heart of non-operative management. Semantically, this process is called modeling; remodeling being the constant homeostatic cycling of calcium and phosphate from bone stores. Everyone understands remodeling so we use it now even though it’s wrong! It is the process by which angulation and translation are corrected to restore acceptable alignment. The potential for remodeling is influenced by a number of factors:
-
Which bone is broken: proximal bones (humerus and femur) of the appendicular skeleton have a greater remodeling capacity than distal bones (forearm and tibia).
-
Proximity to the physis: 75% of angular remodeling takes place at the physis.2 Remodeling occurs with asymmetric bone growth characterised by greater growth on the concave side of the deformity to align the physis perpendicular to the axis of the bone.3 In diaphyseal bone, where 25% of remodeling can be expected, Wolff’s law is followed. Bone is laid down where it is mechanically required and reabsorbed where it is not.3 Increased pressure on the concave side of the fracture stimulates bone growth, and the convex or tension side undergoes reabsorption.4 Asymmetric bone growth serves to restore the normal mechanical axis. The entire bone, and not just the fracture site, should therefore be included in follow-up radiographs to monitor the extent of the remodeling.
-
Which physis is affected: growth is not uniform across all physes. The proximal humeral physis contributes to 80% of humeral growth and 20% occurs distally. The distal radial physis contributes 75% of radial longitudinal growth. In the femur, 30% of growth takes place proximally and 70% distally. Growth is more evenly distributed in the tibia with 55% occurring proximally and 45% distally.5
-
Location of the fracture: the metaphysis has rich blood supply and high osteogenic potential. The high bone turnover and proximity to the physis increases the remodeling capacity. Conversely, the osteogenic potential of diaphyseal bone is lower with a longer time to healing and decreased remodeling potential.4
-
Plane of the deformity: fractures that occur in the same plane as the adjacent joint have the greatest remodeling potential with translation generally remodeling fully, and angulation somewhat; little correction is seen with rotational deformities.
-
Bone age: the limbs of the average girl will stop growing aged 14, the average boy at aged 16. The younger the child, the greater the remodeling potential.
-
Underlying bone disease: the capacity of the bone to heal and remodel is influenced by such conditions as rickets, osteogenesis imperfecta, epiphyseal dysplasia, and fractures through pathological bone. In such circumstances, the standard principles of fracture management may not be appropriate.
Clavicle fractures
The clavicle is a commonly fractured bone in children and accounts for 10% to 15% of all fractures.6 The overwhelming majority heal and remodel without problems. In a series of 35 head injury patients with clavicle fractures treated without immobilisation, Wilkes and Hoffer reported 100% union and excellent remodeling with recovery of normal range of motion.7 The results of recent randomised clinical trials have prompted a shift towards operative intervention for displaced clavicle fractures in adults and data from such studies have been extrapolated to the paediatric population, particularly adolescents aged 15 to 19, with an increasing trend towards operative intervention.8 Potential indications for surgical fixation include open injuries, impending skin compromise, polytrauma patients, floating shoulder, and neurovascular compromise. However, these are not absolute indications as they are in adults. In children, open fractures may just require wound management, and paediatric patients can tolerate longer periods of immobilisation, as may be required in the conservative management of floating shoulder injuries, without problematic stiffness.
Our practice
Most patients under the age of 16 are treated conservatively with a broad-arm sling for pain relief for ten to 21 days before commencing gentle mobilisation. Patients are advised to avoid contact sports for five weeks. Parents are made aware that the fracture will heal with a bump but are reassured that this will have no functional implications. Like Mercer Rang, we do not take a follow-up radiograph.1 Patients are discharged with an open appointment and a standard information leaflet at the first fracture clinic appointment.
Proximal humerus
Fractures of the proximal humerus are comparatively rare, accounting for 0.45% of all paediatric fractures and up to 7% of epiphyseal fractures.9 They are, however, extremely forgiving and the glenohumeral joint demonstrates unrivalled mobility. The proximal humerus is encased in thick periosteum and 80% of humeral growth occurs at the proximal physis.10 As a consequence, both physeal and metaphyseal fractures heal rapidly and remodel well. Because of soft-tissue attachments, these fractures are predisposed to angular deformity and displacement, however, despite this, the overwhelming majority can be managed conservatively with simple collar and cuff immobilisation.
In 1956, FM Smith reported that proximal humeral epiphyseal fractures were ‘much overtreated’ and advocated a laissez-faire policy towards their management.11 Baxter and Wiley found residual varus angulation to be insignificant in 57 patients followed up for between two and eight years after injury. They reported a maximum shortening of 2 cm and found that manipulation of the fracture failed to improve final outcome.10 Some authors advocate reduction +/- fixation for displaced unstable fractures in children over the age of 11 where the opportunity for remodeling is limited.12,13
Our practice
We treat all proximal humeral fractures with collar and cuff immobilisation for symptom control for three weeks. Gentle mobilisation commences at ten days with full mobilisation discarding the collar and cuff at three weeks. The only exceptions are fractures where the epiphysis has become rotated 90° or more and is locked under the acromion. In general, we follow Rang in that full displacement can be accepted in these fractures if two years’ growth remains.1
Supracondylar humeral fractures
Supracondylar humeral fractures are the most common elbow fracture in children and account for 3% of all paediatric fractures.14 The Gartland classification is the most commonly utilised classification system but repeated modifications have rendered it confusing and of limited value. We prefer the AO classification where supracondylar humeral fractures are coded as 13-M/3 and subdivided into I-IV based upon displacement.15
Treatment strategies for supracondylar fractures have been the focus of much debate. AO Type I fractures can be immobilised in a collar and cuff in flexion for three weeks before commencing mobilisation. Type II fractures with an intact posterior hinge are reduced with high flexion and can then be treated as per Type I fractures. Flexion beyond 120° should be avoided because of the risk of vascular compromise and compartment syndrome.16,17 However, AO Type III and IV fractures require reduction and fixation. This can generally be achieved with closed manipulation. In cases when closed reduction is inadequate, open reduction, traction or external fixation may be considered. The American Academy of Orthopaedic Surgeons suggests that open reduction might be performed for fractures with varus or other malposition after closed reduction but acknowledges that supporting evidence for this is unconvincing.18 We would not recommend open reduction in the absence of neurovascular compromise given the risks of complications and associated morbidity, and the extent of available remodeling. In our opinion the moment of failed closed reduction is the worst time to open a supracondylar fracture. In cases of comminuted unstable supracondylar fractures, skin or skeletal traction (via olecranon fixation) or external fixation are preferable to open reduction (Fig. 1). Gadgil et al successfully treated 112 children with closed, displaced supracondylar humeral fractures with elevated straight arm traction. Of the 112 patients, 104 had excellent or good clinical results (determined by change in carrying angle, measurement of rotational deformity, and loss of flexion-extension) and good radiological results (based on the change in metaphyseal-diaphyseal and humerocapitellar angles).19 The authors of this convincing study concluded that results of elevated straight-arm traction were comparable with closed or open K-wire fixation for such fractures. Similar results were obtained in our unit for olecranon screw traction20,21 but ‘parent power’ led to the rapid introduction of closed reduction and wiring in the late 1990s. Slongo and Wilkins have reported the successful use of external fixation in this situation .22
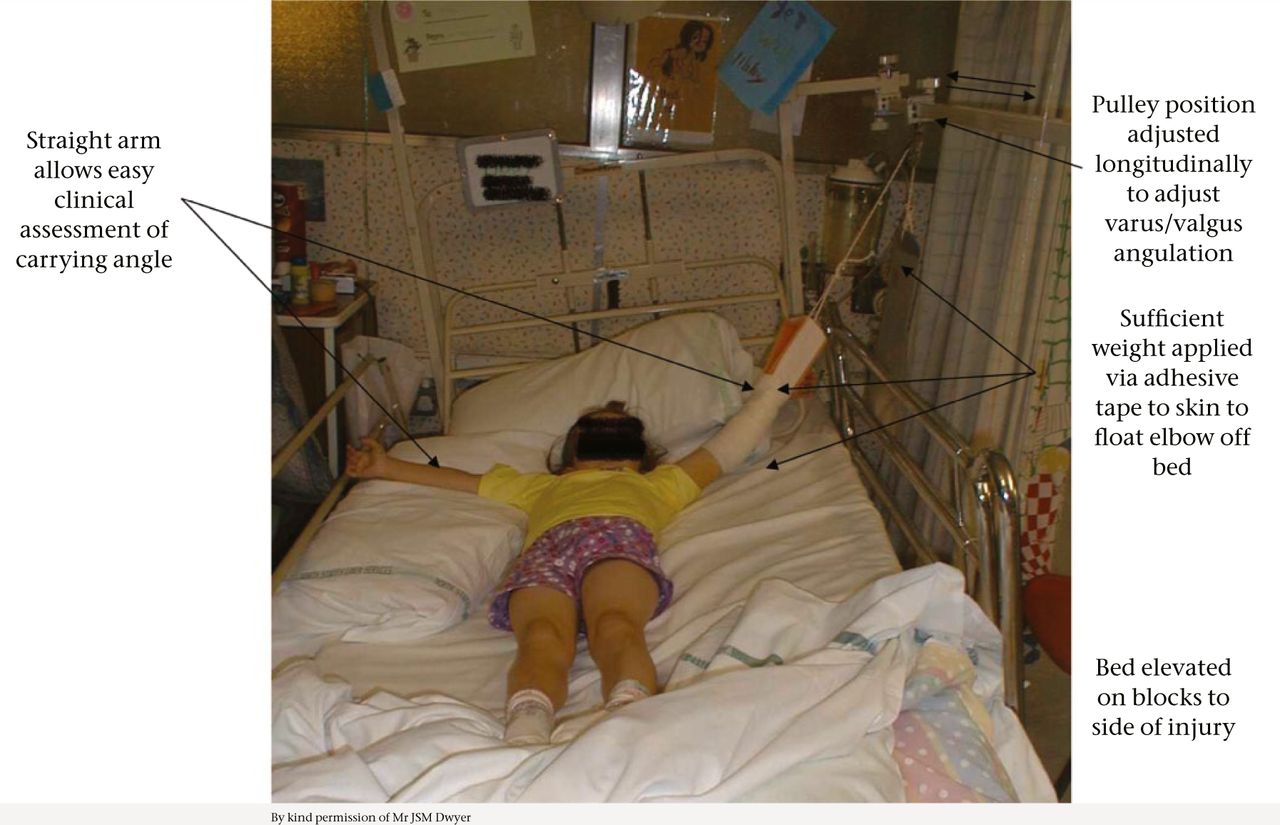
Fig. 1
Elevated straight-arm traction for conservative management of supracondylar humeral fractures.
Twenty percent of growth occurs at the distal humerus and the potential for remodeling is limited, with 10% of distal humeral growth remaining in the child who is eight to ten years of age.23 According to Wilkins, varus and valgus deformities cannot be expected to remodel but translation of up to 100% can be accepted4 while posterior angulation will reliably remodel, at least to neutral. Traction can successfully overcome rotation and varus/valgus deformity. The application of elevated straight-arm traction with forearm supination and wide shoulder abduction is capable of correcting posteromedial displacement and varus angulation, while posterolateral displacement and valgus deformity is addressed by ensuring that the traction is applied with shoulder abduction < 90°.19
Given that the peak age for supracondylar humeral fractures is five to seven years,17 most children will have >10% distal humeral growth remaining and the treating surgeon can assume that some remodeling—perhaps more than we generally expect—will occur.
Our practice
All AO type I and II fractures are treated with collar and cuff in flexion beyond 100o but not extreme. Type III and IV fractures are treated with closed reduction and Kirschner wire fixation as per British Orthopaedic Association Standards for Trauma (BOAST 11) guidelines. In rare cases where reduction cannot be achieved, closed traction or external fixation are used.
Fractures of the distal radius
Fractures of the distal radius and ulna are the most common fractures in children and account for more than three quarters of all forearm fractures,24 and approximately 15% of distal radial fractures affect the radial physis.25 Physeal fractures demonstrate considerable remodeling capacity and 50% displacement can be accepted in a child with 18 months’ growth remaining.16,26 Consequently, such fractures can be immobilised in plaster cast on the safe assumption that remodeling will take place within a year. In a review of remodeling of Salter-Harris II distal radial physeal fractures, Houshian et al established that remodeling occurred in all patients. Remodeling was complete in patients less than ten years of age, and even in those with incomplete radiographic remodeling wrist mobility and grip strength was unaffected.25 Physeal injuries should not be manipulated after ten days post injury due to the significant risk of radial growth arrest.
Metaphyseal distal radial fractures also remodel well, and Do et al evaluated 34 patients presenting with less than 15° of residual angulation and < 1 cm shortening who had been left to heal. On average, full remodeling was complete at 7.5 months, with no significant clinical or functional deficits present.27 Izuka from Hawaii similarly treated 51 patients under the age of ten years, all with off-ended and overriding distal radial fractures with a moulded cast to partially correct angulation in the outpatient clinic. No conscious sedation was used. All patients achieved clinical and radiographic union and correction of deformity at one-year follow-up28 (Fig. 2). The efficacy and safety of such an approach appears increasingly appealing when contrasted with studies revealing a 17% complication rate with percutaneous Kirschner wire fixation.29
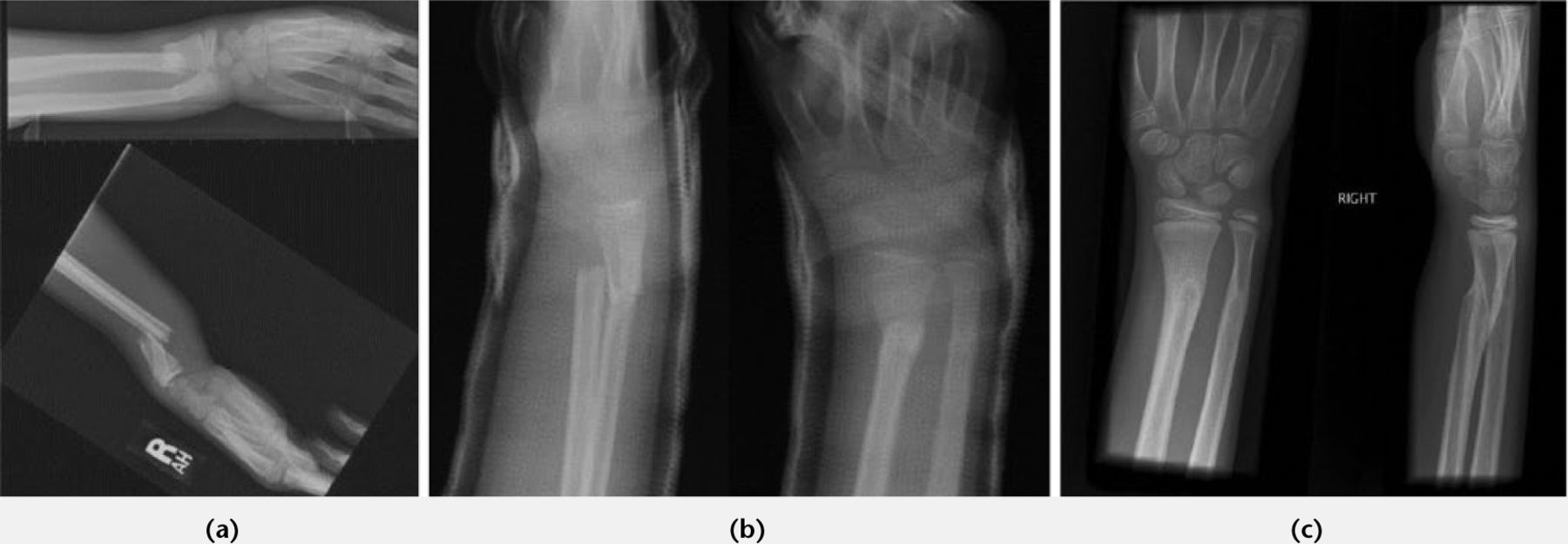
Fig. 2
Remodeling following conservative management of overriding distal radial fracture. a) Initial films; b) At 1 week; c) At 4 months.
Casting technique has also been evaluated, and Bohm et al conducted a randomised control trial (RCT) with no difference in fracture re-displacement when using a below- or above-elbow cast,30 findings confirmed in an RCT reported by Webb et al.31 In addition, this study confirmed children missed fewer school days and required less assistance with activities of daily living in the below-elbow plaster.31
Our practice
Simple buckle fractures are stable injuries and are managed with futura splint immobilisation for three weeks before commencing mobilisation. Splints and information leaflets are given in our Emergency Department (ED) and patients are discharged from hospital without further follow-up.
We manipulate all angulated Salter-Harris I and II fractures, metaphyseal fractures, and angulated diaphyseal fractures of the forearm in our ED, as long as there is no shortening. Entonox and diamorphine analgesia are used and the patients are discharged home. In a review of the first 100 patients treated, average dorsal angulation was corrected from 28° to 7°, there were no complications, and over £45,000 was saved when compared with in-hospital/theatre treatment.32 Acceptable angulations after reduction are shown in Table I.
Table I.
Acceptable angulation after reduction for forearm fractures32
| Age | Diaphyseal forearm | Metaphyseal | Salter-Harris types I & II |
|---|---|---|---|
| < 9 years | < 15° | - | - |
| ≥ 9 years | < 10° | - | - |
| < 10 years | - | < 20° | < 20° |
| ≥ 10 years | - | < 10° | < 10° |
Offended distal radial fractures are currently treated by manipulation under anaesthetic and below-elbow plaster application. The use of additional percutaneous fixation is left to the surgeon’s discretion.
Femoral shaft fractures
Femoral shaft fractures are the second most common paediatric long bone fracture after fractures of the radius and the ulna.33 Of all the fractures to the appendicular skeleton, diaphyseal femoral fractures have the greatest impact on mobility, with attendant implications for schooling and for the family. Conservative options include Pavlik harness, skin traction, spica cast, or a combination of the latter two. Gallows traction is our preferred treatment of choice for children under 18 months or 10 kg. This involves the application of skin traction with the child's legs suspended overhead, with the hips flexed to 90° and the knees extended utilising the child’s bodyweight to apply traction to the fracture (Fig. 3). This form of treatment does carry the risk of vascular complications, including Volkmann ischaemic contracture and compartment syndrome.34 Though such complications are rare, meticulous examination of skin around the bandaging is required. Traction may be used as definitive treatment, and as rule of thumb one week of traction for every year of age is often required. Traction has the benefit of correcting the flexion/abduction deformity of the proximal fragment and prevents rotational malalignment but requires weekly radiographs to check for healing. Once there is visible callus formation the child can be discharged non-weight bearing in a pushchair. The use of traction has the added benefit of affording time for the assessment and investigation of Non-Accidental Injury (NAI). Eighty percent of fractures from NAI are seen in children under 18 months,35 and 80% of non-ambulatory children with femoral fractures are victims of abuse.36,37
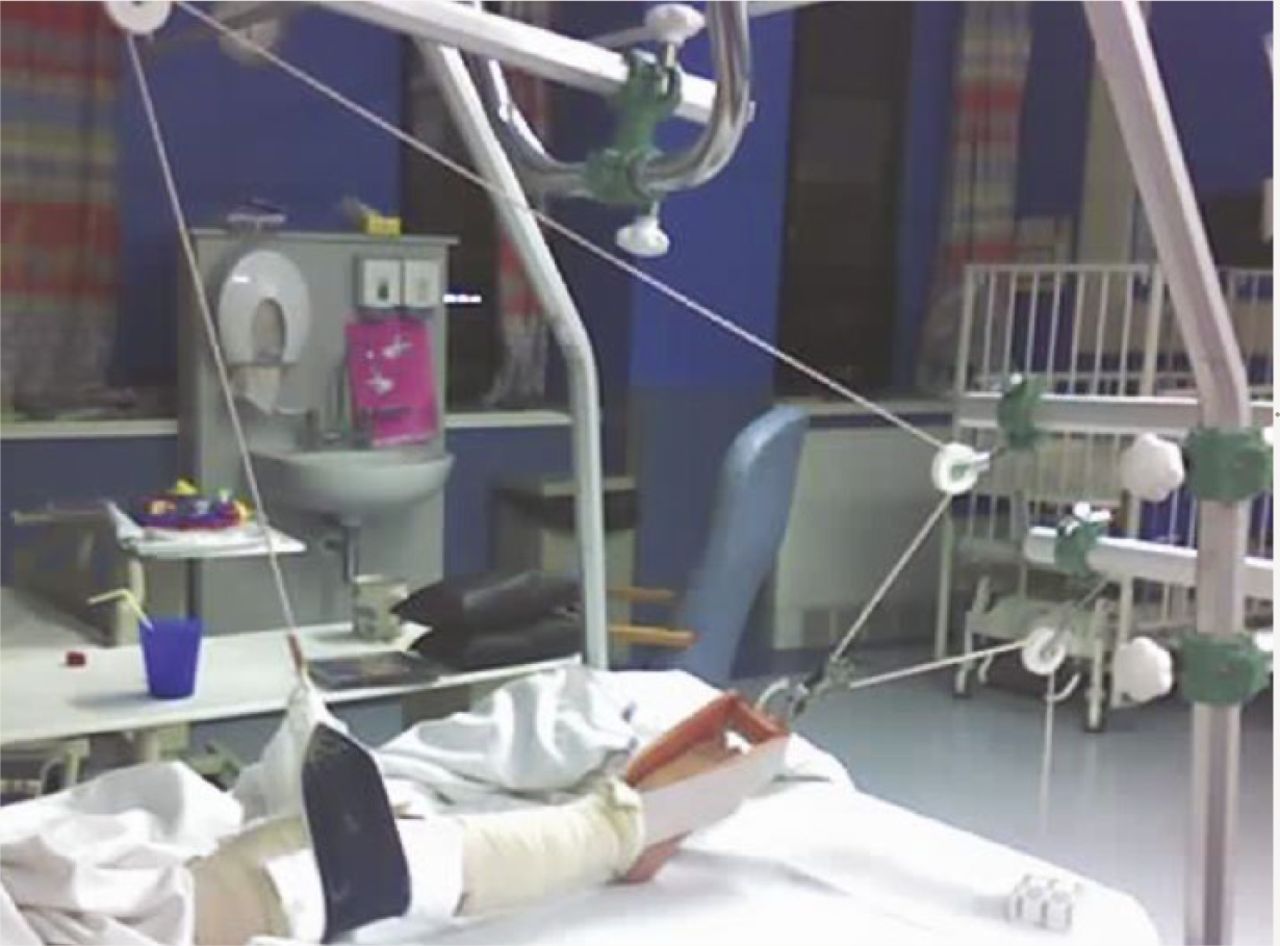
Fig. 3
Hamilton-Russell traction.
Immediate spica cast or Pavlik harness may be used as an alternative in patients under six months of age. The Pavlik harness has been successfully used in the management of small non-ambulatory children with < 2 cm of shortening38 and avoids the need for general anaesthetic often required for early spica cast application. Skin complications are more frequent and were seen in a third of patients in one study.39 The spica should be applied with no more than 60° of flexion in the hip or knee,40 and care must be taken to ensure that the fracture is reduced in a position of extension and valgus to prevent flexion and varus deformity. It is possible to convert from traction to late spica cast but there is limited evidence, or clinical indication, to support this in children under 18 months. The rapidity of healing of paediatric femoral shaft fractures means that when the time comes to convert to spica cast, the benefits of doing so will be minimal and the procedure largely unnecessary.34
Children over 18 months or 10 kg are not candidates for Gallows traction, and instead balanced traction in the form of Hamilton-Russell traction can be used with each 1 lb, and one week for every year of age (Fig. 3). For children between six months and five years, the AAOS found no evidence to suggest superiority of spica casting over traction, or vice versa, but advocated spica casting on the basis of social and economic factors including length of hospital stay.41 When early spica casting is utilised, close observation is required to monitor for shortening; > 15 mm of shortening should be sufficient to prompt conversion to traction.34 In children between four and 12 years of age, conservative strategies become less appealing. Hamilton-Russell traction may still be employed, but in a fit and well child, the duration of fracture healing makes it a less attractive option. Similarly, spica casting is not used in patients over the age of four unless there is a clinical reason.
Femoral shaft fractures frequently demonstrate overgrowth averaging just under 1 cm.42 Acceptable limits of angular deformity are variously reported (Table II). Wallace and Hoffman followed patients for an average of 45 months and concluded that angular deformity of up to 25° in any plane in children under 13 years will remodel satisfactorily.2
Table II.
Acceptable angulation and shortening for femoral shaft fractures based on patient age43
| Age | Varus/valgus | Anterior/posterior | Shortening |
|---|---|---|---|
| < 2 years | 30° | 30° | 15 mm |
| 2-5 years | 15° | 20° | 20 mm |
| 6-10 years | 10° | 15° | 15 mm |
| 11 years + | 5° | 10° | 10 mm |
Our practice
All children < 18 months or 10 kg are treated with Gallows traction. Children between 18 months and six years are treated in Hamilton-Russell traction. In both groups, traction is continued until callus formation and the fracture is clinically healed, at which point children are discharged home non-weight bearing in a pushchair. Children aged over six years are managed with internal fixation unless contraindicated.
Tibial shaft fractures
Tibial shaft fractures account for 15% of all paediatric fractures44,45 and the majority can be managed non-operatively with an above-knee plaster cast.
Fractures of the proximal third of the tibia are the least common but can prove the most challenging due to the risk of late angular deformity.44 The Cozen fracture is the eponymous often incomplete fracture of the proximal tibial metaphysis (caused by valgus force on an extended knee). Treatment is with an above-knee plaster with varus moulding for approximately six weeks. Such fractures have a propensity to develop late valgus deformity and parents should be made aware of this at the outset. Deformities of 18°, which can take up to 18 months to develop, may occur but resolve spontaneously, supporting a conservative approach to both the initial injury and the resultant angular deformity.46,47
The tibial shaft is the most common site of fracture. The ‘Toddler’s fracture’ is an undisplaced spiral fracture in an ambulant child (< four years), however, in non-ambulant children, this fracture should immediately raise the suspicion of NAI. This stable injury can be managed in an above-knee plaster for approximately three weeks (depending on the age of the child), and more recent work suggests a below-knee cast or just a bandage may be sufficient. Interval radiographs are not required unless suspicion exists surrounding the diagnosis, and evidence of periosteal reaction is required for confirmation and to guide duration. Upon removal of the cast the child should be allowed to fully weight-bear though parents should be made aware that the child will initially be reluctant to do so, will invariably walk with a limp and the foot externally rotated, and that it can take several weeks for this to resolve.
In children under 11 years, oblique or spiral fractures predominate, and in around a third of cases the fibula is also fractured.44 Where the fibula is intact, it acts to prevent shortening but also predisposes to varus deformity as the long flexors act upon the distal fragment.45 Tibial shaft fractures have limited capacity to remodel owing to the quantity of diaphyseal bone,4 and although acceptable parameters of angulation have been published (Table III), the evidence to support such values is limited.
Table III.
Recommendations for acceptable deformities in tibial diaphyseal fractures44
| Deformity | Age < 8 years | Age ≥ 8 years |
|---|---|---|
| Varus | 10° | 5° |
| Valgus | 5° | 5° |
| Anterior angulation | 10° | 5° |
| Posterior angulation | 5° | 0 |
| Rotation | 5° | 5° |
| Shortening | 10 mm | 5 mm |
Treatment of tibial shaft fractures requires an above-knee plaster cast. The knee should be slightly flexed and moulded around the supracondylar region of the distal femur to help control rotation. Duration of immobilisation is influenced by the age of the child. After a period of between four and six weeks when the fracture has become sticky, the above-knee cast can be converted to a below-knee weight-bearing or Sarmiento cast. Because of the potential for displacement or angulation, particularly in the presence of an intact fibula, close monitoring with serial radiographs is mandatory. If the position does slip, wedging can be performed to correct angulation (Fig. 4; Table IV). Opening or closing wedging may be used. Opening wedge may be preferable because it obviates the risk of pinching the skin between the cut edges of plaster, but does carry the risk of introducing some distraction at the fracture site.
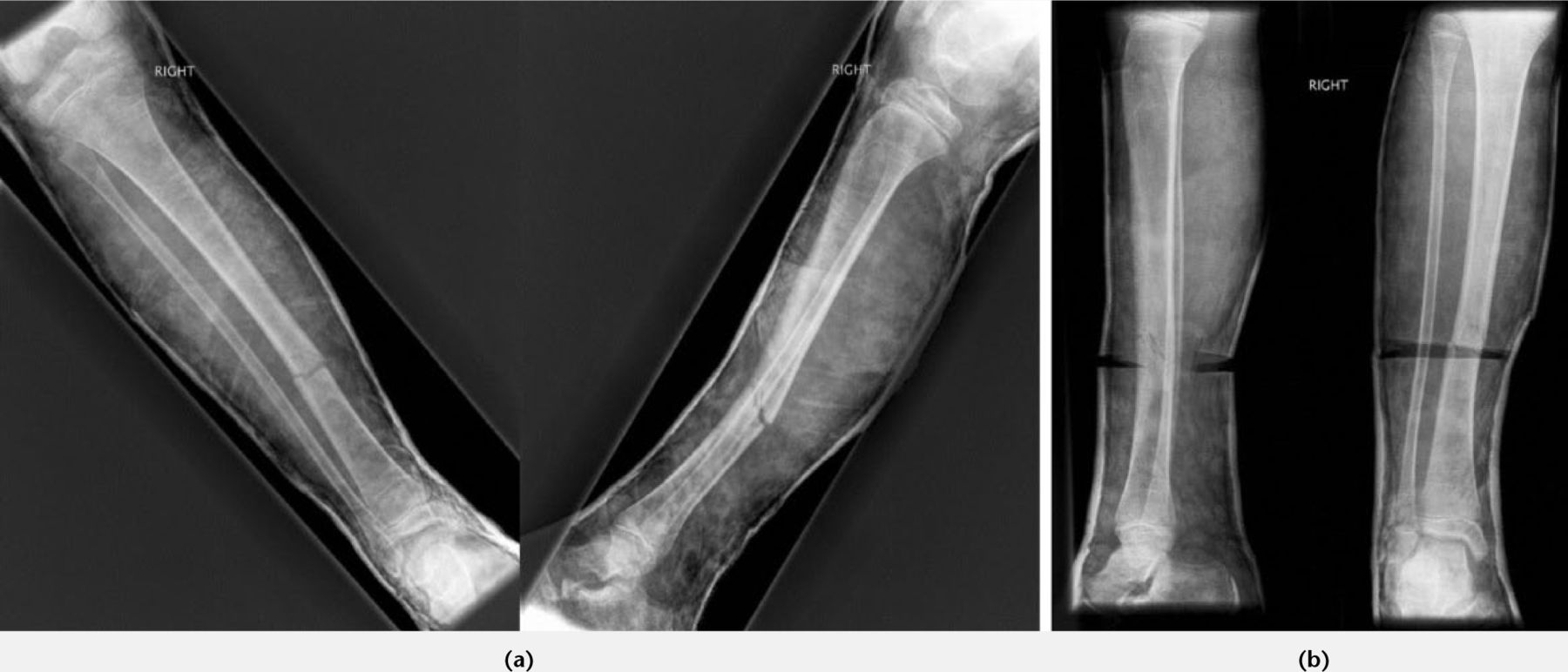
Fig. 4
a) Tibial fracture in cast with valgus and recurvatum deformity (apex posteromedial); b) Position corrected after wedging.
Table IV.
An introduction to wedging
| Step 1 | Calculate the degree of correction required |
| Step 2 | Calculate the distance you need to open the plaster based on an angle hinging on the plaster at the apex of the fracture |
| Step 3 | Mark the fracture site on the radiograph |
| Step 4 | Cut the plaster at this level 80-90% circumferentially |
| Step 5 | Open on the concave side with spreaders |
| Step 6 | Insert a wooded wedge of calculated size (step 2) |
| Step 7 | Wrap the area wedged in plaster |
| Step 8 | Perform new radiograph |
For fractures of the distal third of the tibia, the same principles for those of the diaphysis apply. The only point to note is that reduction and casting may require the foot to be kept in equinus to minimise the risk of apex posterior (recurvatum) angulation at the fracture site. An above-knee plaster cast is mandatory and remains in place until callus is seen, at which point the child is converted to a below-knee weight-bearing plaster cast.
Our practice
Toddlers’ fractures are managed with an above-knee cast for three to four weeks depending on the age of the child. Follow-up radiographs are not routinely performed. The child is permitted to fully weight-bear upon removal of the cast, though parents are made aware that they will not do so with a normal gait for up to several weeks. The child is discharged with an open appointment when the cast is removed. Tibial shaft fractures, including distal third fractures, are managed with an above-knee cast for four to six weeks before conversion to a below-knee weight-bearing or Sarmiento-type cast for a duration determined by the child’s age and radiological features.
Conclusion
The overwhelming majority of paediatric extra-articular fractures can be managed, and are perhaps best managed, conservatively. The orthopaedic surgeon managing paediatric fractures must have an awareness of the options for non-operative management and should appreciate the remodeling potential of the paediatric skeleton and the limits of acceptable deformities. The evidence for the non-operative treatment of some commonly encountered paediatric fractures supports this practice. Our preferences are not meant to be prescriptive and we readily acknowledge that other options and protocols work equally well. Surgeons treating children’s fractures need to have a full range of options within their skillset; perhaps the poorest outcome for patient, surgeon and parent is the poorly explained operation with an associated complication.
References
1. Rang M . Children’s fractures. Second ed. Philadelphia: JB Lippincott, 1983. Google Scholar
2. Wallace ME , HoffmanEB. Remodelling of angular deformity after femoral shaft fractures in children. J Bone Joint Surg [Br]1992;74-B:765-769.CrossrefPubMed Google Scholar
3. Gascó J , De PablosJ. Bone remodeling in malunited fractures in children. Is it reliable?J Pediatr Orthop B1997;6:126-132.CrossrefPubMed Google Scholar
4. Wilkins KE . Principles of fracture remodeling in children. Injury2005;36(Suppl 1): A3-A11.CrossrefPubMed Google Scholar
5. Herring JA . Growth and development. In: HerringJA, ed. Tachdjian’s paediatric orthopaedics. Vol. 1, Fifth ed. Philadelphia: Elsevier Saunders, 2014.CrossrefPubMed Google Scholar
6. Caird MS . Clavicle shaft fractures: are children little adults?J Pediatr Orthop2012;32(Suppl 1):S1-S4.CrossrefPubMed Google Scholar
7. Wilkes JA , HofferMM. Clavicle fractures in head-injured children. J Orthop Trauma1987;1:55-58.CrossrefPubMed Google Scholar
8. Yang S , WernerBC, GwathmeyFWJr. Treatment trends in adolescent clavicle fractures. J Pediatr Orthop2015;35:229-233.CrossrefPubMed Google Scholar
9. Pahlavan S , BaldwinKD, PandyaNK, et al.. Proximal humerus fractures in the pediatric population: a systematic review. J Child Orthop2011;5:187-194.CrossrefPubMed Google Scholar
10. Baxter MP , WileyJJ. Fractures of the proximal humerus epiphysis. Their influence on humeral growth. J Bone Joint Surg [Br]1986;68-B:570-573. Google Scholar
11. Smith FM . Fracture-separation of the proximal humeral epiphysis: A study of cases seen at the Presbyterian Hospital from 1929-1953. Am J Surg1956;91:627-635. Google Scholar
12. Sarwark JF , KingEC, JanickiJA. Proximal humerus, scapula and clavicle. In: BeatyJH, KasserJR, eds. Rockwood and Wilkins’ fractures in children. Seventh ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Google Scholar
13. Dobbs MB , LuhmannSL, GordonJE, et al.. Severely displaced proximal humeral epiphyseal fractures. J Pediatr Orthop2003;23:208-215.PubMed Google Scholar
14. Abzug JM , HermanMJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg2012;20;69-77.CrossrefPubMed Google Scholar
15. Slongo T , AudigéL. AO pediatric classification of long-bone fractures (PCCF). https://www.aofoundation.org/Documents/2008-09-PediatricClassificationBrochure.pdf (date last accessed 10 December 2015). Google Scholar
16. Wilkins KE . Nonoperative management of pediatric upper extremity fractures or ‘Don’t throw away the cast’. Techniques in Orthopaedics2005;20:115-141. Google Scholar
17. Omid R , ChoiPD, SkaggsDL. Supracondylar humeral fractures in children. J Bone Joint Surg [Am]2008;90-A:1121-1132. Google Scholar
18. No authors listed. AAOS. The treatment of pediatric supracondylar humerus fractures: evidence-based guideline and evidence report. http://www.aaos.org/cc_files/aaosorg/research/guidelines/supracondylarfracture/supconfullguideline.pdf (date last accessed 9 December 2015). Google Scholar
19. Gadgil A , HayhurstC, MaffulliN, et al.. Elevated, straight-arm traction for supracondylar fractures of the humerus in children. J Bone Joint Surg [Br]2005;87-B:82-87.PubMed Google Scholar
20. Worlock PH , ColtonC. Severely displaced supracondylar fractures of the humerus in children: a simple method of treatment. J Pediatr Orthop1987;7:49-53.CrossrefPubMed Google Scholar
21. Lewis JR , MonkJ, ChandrateyaAP, HunterJB. Changing the management from Olecranon screw traction to percutaneous wiring for displaced supracondylar fractures of the humerus in children. A justified decision?European J of Trauma and Emergency Surgery2007;33:256-261.CrossrefPubMed Google Scholar
22. Slongo T , SchmidT, WilkinsK, et al.. Lateral external fixation - a new surgical technique for displaced unreducible supracondylar humeral fractures in children. J Bone Joint Surg [Am]2008;90-A:1690-1697. Google Scholar
23. Skaggs DL , FlynnJM. Supracondylar fractures of the distal humerus. In: BeatyJH, KasserJR, eds. Rockwood and Wilkins’ fractures in children. Seventh ed. Philadelphia: Lippincott Williams and Wilkins, 2010.CrossrefPubMed Google Scholar
24. Green JS , WilliamsSC, FinlayD, et al.. Distal forearm fractures in children: the role of radiographs during follow up. Injury1998;29:309-312. Google Scholar
25. Houshian S , HolstAK, LarsenMS, TorfingT. Remodeling of Salter-Harris type II epiphyseal plate injury of the distal radius. J Pediatr Orthop2004;24:472-476.CrossrefPubMed Google Scholar
26. Aitken AP . The end results of fractured distal radial epiphysis. J Bone Joint Surg [Am]1935;17-A:302-308. Google Scholar
27. Do TT , StrubWM, FoadSL, et al.. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop2003;12:109-115.CrossrefPubMed Google Scholar
28. Crawford SN , LeeLS, IzukaBH. Closed treatment of overriding distal radial fractures without reduction in children. J Bone Joint Surg [Am]2012;94-A:246-252.CrossrefPubMed Google Scholar
29. Ramoutar DN , ShivjiFS, RodriguesJN. The outcomes of displaced paediatric distal radius fractures treated with percutaneous Kirschner wire fixation: a review of 248 cases. Eur J Orthop Surg Traumatol2015;25:471-476.CrossrefPubMed Google Scholar
30. Bohm ER , BubbarV, Yong HingK, DzusA. Above and below-the-elbow plaster casts for distal forearm fractures in children. A randomized controlled trial. J Bone Joint Surg [Am]2006;88-A:1-8.CrossrefPubMed Google Scholar
31. Webb GR , GalpinRD, ArmstrongDG. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children. J Bone Joint Surg [Am]2006;88-A:9-17.CrossrefPubMed Google Scholar
32. Kurien T , PriceKR, DieppeC, PearsonRG, HunterJB. Manipulation and reduction of paediatric fractures of the distal radius and forearm using intranasal diamorphine and 50% oxygen and nitrous oxide in the emergency department: a 2.5-year study. Bone Joint J2016;98-A;1:131-136. (In press).CrossrefPubMed Google Scholar
33. Brousil J , HunterJB. Femoral fractures in children. Curr Opin Pediatr2013;25:52-57. Google Scholar
34. Hunter JB . Femoral shaft fractures in children. Injury2005;36:S86-S93.CrossrefPubMed Google Scholar
35. Worlock P , StowerM, BarborP. Patterns of fractures in accidental and non-accidental injury in children: a comparative study. Br Med J (Clin Res Ed)1986;293:100-102.CrossrefPubMed Google Scholar
36. Anglen JO , ChoiL. Treatment options in pediatric femoral shaft fractures. J Orthop Trauma2005;19:724-733.CrossrefPubMed Google Scholar
37. Gross RH , StrangerM. Causative factors responsible for femoral fractures in infants and young children. J Pediatr Orthop1983;3:341-343.CrossrefPubMed Google Scholar
38. Stannard JP , ChristensenKP, WilkinsKE. Femur fractures in infants: A new therapeutic approach. J Pediatr Orthop1995;15:461-466.CrossrefPubMed Google Scholar
39. Podeszwa DA , MooneyJF3rd, CramerKE, et al.. Comparison of Pavlik harness application and immediate spica casting for femur fractures in infants. J Pediatr Orthop2004;24:460-462.CrossrefPubMed Google Scholar
40. Morrissey ART , WeinsteinSL. Atlas of pediatric orthopaedic surgery. Third ed. Philadelphia: Lippincott Williams and Wilkins, 2001. Google Scholar
41. No authors listed. AAOS. The treatment of pediatric disphyseal femur fractures: evidence-based clinical practice guideline, June122015. http://www.aaos.org/research/guidelines/PDFF_ReIssue.pdf (date last accessed 10 December 2015). Google Scholar
42. Shapiro F . Fractures of the femoral shaft in children. The overgrowth phenomenon. Acta Orthop Scand1981;52:649-655.CrossrefPubMed Google Scholar
43. Flynn JM , SkaggsDL. Femoral shaft fractures. In: BeatyJH, KasserJR, eds. Rockwood and Wilkins’ fractures in children. Seventh ed. Philadelphia: Lippincott Williams and Wilkins, 2010.CrossrefPubMed Google Scholar
44. Heinreich SD , MooneyJF. Fractures of the shaft of the tibia and fibula. In: BeatyJH, KasserJR, eds. Rockwood and Wilkins’ fractures in children. Seventh ed. Philadelphia: Lippincott Williams and Wilkins, 2010.CrossrefPubMed Google Scholar
45. Mashru RP , HermanMJ, PizzutilloPD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg2005;13:345-352.CrossrefPubMed Google Scholar
46. Tuten HR , KeelerKA, GabosPG, ZiontsLE, MacKenzieWG. Posttraumatic tibia valga in children. A long-term follow-up note. J Bone Joint Surg [Am]1999;81-A:799-810.CrossrefPubMed Google Scholar
47. Zionts LE , MacEwenGD. Spontaneous improvement of post-traumatic tibia valga. J Bone Joint Surg [Am]1986;68-A:680-687.PubMed Google Scholar
Fig.