Abstract
Aims
For cementless implants, stability is initially attained by an interference fit into the bone and osteo-integration may be encouraged by coating the implant with bioactive substances. Blood based autologous glue provides an easy, cost-effective way of obtaining high concentrations of growth factors for tissue healing and regeneration with the intention of spraying it onto the implant surface during surgery. The aim of this study was to incorporate nucleated cells from autologous bone marrow (BM) aspirate into gels made from the patient’s own blood, and to investigate the effects of incorporating three different concentrations of platelet rich plasma (PRP) on the proliferation and viability of the cells in the gel.
Methods
The autologous blood glue (ABG) that constituted 1.25, 2.5, and 5 times concentration PRP were made with and without equal volumes of BM nucleated cells. Proliferation, morphology, and viability of the cells in the glue was measured at days 7 and 14 and compared to cells seeded in fibrin glue.
Results
Overall, 2.5 times concentration of PRP in ABG was capable of supporting the maximum growth of cells isolated from the BM aspirate and maintain their characteristics. Irrespective of PRP concentration, cells in ABG had statistically significantly higher viability compared to cells in fibrin glue.
Conclusion
In vitro this novel autologous gel is more capable of supporting the growth of cells in its structure for up to 14 days, compared to commercially available fibrin-based sealants, and this difference was statistically significant.
Cite this article: Bone Joint Res 2020;9(7):402–411.
Article focus
-
The aim of this study was to incorporate the buffy layer containing nucleated cells including mesenchymal stem cells (MSCs) from autologous bone marrow (BM) aspirate into gels made from the patient’s own blood, and to investigate the effects of incorporating three different concentrations of PRP on the proliferation and viability of the cells in the gel.
Key messages
-
This novel autologous blood glue (ABG) can be prepared cost-effectively and it has the potential to be incorporated with stem cells from the BM, which remain viable in it and retain their characteristic.
-
Due to the presence of platelet rich plasma (PRP) in the glue, the proliferation of cells is further boosted by growth factors released by platelets.
Strengths and limitations
-
This novel autologous gel can be easily prepared in surgery and it can be sprayed onto implants, potentially improving osseointegration of the implant.
-
This study could benefit from investigating the potential of stem cells in the ABG to differentiate to bone.
Introduction
Each year over one million total hip arthroplasties (THAs) are carried out worldwide and provide patients with pain relief, as well as an enhanced quality of life.1,2 Despite the success of this surgical intervention, there is still controversy over the optimal choice of implant and fixation methods. The lifespan of a hip implant can be described in two aspects, the initial stage where an implant must become firmly fixed and the remainder of its life cycle in which its stability is either preserved or lost.3 For cementless implants stability is initially attained by an interference fit into the bone and over time the surrounding bone integrates with the implant surface, which may be coated with bioactive substance that encourages bone on-growth, otherwise known as osteo-integration. Depending on the design of the implant and on patient factors such as their activity level, the mean survivorship of a hip arthroplasty is around 90% to 95% at ten years.4
In revision cases the amount of bone stock is often compromised due to the loss of bone associated with loosening of the primary implant, adjacent osteolysis, and the destruction of bone caused by the removal of the failed primary. In these cases bone grafts may be used, while works have also demonstrated the utility of cells incorporated in gels such as fibrin glue or pre-seeded onto the implants to aid their retention at the bone-implant interface and help with bone ingrowth.5-10
Fibrin-based sealants are commonly used to reduce blood loss during and after surgery. These sealants are composed of a mixture of fibrinogen, thrombin, and calcium ions. Currently a commercial fibrinogen concentrate, Tisseel (Baxter Healthcare Corp, Deerfield, Illinois, USA), is used in surgery. However, the fibrinogen in Tisseel is isolated from pooled human plasma and although it is heat treated, it carries a risk of viral infection. The advantages of autologous glue include an easy, cost-effective way of obtaining high concentrations of growth factors for tissue healing and regeneration by incorporating platelet rich plasma (PRP) that contains beneficial cytokines, such as platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and epidermal growth factor (EGF). These proteins encourage cellular chemotaxis, proliferation and differentiation, removal of tissue debris, angiogenesis, and the laying down of extracellular matrix.11,12 Autologous PRP have been used in various orthopaedic cases, with positive outcomes.11,13 It would be ideal to spray any form of autologous PRP gel onto the implant surface during surgery, and therefore it must be prepared relatively rapidly perioperatively and must gelate quickly and form a mechanical robust layer when sprayed onto a surface.
The aim of this study was to incorporate the buffy layer containing nucleated cells including mesenchymal stem cells (MSCs) from autologous bone marrow (BM) aspirate into gels made from the patient’s own blood, and to investigate the effects of incorporating three different concentrations of PRP on the proliferation and viability of the cells in the gel.
Methods
Informed consent was obtained from all human volunteers prior to blood donation and for the BM samples. The study was conducted with the approval of the University College London Research Ethics Committee (07/Q0506/10). BM and blood samples were harvested from healthy, non-smoking patients; those with inflammatory, haematological, and endocrine conditions were excluded; all subjects were American Society of Anesthesiologists grade one.14 The age of patients ranged from 49 to 60 years old (n = 9). All blood samples (approximately 50 ml) were taken in citrated and plain blood sample tubes (BD Biosciences Vacutainer, BD, Oxford, UK). The blood and BM samples were collected from the same nine patients, all of whom were undergoing elective joint arthroplasty surgery. The glue was prepared under sterile conditions.
Harvesting the bone marrow aspirate from patients
BM aspirate was harvested from the patient's iliac crest by vacuum aspiration; all patients were undergoing THA for end-stage osteoarthritis, and consented to intraoperative marrow aspiration preoperatively. After aspiration the mono-nucleated cells were concentrated using a separation tube (ABMC harvest kit; NTL Biologica, Wantage, UK). One 20 ml syringe was filled with 2 ml of citrate anticoagulant (ACD-A, Anticoagulant Citrate Dextrose Solution; Biomet Biologics, Warsaw, Indiana. USA) and 18 ml of BM. The BM was harvested using a unique jamshidi needle (NTL Biologica) that is perforated along its length to access the multiple sites within the iliac crest simultaneously. The BM was then spun to separate the cell-free plasma/ACD-A mixture from red blood cells. From a 20 ml BM aspirate that was spun, approximately 1.5 ml of buffy layer would be obtained that would contain nucleated cells of which a small percentage would be MSCs. For all samples collected from the nine patients, all experiments were completed in triplicate within each experimental condition.
Preparation of the gel
Autologous blood-derived gel (ABG) was prepared by spinning the blood using the ABMC kit (NTL Biologica). Blood was spun at 3,300 rpm for three minutes, to obtain the plasma which was then activated to obtain the thrombin component using acetic acid. Blood was also spun at 3,300 rpm for three minutes to obtain PRP and BM aspirate. PRP and BM aspirate were combined in equal ratios and this final mixture was then combined with blood serum. To form the ABG gel with the BM aspirate, three different concentrations of PRP were investigated: 1.25 times; 2.5 times; and 5 times concentration. This is the concentration of the PRP after mixing it with equal volumes of BM aspirate and then combining it with the rest of the gel components. Mixing was carried out using a double-barrelled syringe that enabled the components to form a gel almost immediately after mixing. In addition, the gel thickness and the area covered by the gel could be controlled using a compressed air spraying system (NTL Biologica).
The same volume of BM aspirate used in the ABG gels was also mixed with the thrombin component, and then combined with aprotonin component to form fibrin glue (Tisseel, Baxter Healthcare Corp, Illinois USA). All ABG gels and fibrin glue gels were 1 ml in volume.
Rheology
Prior to assessing the utility of ABG as a coating, its viscoelastic properties were optimized by adjusting the proportion of each component. Eight thrombin:PRP:serum ratios were investigated. The viscoelastic properties of each ABG produced were quantified using a shear rheometer (Bohlin CVO 100 Rheometer; Malvern Instruments, Malvern, UK). Next 40 ml of blood for ABG production was donated from four volunteers (n = 4) each. Then 1.5 ml ABG was formed on the mounting plate (4 mm diameter parallel plates; 0.5 mm gap). A frequency sweep was performed in dynamic mode and a frequency amplitude (ω) range of 0.628 to 62.8 at 22 rad/s (0.001 to 10 Hz) (Bohlin CVO 100 software) (Malvern Instruments, Inc., Westborough, Massachusetts, USA). All sweeps were conducted at room temperature (approximately 23°C). The storage modulus (G’, relating to elastic properties) and loss modulus (G’’, relating to viscous properties) of each sample was measured.
Characterization of bone marrow mesenchymal stem cells
The bone marrow mesenchymal stem cells (BMSCs) isolated with the ABMC kit were cultured in a flask and characterized according to the International Society for Stem Cell Research.15 Once colony forming units were established after approximately 14 days, the cells were passaged and characterized using flow cytometry for the positive CD marker expression of CD105, CD29, CD90, and negative CD marker expression of CD34 and CD45.16 Briefly, 100 000 BMCs were fixed in 4% formalin for 15 minutes at room temperature, washed with 0.5% bovine serum albumin (BSA), and stained with the conjugated primary antibody for one hour at room temperature in the dark. The cells were labelled with antimouse/rat CD29-fluorescein (Thermo Fisher Scientific, Walham, Massachusetts, USA), 17 anti-mouse/rat CD90-APC (Thermo Fisher Scientific), antirat CD45-APC (Thermo Fisher Scientific), and CD34-PE (Abcam, Cambridge, UK). The CD expression was compared with the isotype control. After one hour, the cells were washed with 0.5% BSA and analyzed on a flow cytometer (Cytoflex; Beckman Coulter, Brea, California, USA).
Metabolic activity/cell viability
A PrestoBlue assay (Bio-Rad, Kidlington, UK) was used to measure the cell proliferation of the BM cells cultured within the ABG gel and fibrin glue at days 7 and 14. Then 10% PrestoBlue solution was added to the culture medium for 30 minutes and excitation at 560 nm and emission at 590 nm were measured using a Tecan plate reader (Infinite Pro 200 series; Tecan, Männedorf, Switzerland). The mean absorbance was determined from triplicate samples. Metabolic activity of the cells in the ABG gel was normalized to their corresponding ABG gels without cells. The metabolic activity of the following gels was investigated: 1) 1.25× concentration with cells; 2) 1.25× concentration without cells; 3) 2.5× concentration with cells; 4) 2.5× concentration without cells; 5) 5× concentration with cells; 6) 5× concentration without cells; 7) fibrin glue with cells; 8) fibrin glue without cells.
Live/dead
To check the viability of the cells in the ABG gels and fibrin glue, a live/dead assay was carried out at days 7 and 14. The gels with and without cells were washed with PBS and incubated in Calcein AM (Thermo Fisher Scientific, UK) and Ethidium Homodimer (Thermo Fisher Scientific, UK) for 30 minutes at 37°C, 5% Co2, in the dark. After incubation, the gels were washed with PBS and viewed under a fluorescent microscope (Apotome.2; Carl Zeiss Microscopy, Jena, Germany).
Phalloidin stain for actin filaments and the cell cytoskeleton
The ABG gels were fixed in 10% buffered Formaldehyde for 30 minutes, washed with PBS, and then incubated with Phalloidin and Hoescht mixture for 30 minutes at room temperature. The stain was then washed off with PBS thrice to remove any excess stain, and the gels were viewed under a fluorescent microscope (Apotome.2).
Histology
Histology was performed as per our previous studies.17 Briefly, the ABG and fibrin glue gels were fixed in 10% buffered Formaldehyde, dehydrated in a series of increasing alcohol concentrations, treated with chloroform for two days to de-fat the tissue, and then embedded in wax. Sections measuring 5 μm thick were made using a microtome at the centre of each gel (Thermo Fisher Scientific). Samples were de-waxed twice in xylene, placed in two changes of 100% alcohol, and then hydrated in serial dilutions of alcohol. After hydration, samples were stained in haematoxylin, a nuclear stain, for five to ten minutes. The excess stain was washed off by immersing the slides in water for five minutes. Samples were then differentiated in 0.5% hydrochloric acid (HCL; made up in 70% alcohol) and washed using water. After removing the acid-alcohol, samples were counterstained in 1% eosin for three to four minutes, washed in water, and dehydrated by serial dilutions of alcohol. Finally, samples were cleaned by xylene and mounted under coverslips using Pertex Mounting Medium (CellPath, Newtown, UK). Samples were observed under a light microscope (KS-300 Zeiss; Carl Zeiss Microscopy) at 10× magnification. The area containing stem cells in the gel was calculated by counting the number of quadrants with spindle shaped cells and dividing this by the total number of quadrants covering the whole gel area. This value was then expressed as a percentage
The retention of stem cell characteristics of cells embedded in the gel
BM aspirate was incorporated in the gels and although it was established that the cells obtained from this aspirate expressed stem cell characteristics, it was important for us to determine whether these characteristics were maintained after 14 days in a gel. As such, after 14 days the gels were fixed, prepared for wax histology (as described above), and stained for CD90 and CD29 using immunofluorescence.
Statistical analysis
Normality was determined using the Kolmogorov-Smirnov and Shapiro-Wilk tests and where the data was normal, comparison was made using independent-samples t-test. Where the data was non-parametric comparison was made using Mann-Whitney U test with a Bonferroni correction. All data was analyzed using SPSS version 24 (IBM, Armonk, New York, USA) with p < 0.05 considered to be significant.
Results
Rheology
To select the optimized gel formulation, it was important for the gel to have the following mechanical properties as measured rheologically: 1) the elastic and viscous modulus should not cross over as this demonstrates episodes of viscosity and elasticity, indicating a lack of network structure typical of an unlinked polymer; and 2) values of elastic modulus (G’) should remain higher than viscous modulus (G’’), indicating predominantly elastic properties and the presence of a 3D network structure.
In accordance with these properties, gel B had the highest values of G’ and G’’ over the higher frequency range (5 to 50 rad/s), with the two different types of moduli not crossing over. Therefore, gel B possesses the highest cohesiveness and network concentration at 23°C (Figure 1).
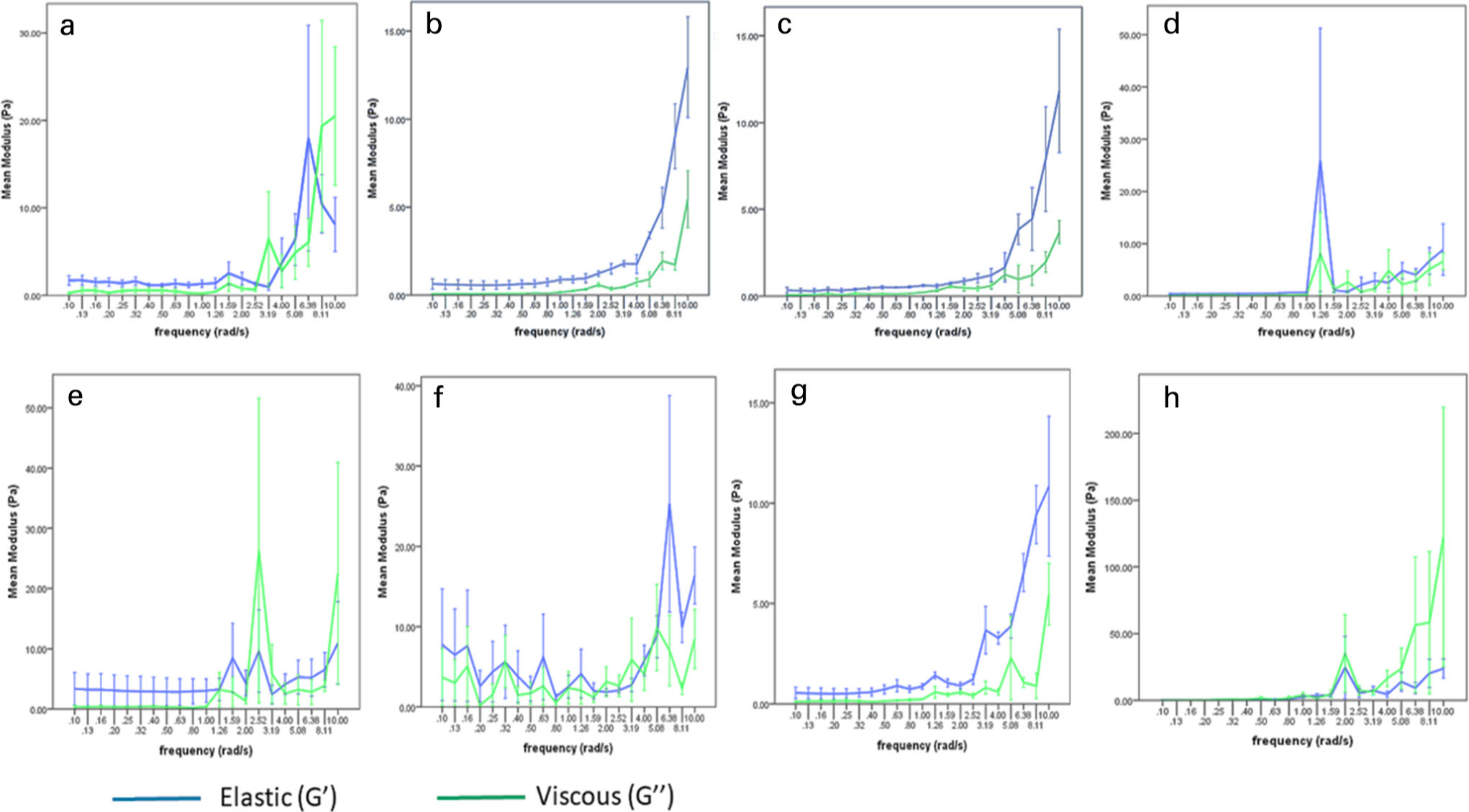
Fig. 1
Rheological analysis of platelet rich plasma (PRP) gels. The graphs show the elastic (blue) and viscous (green) modulus of the different types of gels (Gel A – H) at different thrombin and platelet concentrations. A higher elastic modulus compared to the viscous modulus without any crossover demonstrates effective cross-linking of the gels. Error bars represent standard error (SE) ± 1 (n = 4).
Cell viability
Phalloidin and live/dead staining of the cells up to 14 days showed that the gels supported the proliferation and growth of cells. At 14 days, it was evident that cells of two different morphologies were present; a rounder type and an elongated type. BM stem cells grown from the BM aspirate on tissue culture surfaces were characterized using CD marker expression at passage 2 (Figures 2 and 3). Over 70% of these cells expressed CD29, CD90, and CD105. Additionally, less than 5% of the cell population from this BM aspirate expressed CD34 and CD45 (Figure 4).
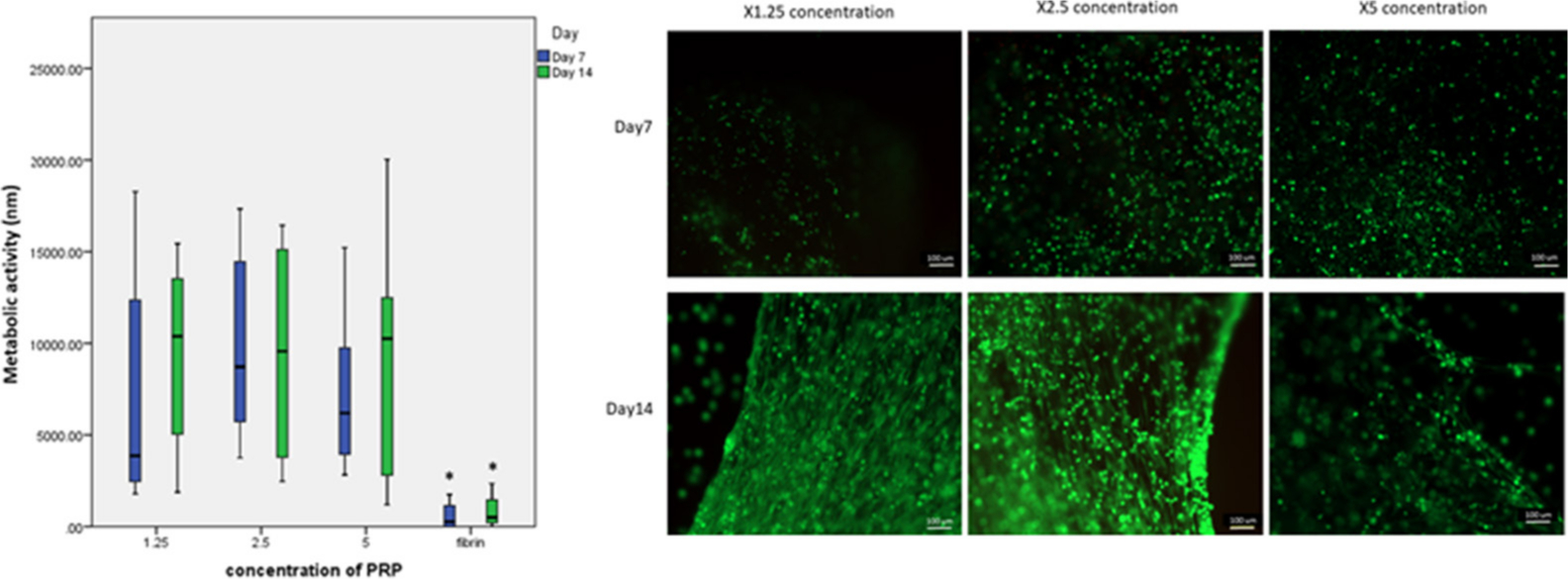
Fig. 2
Proliferation and Live/Dead stain of bone marrow mesenchymal stem cells (BMSCs) at days 7 and day 14 for different concentration of platelet rich plasma (PRP) in gels; 1.25, 2.5, and 5 times concentration of PRP. These photos are taken of gels made from one patient at 2.5× magnification. The 5 times concentration of PRP in the autologous gel was observed to reduce the proliferation of BMSCs, although quantification using PrestoBlue (metabolic activity) showed that this was not significantly different to the other concentrations of gels (n = 8). However, there was a significant difference for the mean metabolic activity between fibrin glue and all concentrations of gels at days 7 and day 14. Magnification ×10.
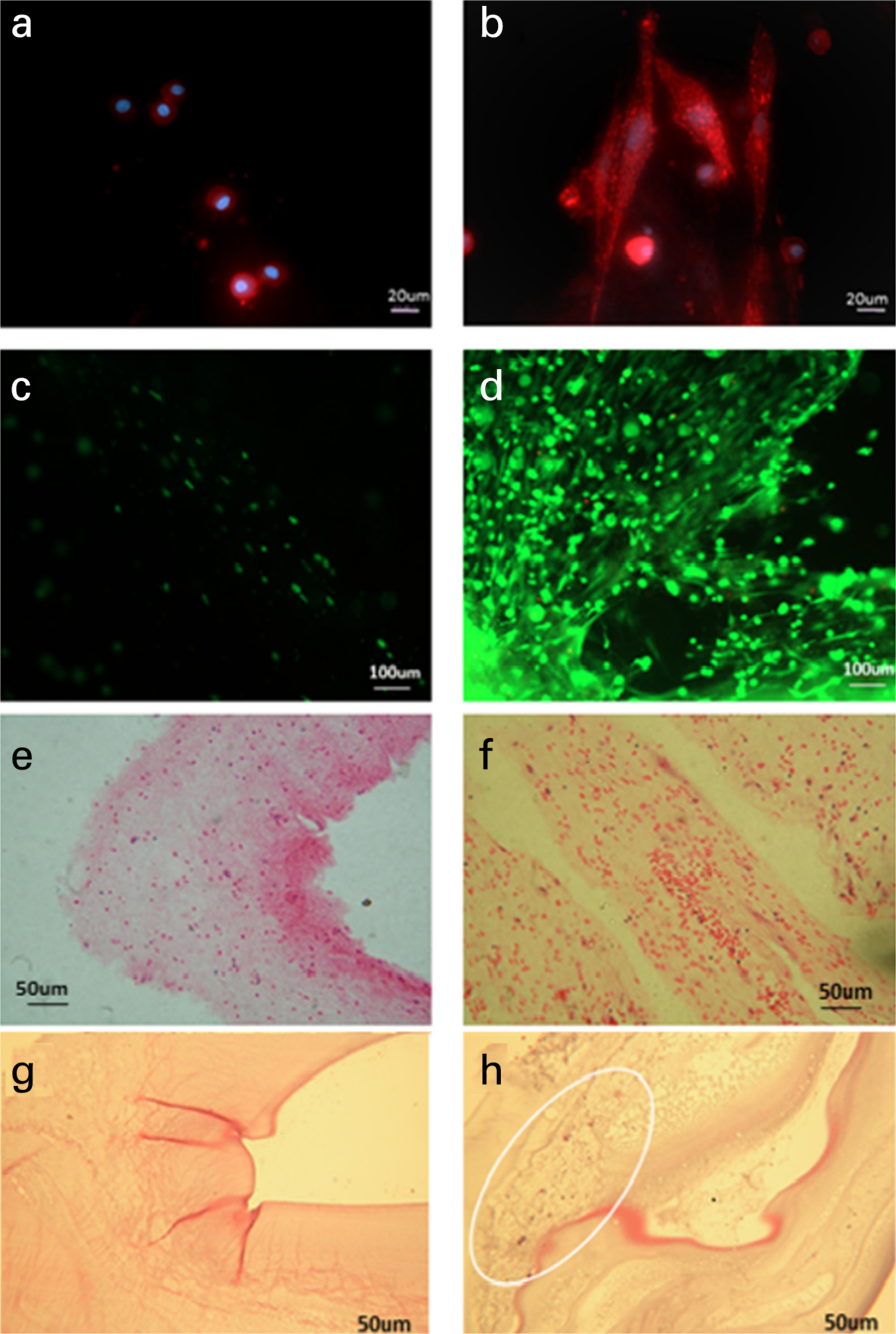
Fig. 3
Phalloidin (magnification ×20), live/dead (magnification ×10), and Haemotoxylin and Eosin staining (magnification ×10) of the cells in the 2.5 times concentration autologous blood gel after 14 days. Images A, C, and E are gels without bone marrow (BM) stem cells and Images B, D, and F are gels with BM stem cells cultured in them. The cells were stained with Phalloidin (A and B), Live/Dead stain (C and D), and Haemotoxylin and Eosin stain (E and F). Haemotoxylin and Eosin staining Fibrin glue without cells (G), and with cells (H), with cells circled.
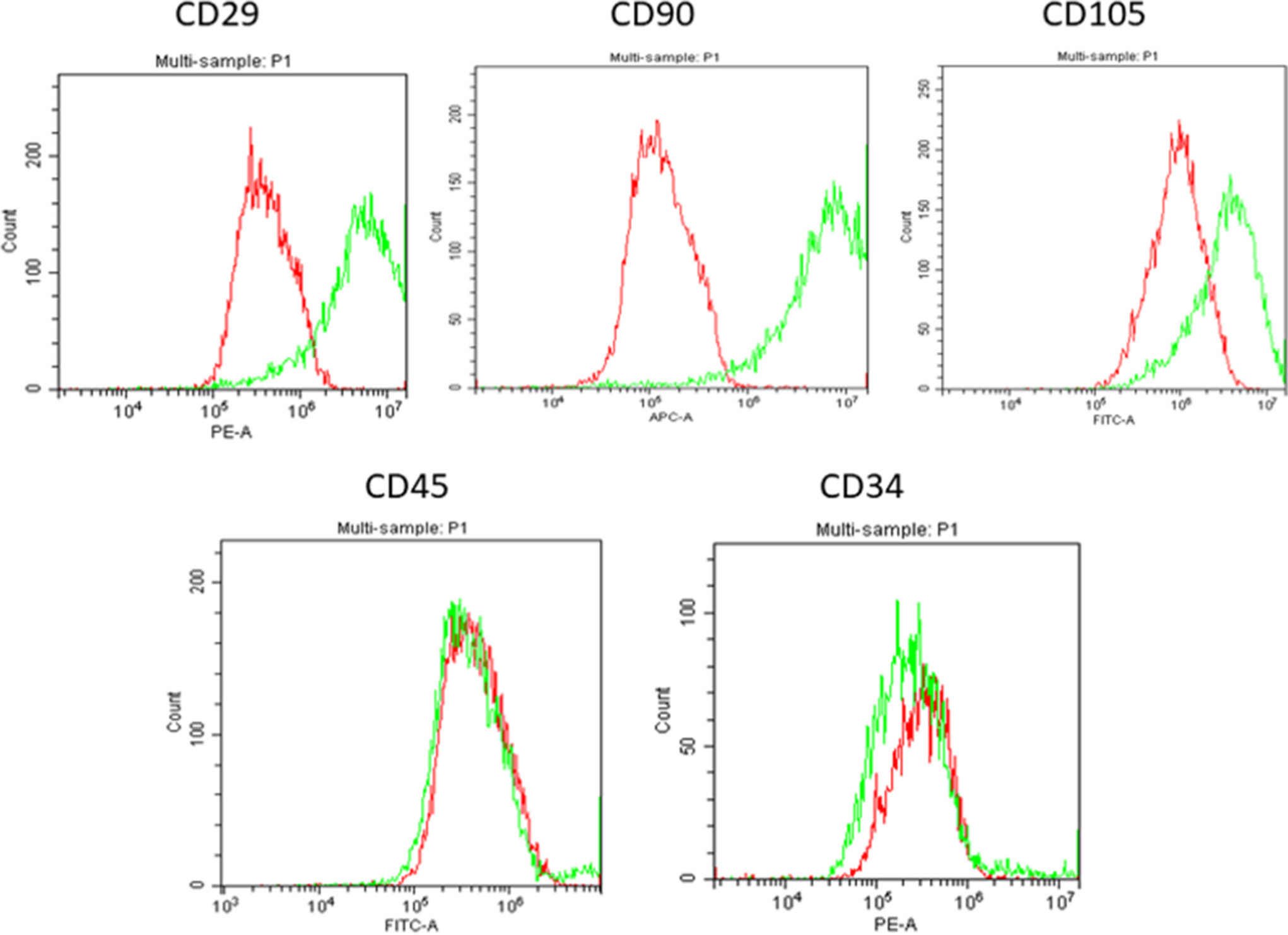
Fig. 4
Flow cytometry results of cells obtained from the bone marrow aspirate isolated from the buffy layer. The cells had positive stem cell markers for CD29, CD90, Stro1, CD105, and negative expression of CD34 and CD45. The red histograms are isotype controls and green histograms show the expression of the CD markers (n = 9). The stains used were Phycoerythrin (PE), Allophycocyanin (APC), and Fluorescein isothiocyanate (FITC).
Effect of PRP concentration on cell viability
It was observed that the concentration of platelets in the gel had an effect on the viability of MSCs embedded in the gels. A 2.5 times concentration of PRP produced optimum conditions for cell proliferation. Interestingly, 5 times concentration of PRP was observed to reduce the growth of the cells in the gel at both days 7 and 14. The 5 times concentration gels were also observed to reabsorb faster than the 2.5 times and 1.25 times concentration gels. Although the differences were not statistically significant, MSCs cultured in the 2.5 times PRP concentration maintained the highest proliferation at day 7 (mean 11,731 nm (SD 4,416)) and at day 14 as well (mean 11,393 nm (SD 6,001)) (Figure 2). High variability in the proliferation data was observed because of variability in PRP between patients.
Retention of stem cell characteristics
Immunofluorescence staining showed that after 14 days in the gel, the human BMSCs retained their stem cell characteristics as they continued to strongly express CD29 and CD90 (Figure 5). The spindle-like shape of the cells was clearly visible. This expression of CD29 and CD90 was not visible in gels without cells. This shows that the components of the gel and PRP did not influence the characteristics of the MSCs and their CD marker expression, and the nucleated cells which were embedded in the gel were able to proliferate into stem cells.
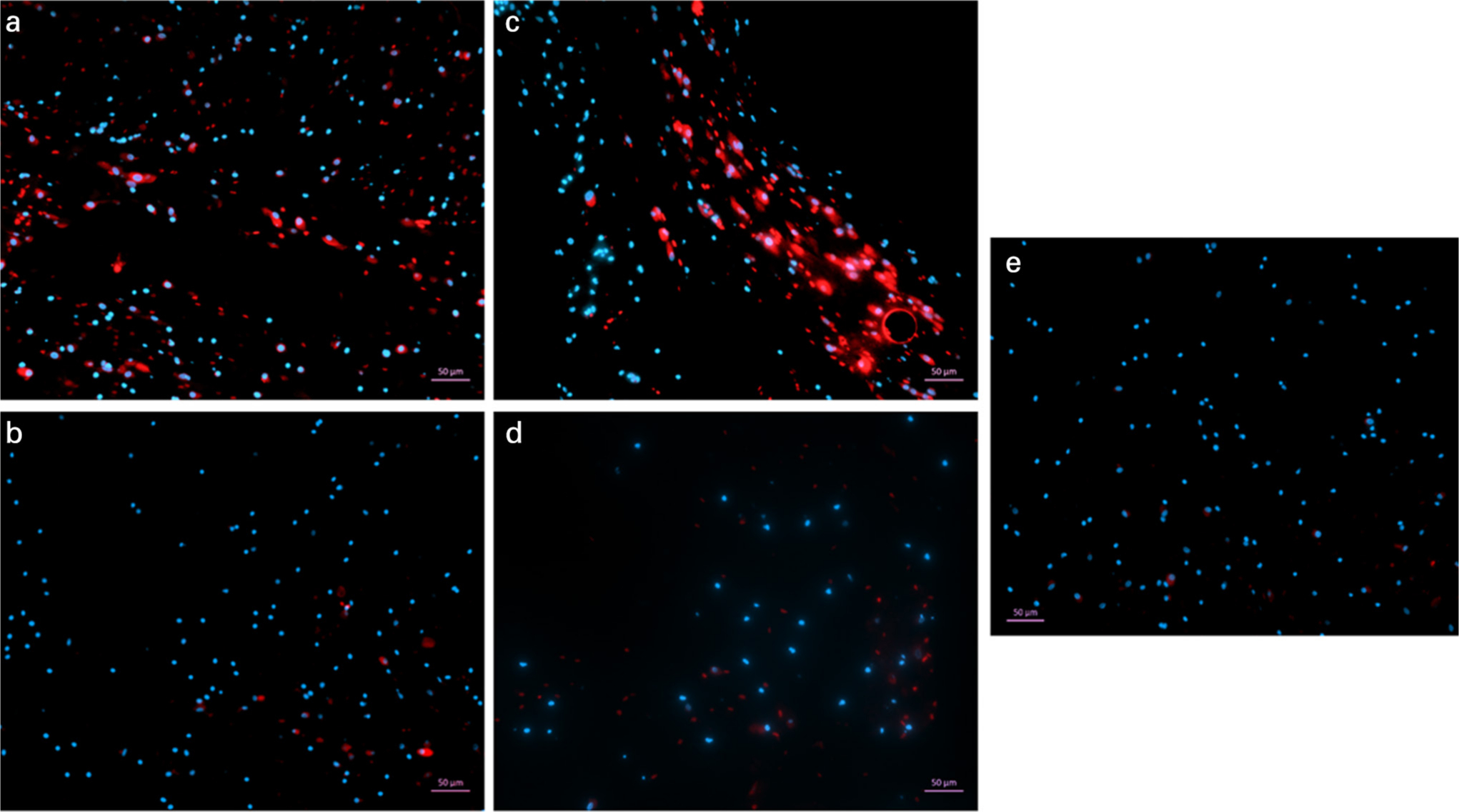
Fig. 5
Immunofluorescence staining of mesenchymal stem cells (MSCs) in the gel after 14 days in 2.5 times concentration gels. The gels containing bone marrow MSCs were stained for CD29-APC (A) and CD90-APC (C). Gels without any cells were also stained for CD29 (B) and CD90 (D). All gels were counter-stained with DAPI and viewed at a magnification of ×5. A control gel containing MSCs was stained with DAPI only to establish any background staining. Spindle-shaped cells visible in the gel had stained positive for CD29 and CD90.
Histological analysis
Histologically at day 7, the percentage area occupied by the cells was statistically significantly higher in the gels with PRP groups compared to gels with no buffy layer cells and cells + fibrin glue. This was also evident at day 14, showing that the ABG gel supported cell growth and allowed the cells to proliferate. Even though the 2.5 times concentration gel had the highest cell number, there was no significant difference between the gels of different PRP concentrations and this could be due to large variability between patients. Between days 7 and 14, a significant difference in cell area was only observed in 1.25 times concentration gels (Figure 6).
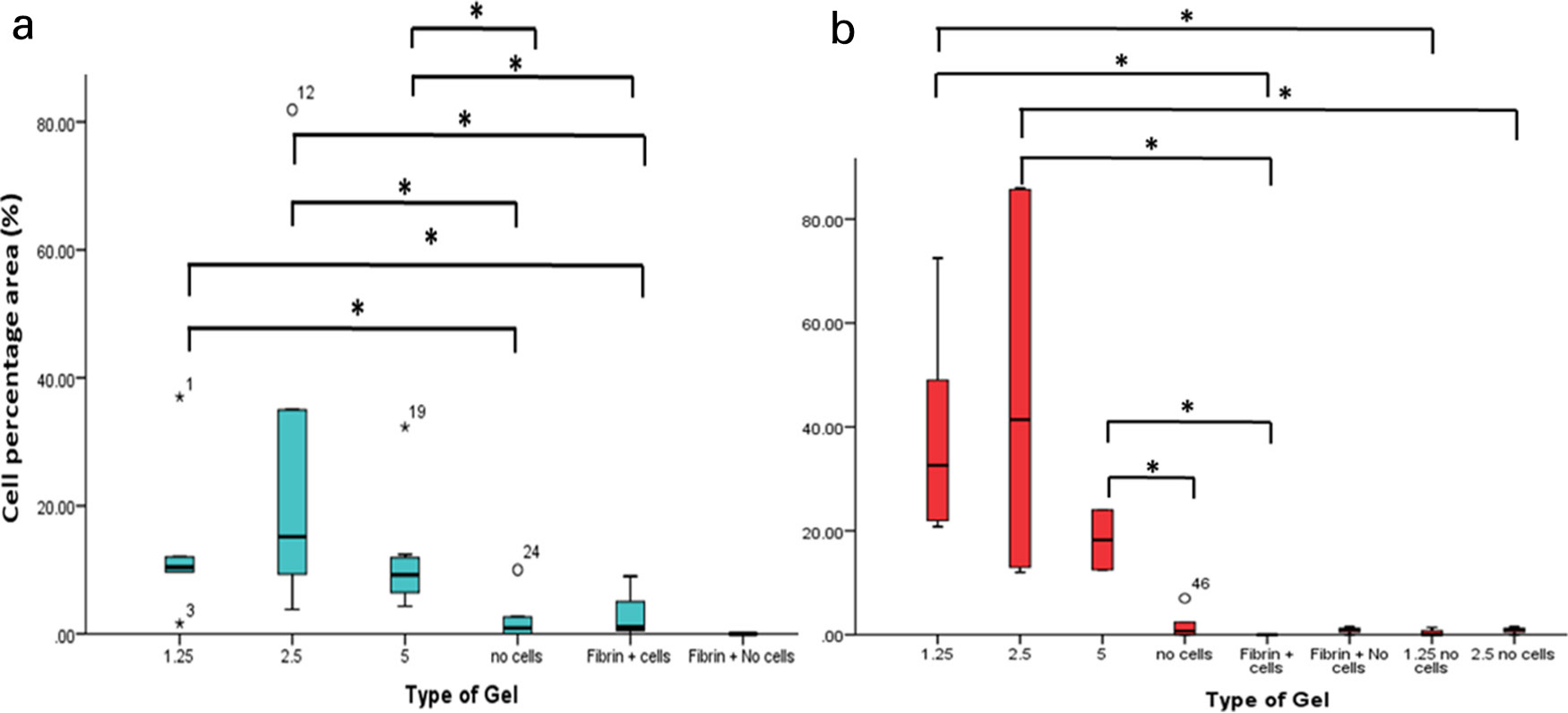
Fig. 6
Percentage cell area for the different platelet rich plasma (PRP) concentration gels and fibrin glue, with and without cells at day 7 (A) and day 14 (B). When implemented with bone marrow aspirate, the ABG had a statistically significantly higher cell area compared to fibrin glue after days 7 and 14. *p < 0.05.
Discussion
This study investigated the behaviour of human MSCs in an autologous gel made from venous blood. The cells isolated by the ABMC kit were identified to be MSCs by flow cytometry and were observed to be viable in the gel up to 14 days, until the gel was reabsorbed. Using Calcein AM and Ethidium Homodimer, as well as PrestoBlue measurements, the cells were also observed to be viable in the gel up to 14 days. Histology and actin staining demonstrated that two different types of cells were present in the gel; cells with a round morphology, most likely to be nucleated blood cells, and spindle-shaped fibroblastic like cells most likely to be MSCs. At the later timepoints cells with a fibroblastic morphology predominated, indicating that the gel enhanced the proliferation of these cells disproportionately compared with the nucleated blood cells. BMSCs are an important component of the gel because they have the potential to differentiate to bone, helping with the integration of the implant to the bone surface.15 Indeed it has been shown that bone contact and ingrowth of hydroxyapatite coated implants was statistically significant, improved by spraying autologous MSCs suspended in fibrin glue in ovine models.9 The difference between this and our current study is that we are using minimally manipulated cells in an autologous glue derived from the patient’s own blood.
Although the use of ABG in orthopaedic treatments needs to be established, the demand for autologous fibrin glue has increased compared to commercially available products. Its application varies from use in wound covering to maxillofacial surgery and all these various gels/glues have different compositions of blood, with PRP forming the main component.18–22 PRP contains growth factors that can encourage the growth of cells. From this study, 5 times concentration was seen to reduce MSC proliferation at day 7 and day 14. In addition, 2.5 times concentration of PRP supported the growth of MSCs in the gel, and cell proliferation was higher at this concentration than at 1.25 times or without any PRP. Although PRP contains growth factors such as transforming growth factor beta (TGF-β), VEGF, and IGF-1, which enhances cell proliferation, it also contains thrombospondin-1 (TSP-1), and high concentrations of this protein inhibit proliferation, cell adhesion, and angiogenesis.23 This could explain why stem cell growth was reduced at 5 times PRP concentrations. Although we did not find any significant differences in cell numbers and metabolic activity between different PRP concentrations, it has been reported that high concentrations of PRP have an inhibitory effect on bone formation in rabbits.24 The highest concentration of PRP did exhibit the lowest cell metabolic activity but results were insignificant, which could be due to variability in platelets between different patients. Yet PrestoBlue assays may not truly represent changes in cell number, as changes in reaction product may be a result of increased metabolic activity or increased cell number. As such, effects relating to cell number may require further investigation by normalizing the PrestoBlue results using DNA assays.
The results from this study are similar to a study by Jalowiec et al.25 They observed that PRP gels, formed using PRP and the thrombin component from Tisseel Baxter, formed an effective carrier for BMSCs in terms of cell viability and proliferation.25 This is similar to our work, where we have shown that an autologous fibrin-base scaffold/gel is much better at supporting the proliferation and viability of chondrocytes and BMSCs compared to the commercial Fibrin glue (Tisseel, Baxter. UK).26
Cravens et al27 have shown that commercial fibrin glues have a higher compressive strength than autologous gels. Although this is an important property, the commercial glue lacks the growth factors provided by PRP and we have shown that the proliferation of cells within the autologous gel increased, and this was statistically significant compared with the fibrin glue. It is also important for such a glue that carries cells to initially act as an effective scaffold to support cell proliferation and differentiation, and also biodegrade within a certain time frame to enable neovascularization. Additionally, it has been reported that Aprotinin, which is added to commercial fibrin glues to provide delayed fibrinolysis of the clot, demonstrates a slower regeneration of granulation tissue, thus aprotinin may not be beneficial where the glue is being used as a carrier for cells and tissue regeneration that replaces the glue is required.28 One of the limitations of this study is that we did not establish the resorption rates of the different concentrations of the gels and whether this is cell mediated or not. Further studies may be needed to answer this important question of this autologous gel’s biodegradability and its ability to act as an effective scaffold for cells.
The reasoning behind encapsulating MSCs into gels was to investigate whether this ABG could be used as a scaffold and delivery system of cells as well as growth factors. This is important because clinically, where patients suffer from impaired wound healing and tissue loss, the ABG could provide stem cells that have the potential to differentiate to the surrounding tissue, and this would be further boosted with growth factors from the PRP in the glue. We also showed that the stem cells embedded in the gel retained their stem cell characteristics. In vivo, autologous fibrin glue generated from the plasma of rabbit blood has been used in healing osteochondral fractures of the distal femur of rabbits, and they found improved cellularity in the glue group compared to the control group. In this study cells were not incorporated into the glue before it was implanted, and possibly the positive results seen could have been further enhanced if stem cells had been included.29
Our study demonstrates that a 2.5 times concentration of PRP in ABG is capable of supporting the growth of cells isolated from the BM aspirate. In vitro this autologous gel is capable of supporting cells in its structure for up to 14 days, and the cells remain viable during this period of time. Further avenues for exploration include the utility of the gel-cell as a suspension that can be applied to implants. If the cell viability is maintained in the spray form as it is in gel, then there may be profound implications for spraying a PRP-stem cell suspension on implants to improve osseointegration.30
References
1. Pivec R , JohnsonAJ, MearsSC, MontMA. Hip arthroplasty. The Lancet. 2012;380(9855):1768–1777.CrossrefPubMed Google Scholar
2. Sporer SM , PaproskyWG. Biologic fixation and bone ingrowth. Orthop Clin North Am. 2005;36(1):105–111.CrossrefPubMed Google Scholar
3. Bauer TW , SchilsJ. The pathology of total joint arthroplasty. Skeletal Radiol. 1999;28(8):423–432.CrossrefPubMed Google Scholar
4. Ulrich SD , SeylerTM, BennettD, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32(5):597–604.CrossrefPubMed Google Scholar
5. Frosch KH , SondergeldI, DresingK, et al. Autologous osteoblasts enhance osseointegration of porous titanium implants. J Orthop Res. 2003;21(2):213–223.CrossrefPubMed Google Scholar
6. Frosch K-H , DrengkA, KrauseP, et al. Stem cell-coated titanium implants for the partial joint resurfacing of the knee. Biomaterials. 2006;27(12):2542–2549.CrossrefPubMed Google Scholar
7. Franchi M , FiniM, MartiniD, et al. Biological fixation of endosseous implants. Micron. 2005;36(7):665–671.CrossrefPubMed Google Scholar
8. Marcacci M , KonE, MoukhachevV, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947–955. Google Scholar
9. Kalia P , BhallaA, CoathupM, et al. Augmentation of massive implant fixation using mesenchymal stem cells. Orthopaedic Proceedings. 2006;88-B(SUPP_III). Google Scholar
10. Kalia P , BlunnGW, MillerJ, et al. Do autologous mesenchymal stem cells augment bone growth and contact to massive bone tumor implants? Tissue Eng. 2006;12(6):1617–1626.CrossrefPubMed Google Scholar
11. Alsousou J , ThompsonM, HulleyP, NobleA, WillettK. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987–996.CrossrefPubMed Google Scholar
12. Everts PAM , KnapeJTA, WeibrichG, et al. Platelet-Rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174.PubMed Google Scholar
13. Spaková T , RosochaJ, LackoM, HarvanováD, GharaibehA. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417.CrossrefPubMed Google Scholar
14. Saklad M . Grading of patients for surgical procedures. Anesthesiol. 1941;2(5):281–284. Google Scholar
15. Dominici M , Le BlancK, MuellerI, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317.CrossrefPubMed Google Scholar
16. Sanghani-Kerai A , Osagie-ClouardL, BlunnG, CoathupM. The influence of age and osteoporosis on bone marrow stem cells from rats. Bone Joint Res. 2018;7(4):289–297.CrossrefPubMed Google Scholar
17. Sanghani A , Osagie-ClouardL, SamizadehS, et al. CXCR4 Has the Potential to Enhance Bone Formation in Osteopenic Rats. Tissue Eng Part A. 2018;24(23-24):1775–1783.CrossrefPubMed Google Scholar
18. Kouketsu A , NogamiS, Yamada-FujiwaraM, et al. Clinical evaluations of complete autologous fibrin glue, produced by the CryoSeal® FS system, and polyglycolic acid sheets as wound coverings after oral surgery. J Craniomaxillofac Surg. 2017;45(9):1458–1463.CrossrefPubMed Google Scholar
19. Kurian A , ReghunadhanI, NairKGR. Autologous blood versus fibrin glue for conjunctival autograft adherence in sutureless pterygium surgery: a randomised controlled trial. Br J Ophthalmol. 2015;99(4):464–470.CrossrefPubMed Google Scholar
20. Kikuchi D , IizukaT, HoteyaS, KaiseM. 379 utility of autologous fibrin glue and polyglycolic acid sheets for prevention of delayed bleeding after gastric endoscopic submucosal dissection under antithrombotic therapy. Gastrointest Endosc. 2017;85(5):AB73. Google Scholar
21. Hexter AT , ThangarajahT, BlunnG, HaddadFS. Biological augmentation of graft healing in anterior cruciate ligament reconstruction: a systematic review. Bone Joint J. 2018;100-B(3):271–284.CrossrefPubMed Google Scholar
22. Ji G , XuR, NiuY, et al. Vascular endothelial growth factor pathway promotes osseointegration and CD31hiEMCNhi endothelium expansion in a mouse tibial implant model: an animal study. Bone Joint J. 2019;101-B(7_Supple_C):108–114CrossrefPubMed Google Scholar
23. Hsu C-W , YuanK, TsengC-C. The negative effect of platelet-rich plasma on the growth of human cells is associated with secreted thrombospondin-1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(2):185–192.CrossrefPubMed Google Scholar
24. Weibrich G , HansenT, KleisW, BuchR, HitzlerWE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671.CrossrefPubMed Google Scholar
25. Jalowiec JM , D'EsteM, BaraJJ, et al. An In Vitro Investigation of Platelet-Rich Plasma-Gel as a Cell and Growth Factor Delivery Vehicle for Tissue Engineering. Tissue Eng Part C Methods. 2016;22(1):49–58.CrossrefPubMed Google Scholar
26. Kasemkijwattana C , RungsinapornV, SiripisitsakT, et al. Autologous Fibrin-Base scaffold for chondrocytes and bone marrow mesenchymal stem cells implantation: the development and comparison to conventional fibrin glue. J Med Assoc Thai. 2016;99 Suppl 8:S99–S104.PubMed Google Scholar
27. Cravens MG , BehnAW, DragooJL. Comparison of mechanical compressive properties of commercial and autologous fibrin glues for tissue engineering applications. Clin Biomech (Bristol, Avon). 2017;49:34–39.CrossrefPubMed Google Scholar
28. Basad E , SealantF. The use of autologous fibrin glue in total knee arthroplasty. US Musculoskeletal Review. 2006;1:77–78. Google Scholar
29. Azarpira MR , VazaniK, AyatollahiM, AzarpiraN, KavianiM. Comparison of healing intra-articular fracture of distal femur using a Kirschner wire and autologous fibrin glue in an animal model. J Pediatr Orthop B. 2017;26(5):454–457.CrossrefPubMed Google Scholar
30. Raphel J , HolodniyM, GoodmanSB, HeilshornSC. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301–314.CrossrefPubMed Google Scholar
Author contributions
A. Sanghani-Kerai: Designed the experiments, Collected the data, Wrote the manuscript.
M. Coathup: Collected the data.
R. Brown: Collected the data.
G. Lodge: Collected the data.
L. Osagie-Clouard: Collected the data.
I. Graney: Collected the data.
J. Skinner: Designed the experiments.
P. Gikas: Designed the experiments.
G. Blunn: Designed the experiments, Wrote the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
Ian Graney, who is an author on this original research article, is the Chief Executive Officer of NTL Biologica Limited. Apart from University College London (UCL) receiving the grant, all other authors or represented academic institutions do not have any other conflict of interest associated with this article.
Acknowledgements
This study was funded by NTL Biologica Limited, an advance regenerative medicine organization based in the UK.
Ethical review statement
This study was carried out with the approval of the University College London Research Ethics Committee (07/Q0506/10), with written informed consent from all subjects.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









