Abstract
Cartilage repair in terms of replacement, or regeneration of damaged or diseased articular cartilage with functional tissue, is the ‘holy grail’ of joint surgery. A wide spectrum of strategies for cartilage repair currently exists and several of these techniques have been reported to be associated with successful clinical outcomes for appropriately selected indications. However, based on respective advantages, disadvantages, and limitations, no single strategy, or even combination of strategies, provides surgeons with viable options for attaining successful long-term outcomes in the majority of patients. As such, development of novel techniques and optimisation of current techniques need to be, and are, the focus of a great deal of research from the basic science level to clinical trials. Translational research that bridges scientific discoveries to clinical application involves the use of animal models in order to assess safety and efficacy for regulatory approval for human use. This review article provides an overview of animal models for cartilage repair.
Cite this article: Bone Joint Res 2014;4:89–94.
History
Animal models have been the mainstay of cartilage repair research for decades and continue to be required for regulatory approval for clinical use of biologics, devices, and methods.1-8In vitro models of cartilage repair have also been developed and can have utility in terms of screening various strategies prior to in vivo validation using animal models9,10; however, in vitro models do not currently provide a direct pathway to clinical use and are not the subject of this review.
For this review, the senior author (JLC) searched PubMed (from 1967 to 2013) using the search terms ‘cartilage’ AND ‘animal’ with a Review Article filter. The reference lists of articles identified by this search strategy were then reviewed and studies judged to be relevant were selected. The reference list was subsequently modified during the peer-review process on the basis of comments from reviewers.
Historically, small animal models including rats and rabbits and large animal models including dogs, sheep, goats and horses have been most commonly used for investigation of cartilage repair strategies.1-8 In each of these models, surgically created focal defects in the knee (stifle) have been the typical methodology employed. While each of these models can be effective in assessing safety and efficacy of cartilage repair strategies to some degree, each also has its limitations.
Rodent and rabbit models are associated with spontaneous intrinsic healing of cartilage not seen in larger animal models and humans. Rodents retain open physes with continued endochondral ossification throughout life.11 The small size of their joints and very thin nature of their articular cartilage limit the types of treatments and outcome measures that can be used. It is also difficult to mimic post-operative manipulations such as bandaging, limited weight-bearing and physical therapy in mice, rats, and rabbits. Additionally, gait and movement characteristics in rats and rabbits are considerably different from that of humans, leading to differences in cartilage loading patterns. However, rodent models are cost- and space-effective, allow for genetic manipulation and use of xenogeneic cells and tissues. This makes rodent models an efficient means for determining mechanisms, assessing variables, and screening drugs, biologics, devices and methods for further investigations in large animal models.
Although financial costs and public perception of ethical aspects must be considered, large animal models address many of the limitations associated with rodent and rabbit models and are typically required for regulatory approval of any cartilage repair strategy. Healing potential of the cartilage is comparable with that seen in humans as noted in the numerous research studies using these models,3,5,6,8,9,12-14 as well as data from the veterinary clinical literature.15,16 The size of the joints and thickness of the articular cartilage in these species are amenable to clinically relevant manipulations and outcomes assessments. Post-operative management strategies used for human cartilage repair patients can be employed in these species – particularly in dogs – in which bandages, orthotics, limited weight-bearing and physical therapy are routinely and effectively used in research and clinical veterinary settings.8,12-14,15,17,18 Importantly, client-owned dogs and horses (rather than research dogs and horses) are also afflicted with cartilage defects resulting from osteochondrosis, trauma, and athletic injuries with symptomatology and treatment options that are essentially identical to those in humans.8,19,20 These spontaneously occurring cartilage defects provide an optimal opportunity for critically assessing cartilage repair strategies for human application, in that the mechanisms of disease are the same as those seen clinically, all of the articular tissues are involved, and assessments of efficacy can be based on clinically relevant measures of return to function.20
ASTM and FDA guidelines
In 2010, the American Society for Testing and Materials (ASTM) published the Standard Guide for in vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage,2 which included descriptions and rationale for various animal models. This guide distinguished cartilage regeneration (the formation of articular-like cartilage that has histologic, biochemical and mechanical properties similar to that of native articular cartilage) from cartilage repair (the process of healing injured cartilage or its replacement through cell proliferation and synthesis of new extracellular matrix) and fibrocartilage (disorganised cartilaginous tissue with an abnormally high content of type I collagen). A critical size defect is defined as the minimum defect dimension (in diameter) that the animal is incapable of repairing without intervention. For the femoral condyle – which was the primary location recommended – ASTM suggested that defect size should not exceed 15% to 20% of the articulating surface, or 50% to 60% of the condylar width, with depth varying from 1 mm to 10 mm depending on intended purpose, animal model, indication, and controls and cohorts used in the study design.2 While methods for reducing joint movement and load are acceptable, the joint should be restored to normal activity and unrestricted movement for an appropriate time prior to final assessment. At the time of sacrifice, outcome measures including gross (synovial fluid, synovium, repair site, surrounding and apposing articular cartilage, surrounding bone, osteophytes),16 histologic (validated scoring systems),21 biochemical (quantity and quality of collagen and proteoglycans) and biomechanical assessments (aggregate modulus, Poisson’s ratio, permeability using confined compression creep testing and/or creep indentation)22-31 along with appropriate statistical analyses, should be performed.2
In December 2011, the United States Food and Drug Administration (FDA) issued a Guidance for Industry regarding the requirements for approval of drugs, biologics, devices or combination products intended to repair or replace cartilage in the knee.1 While the FDA’s guidance documents do not establish legally enforceable responsibilities, the recommendations provided are typically considered to be the best ‘road map’ for gaining approval for clinical use of a given technology. In this document, the FDA outlined the components of translational (animal model) research that were suggested for applications for conducting clinical studies in the United States for cartilage repair or replacement in the knee, such as
Nonclinical data should establish scientific support for clinical investigation of the product by demonstrating an acceptable safety profile in the anticipated performance of the product, which can be derived from animal studies, mechanical testing, or a combination of both. Ideally, animal and mechanical testing should be combined in single studies.
Animal studies are used to assess biological responses (proof of concept for clinical efficacy and safety data), durability (time for repair, wear resistance, degradation and withstanding loads over time), toxicology (local and systemic), dose response, lesion size and location (location analogous to intended use in humans), appropriate endpoints (should mirror eventual clinical study) and use of arthroscopic and/or MRI evaluations (to allow for longitudinal, interim assessments before sacrifice).
At the time of each sacrifice, gross examination and mechanical integrity of the cartilage should be assessed. Mechanical testing should address the ability of the implant to withstand expected in vivo static and dynamic loading, analysis of fixation method and strength of integration, and propensity to generate wear debris.
Large animal models are recommended and researchers should carefully consider the model’s ability to reflect the intended clinical use of the product and the ASTM’s Standard Guide.2
In general, pivotal animal studies that are a minimum of one year in length are necessary; however, pilot studies designed to confirm the suitability of a specific animal model are recommended.
For cell-based therapies, the use of analogous cellular products from the animal species used for testing are recommended to avoid the need for immunosuppressive agents or rejection.
Complete reports of animal studies should be provided and include, at a minimum: 1) the purpose of the study; 2) the rationale for the animal model; 3) detailed methods; 4) the period of immobilisation; 5) gait analysis reports; 6) all tested parameters from bullet point two (page 90); and 7) pathological, histological and radiological evaluations. In addition, any differences between the product used in animal studies and the product proposed for clinical use should be explicitly described, and a statement regarding Good Laboratory Practice (GLP) compliance provided.
The FDA recognised that there is no perfect animal model of articular cartilage injury and recommended contacting them prior to nonclinical testing in order to best determine and define specific study design components.
Comparison of current models
Table I provides a comparison of common currently used animal models for articular cartilage repair.
Table I
Comparison of common currently used animal models for articular cartilage repair
| Species | Joint(s) | Cartilage thickness (mm)* | Defect type/ size | Primary use | Post-operative management capabilities | Outcomes assessments† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat | Knee | ~0.1‡ | Surgically created 0.75 mm to 3 mm chondral/osteochondral. Critical size = unknown | Mechanisms of action; use of xenogeneic cells or tissues; screening of treatments for pivotal study in large animal model | Laboratory rat caging. Running wheel. Hindlimb suspension | MRI if 7T or greater capabilities, microCT. Gross. Histology. Biochemical. Biomechanical | ||||||
| Rabbit | Knee, shoulder | 0.21 to 0.56 | Surgically created 2 mm to 4 mm (3 mm most common) chondral/osteochondral. Critical size = 3 mm | Mechanisms of action; screening of treatments for pivotal study in large animal model | Laboratory rabbit caging | MRI if 7T or greater capabilities, microCT. Gross. Histology. Biochemical. Biomechanical | ||||||
| Sheep/Goat | Knee | 0.4 to 1.5 | Surgically created 4 mm to 15 mm chondral/osteochondral. Impact injury. Critical size = 6 mm to 7 mm | Pivotal studies using surgically created defects for which post-operative management variables are not critical | Stall/pasture. Schroeder–Thomas splint | MRI, CT, radiography. Subjective function. Gross. India ink staining. Histology. Biochemical. Biomechanical | ||||||
| Dog | Knee, shoulder, elbow, hip, ankle | 0.95 to 1.3 | Surgically created 3 mm to 12 mm chondral/osteochondral. Impact injury. Osteochondrosis. Secondary osteoarthritis. Elbow dysplasia. Critical size = 4 mm | Pivotal studies using surgically created or spontaneous defects; post-operative assessments and management most closely mimic human | Kennel/run/group housed. Bandages, casts, splints, orthotics, external skeletal fixators, non-weight-bearing slings. Dedicated exercise. Physical therapy | Arthroscopic scoring. MRI, CT, radiography. VAS for pain, function, effusion and QoL. ROM. Muscle mass. Kinetics and kinematics. Gross. India ink staining. Histology. Biochemical. Biomechanical | ||||||
| Horse | Knee, carpus, ankle | 1.5 to 2.0 | Surgically created 6 mm to 20 mm chondral/osteochondral. Chip fracture. Osteochondrosis. Critical size = 9 mm | Pivotal studies using surgically created or spontaneous defects; cartilage thickness and cartilage biomechanics most closely resemble human | Stall/pasture. Dedicated exercise | Arthroscopic scoring. MRI and CT if special capabilities, radiography. Subjective function. Kinetics and kinematics. Gross. India ink staining. Histology. Biochemical. Biomechanical | ||||||
Recommendations
In addition to addressing the FDA and ASTM guidelines, it is critical to involve a team of professionals during the earliest stages of study design in order to optimise the results of the research and ensure the most ethical and effective use of animals. Key members of the team include a biostatistician to determine required animal numbers and data handling, a veterinarian and laboratory animal personnel to optimise animal care and use, experts in biomechanical testing, musculoskeletal histology and diagnostic imaging, and an orthopaedic surgeon who is very familiar with cartilage surgery. It would also be ideal to include regulatory experts and representatives from any industry partners from the onset to ensure feasibility for approval and clinical application. A pre-investigational device exemption (IDE) or pre-investigational new drug (IND) meeting and/or submission should be performed with the appropriate FDA agency well in advance of any pivotal study.
Researchers must weigh the needs for using a small or large animal study model with their outcome goals. Rodent and rabbit models are recommended for ‘mechanism’ or ‘proof of principle’ studies when data regarding toxicity, formulation, dose response and/or safety are needed before further pivotal studies. Large animal models are necessary for truly translational research aimed at gaining regulatory approval for clinical use in humans.
When deciding between large animal models, important factors to consider are those outlined above and in Table I, including feasible joints, cartilage thickness, defect type, size and location, post-operative management capabilities and availability and validated outcomes measures. In addition, it is important to consider the age of skeletal maturity and cartilage maturity of the animals chosen, the gastrointestinal physiology of the species, and involvement of all articular tissues that comprise the diarthrodial joint organ.
As outlined in the recommendations of the International Cartilage Repair Society (ICRS),5 cartilage maturity is thought to be a more important consideration than skeletal maturity (age at which the epiphyseal plates are fused). Cartilage maturity is typically determined by a well-defined zonal architecture, with an intact calcified cartilage layer (tidemark) and a fully-formed subchondral bone plate that is minimally vascularised. This helps to ensure that the articular cartilage has the appropriate cellular, biochemical, biomechanical and healing characteristics for relevant human clinical application. Gastrointestinal physiology has important ramifications for any studies that involve the use of pharmaceuticals, nutraceuticals, or other substances delivered via enteral absorption or uptake. Because goats and sheep are ruminants and horses and rabbits are hindgut fermenters, important differences in uptake, absorption, efficacy and side effects can occur in these species when compared with monogastrics such as dogs and humans.
Lastly, consideration of all articular tissues with respect to treatment of cartilage pathology is vitally important, but often overlooked. The joint is an organ and even early focal cartilage defects are associated with pathology of perilesional and apposing articular cartilage, subchondral bone and synovium, as well as other tissues such as meniscus, intra-articular ligaments, and labrum in respective joints. If the whole joint is not taken into consideration when developing and assessing cartilage repair strategies, clinical applicability of the data will be severely limited, especially with respect to pain relief, level of function attained and effects on disease progression. To this point, chronic defect models or spontaneously occurring disease in clinical veterinary patients are preferred and arthroscopic, MRI, gross and histologic outcome measures should include whole-joint assessments.11,16
In balancing all factors for optimising the translational potential of an animal model for testing cartilage repair strategies, the following are considered key characteristics for pivotal studies1-8,16,32,33:
1. Large animal model (dog, sheep, goat or horse) at age of skeletal and cartilage maturity, appropriately powered.
2. Critical size chondral or osteochondral defect (protection or violation of calcified layer verified arthroscopically and/or histologically).
3. Inclusion of a relevant control and/or cohort group.
4. Study duration ≥ six months.
5. Include diagnostic imaging (arthroscopy, MRI), clinically relevant (pain, effusion, muscle mass), functional (subjective with blinding and/or objective gait analysis, range of movement [ROM]), gross, biomechanical and histologic outcomes measures at a minimum.
6. Good Laboratory Practices (GLP)-level documentation.
In our research centre, these characteristics are addressed best by implementing canine models using adult (> 18 months old) purpose-bred hounds (> 20 kg body weight) of both genders. Types of defects include surgically-created critical size (≥ 5 mm) chondral or osteochondral defects in the knee, shoulder, elbow or hip, osteochondrosis lesions of the femoral condyle or medial humeral condyle, or ICRS grade 3 or 434 femoral condylar lesions occurring secondary to impact injury or meniscal release.35-38 Controls include the contralateral normal joint and/or untreated defects and cohorts are one or more relevant standard-of-care treatments such as microfracture, osteochondral autografts or allografts, or scaffold-only implants based on intended indication for the technology being evaluated. Dogs are evaluated longitudinally using clinical (pain, effusion, muscle mass, quality of life), functional (subjective and/or objective gait analysis, ROM), and diagnostic imaging (arthroscopy, radiography and/or MRI) assessments and at study endpoint (≥ six months) using gross, biomechanical, histologic and biochemical outcome measures (Fig. 1).
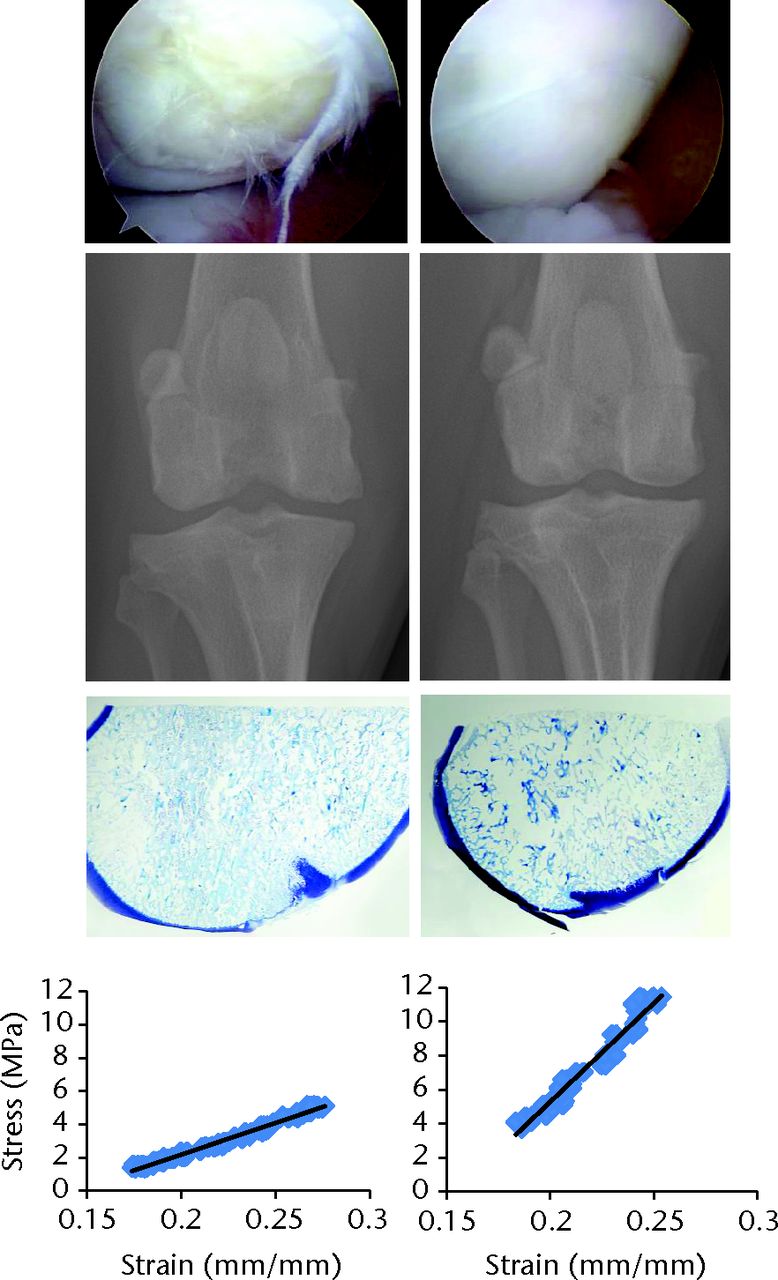
Fig. 1
Corresponding arthroscopic (top), radiological, histologic (toluidine blue stain) and biomechanical (Young’s Modulus) assessments of two different focal (10 mm) osteochondral defect treatments in the femoral condyles of dogs at six months after implantation. The defect on the left was treated with an osteochondral allograft that was preserved such that chondrocyte viability was < 70% at the time of implantation. The defect on the right was treated with an osteochondral allograft that was preserved such that chondrocyte viability was > 90% at the time of implantation.
Conclusions
Cartilage repair is a critical area of research for which translational animal models provide an essential component to safe and effective clinical use of new treatment strategies. While no perfect animal model exists, several good resources for optimising animal care and use within a sound experimental design are available.1-8,16,33 Adopting a team approach that includes basic scientists, veterinarians and laboratory animal personnel, physicians and industry representatives with the guidance of regulatory bodies, is highly recommended.
1 No authors listed. US Food and Drug Administration: Guidance for industry: Preparation of IDEs and INDs for products intended to repair or replace knee cartilage. http://www.regulations.gov/#!home (date last accessed 01 April 2014). Google Scholar
2 No authors listed. American Society for Testing and Materials. ASTM F2451 - 05(2010) Standard Guide for in vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage. http://www.astm.org/Standards/F2451.htm (date last accessed 28 November 2013). Google Scholar
3 Ahern BJ , ParviziJ, BostonR, SchaerTP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage2009;17:705–713.CrossrefPubMed Google Scholar
4 McGowan KB , StiegmanG. Regulatory challenges for cartilage repair technologies. Cartilage2013;4:4–11.CrossrefPubMed Google Scholar
5 Hurtig MG , BuschmannMD, FortierLA, et al.Preclinical studies for cartilage repair: recommendations from the International Cartilage Repair Society. Cartilage2011;2:137–152.CrossrefPubMed Google Scholar
6 Chu CR , SzczodryM, BrunoS. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev2010;16:105–115.CrossrefPubMed Google Scholar
7 Mithoefer K , SarisDB, FarrJ, et al.Guidelines for the design and conduct of clinical studies in knee articular cartilage repair: International Cartilage Repair Society recommendations based on current scientific evidence and standards of clinical care. Cartilage2011;2:100–121.CrossrefPubMed Google Scholar
8 Gregory MH , CapitoN, KurokiK, et al.A review of translational animal models for knee osteoarthritis. Arthritis2012;2012:764621.CrossrefPubMed Google Scholar
9 Tam HK , SrivastavaA, ColwellCW, D’LimaDD. In vitro model of full-thickness cartilage defect healing. J Orthop Res2007;25:1136–1144.CrossrefPubMed Google Scholar
10 Ng KW , WanivenhausF, ChenT, et al.A novel macroporous polyvinyl alcohol scaffold promotes chondrocyte migration and interface formation in an in vitro cartilage defect model. Tissue Eng2012;18:1273–1281.CrossrefPubMed Google Scholar
11 Dawson AB . The age order of epiphyseal union in the long bones of the albino rat. Anat Res1925;31:1–17. Google Scholar
12 Cook JL , FoxDB, MalaviyaP, et al.Long-term evaluation of treatment of large meniscal defects using small intestinal submucosa in a dog model. Am J Sports Med2006;34:32–42. Google Scholar
13 Jerre S . Rehabilitation after extra-articular stabilisation of cranial cruciate ligament rupture in dogs. Vet Comp Orthop Traumatol2009;22:148–152.CrossrefPubMed Google Scholar
14 Canapp S , AccianiD, HulseD, SchulzK, CanappD. Rehabilitation therapy for elbow disorders in dogs. Vet Surg2009;38:301–307.CrossrefPubMed Google Scholar
15 Ng KW , LimaEG, BianL, et al.Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A2010;16:1041–1051.CrossrefPubMed Google Scholar
16 Cook JL , KurokiK, ViscoD, et al.The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage2010;18(Suppl):S66–S79. Google Scholar
17 Cook JL , TomlinsonJL, ArnoczkySP, et al.Kinetic study of the replacement of porcine small intestinal submucosa grafts and the regeneration of meniscal-like tissue in large avascular meniscal defects in dogs. Tissue Eng2001;7:321–334.CrossrefPubMed Google Scholar
18 Canapp SO Jr . The canine stifle. Clin Tech Small Anim Pract2007;22:195–205. Google Scholar
19 Cook JL , HudsonCT, KurokiK. Autogenous osteochondral grafting for treatment of stifle osteochondrosis in dogs. Vet Surg2008;37:311–321.CrossrefPubMed Google Scholar
20 Cook JL , EvansR, ConzemiusMG, et al.Proposed definitions and criteria for reporting time frame, outcome, and complications for clinical orthopedic studies in veterinary medicine. Vet Surg2010;39:905–908.CrossrefPubMed Google Scholar
21 Mainil-Varlet P , Van DammeB, et al.A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med2010;38:880–890.CrossrefPubMed Google Scholar
22 Mow VC , LaiWM. Recent development in synovial joint biomechanics. SIAM Review1980;22:275. Google Scholar
23 Mak AF , LaiWM, MowVC. Biphasic indentation of articular cartilage: i: theoretical analysis. J. Biomech1987;20:703–714. Google Scholar
24 Mow VC , KueiSC, LaiWM, ArmstrongCG. Biphasic creep and stress relaxation of articular cartilage in compression?: theory and experiments. J Biomech Eng1980;102:73–84. Google Scholar
25 Mow VC , GibbsMC, LaiWM, ZhuWB, AthanasiouKA. Biphasic indentation of articular cartilage: ii: a numerical algorithm and experimental study. J Biomech1989;22:853–861. Google Scholar
26 Athanasiou KA , AgarwalA, DzidaFJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res1994;12:340–349.CrossrefPubMed Google Scholar
27 Alberts LR , NeffJR, BruggemanNB, KeenanSC. Effects of preservation technique on the viscoelastic properties of human articular cartilage. Trans Orthop Res Soc2001;26:0421. Google Scholar
28 Athanasiou KA , NiederauerGG, SchenkckRC Jr. Biomechanical topography of human ankle cartilage. Ann Biomed Eng1995;23:697–704.CrossrefPubMed Google Scholar
29 Schenck RC Jr , AthanasiouKA, ConstantinedesG, GomezEA. Biomechanical analysis of articular cartilage of the human elbow and a potential relationship to osteochondritis dessicans. Clin Orthop Relat Res1994;299:305–312. Google Scholar
30 Athanasiou KA , AgarwalA, MuffolettoA, DzidazFJ, ConstiantimidesG. Biomechanical properties of hip cartilage in experimental animal modelsClin Orthop. Relat Res1995;316:254–266. Google Scholar
31 Athanasiou KA , RosenwasserMP, BuckwalterJA, MowVC. Interspecies comparisons of in situ intrinsic mechanical properties of knee joint cartilages. J Orthop Res1991;9:330–340. Google Scholar
32 Frisbie DD , CrossMW, McIlwraithCW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol2006;19:142–146.PubMed Google Scholar
33 Hoemann C , Kandel R. RobertsS, et al.International Cartilage Repair Society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage2011;2:153–172.CrossrefPubMed Google Scholar
34 No authors listed. International Cartilage Repair Society. http://www.cartilage.org/ (date last accessed 14 March 2014). Google Scholar
35 Luther JK , CookCR, CookJL. Meniscal release in cruciate ligament intact stifles causes lameness and medial compartment cartilage pathology in dogs 12 weeks post operatively. Vet Surg2009;38:520–529. Google Scholar
36 Kuroki K , CookCR, CookJL. Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage2011;19:1142–1149.CrossrefPubMed Google Scholar
37 Garner BC , StokerAM, KurokiK, et al.Using animal models in osteoarthritis biomarker research. J Knee Surg2011;24:251–264.CrossrefPubMed Google Scholar
38 O’Connell GD , LimaEG, LimingB, et al.Toward engineering a biological joint replacement. J Knee Surg2012;25:187–196.CrossrefPubMed Google Scholar
Funding statement:
This review article used no specific grant from any funding agency in the public, commercial or not-for-profit sectors in its preparation.
Author contributions:
J. L. Cook: Senior author
C. T. Hung: Literature research, Article writing and editing
K. Kuroki: Literature research, Article writing and editing
A. M. Stoker: Literature research, Article writing and editing
C. R. Cook: Literature research, Article writing and editing
F. M. Pfeiffer: Literature research, Article writing and editing
S. L. Sherman: Literature research, Article writing and editing
J. P. Stannard: Literature research, Article writing and editing
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









