Abstract
Aims
Large-diameter metal-on-metal (MoM) total hip arthroplasty (THA) has demonstrated unexpected high failure rates and pseudotumour formation. The purpose of this prospective cohort study is to report ten-year results in order to establish revision rate, prevalence of pseudotumour formation, and relation with whole blood cobalt levels.
Methods
All patients were recalled according to the guidelines of the Dutch Orthopaedic Association. They underwent clinical and radiographical assessments (radiograph and CT scan) of the hip prosthesis and whole blood cobalt ion measurements. Overall, 94 patients (95 hips) fulfilled our requirements for a minimum ten-year follow-up.
Results
Mean follow-up was 10.9 years (10 to 12), with a cumulative survival rate of 82.4%. Reason for revision was predominantly pseudotumour formation (68%), apart from loosening, pain, infection, and osteolysis. The prevalence of pseudotumour formation around the prostheses was 41%, while our previous report of this cohort (with a mean follow-up of 3.6 years) revealed a 39% prevalence. The ten-year revision-free survival with pseudotumour was 66.7% and without pseudotumour 92.4% (p < 0.05). There was poor discriminatory ability for cobalt for pseudotumour formation.
Conclusion
This prospective study reports a minimum ten-year follow-up of large-head MoM THA. Revision rates are high, with the main reason being the sequelae of pseudotumour formation, which were rarely observed after five years of implantation. Blood ion measurements show limited discriminatory capacity in diagnosing pseudotumour formation. Our results evidence that an early comprehensive follow-up strategy is essential for MoM THA to promptly identify and manage early complications and revise on time. After ten years follow-up, we do not recommend continuing routine CT scanning or whole cobalt blood measurements, but instead enrolling these patients in routine follow-up protocols for THA.
Cite this article: Bone Jt Open 2022;3(1):61–67.
Take home message
The decision for revision a large-diameter metal-on-metal (MoM) total hip arthroplasty (THA) should be based on the clinical scenario of symptoms, radiological findings, and, to a lesser extent, systemic metal ions.
In the absence of significant abnormalities on initial screening, routine repeated cross-sectional imaging or measurement of systemic metal ions in asymptomatic patients with a large-diameter MoM THA is not necessary.
Introduction
Large-diameter metal-on-metal (MoM) total hip arthroplasty (THA) has resulted in unexpected high failure rates, leading to early revision. Wear of the MoM articulation is associated with an increase of metal particles around the joint. These particles are thought to cause a condition referred to as adverse reactions to metal debris (ARMD). The spectrum of ARMD ranges from formation of pseudotumours to osteolysis, increased blood metal ions, and metallosis.1-7
Popularity of the MoM articulation fell rapidly after problems were first reported in 2010, and has largely been discontinued, first in the Netherlands.8 Although there seems to be a general consensus not to use large-diameter MoM THA, there is much less consensus on the clinical management of our patients with MoM hip devices. In an attempt to identify ARMD early, worldwide regulatory authorities recommend regular patient follow-up, but guidelines for MoM THA follow-up differ considerably.9-11 This can partially be attributed to missing or conflicting evidence.12 We previously reported on the early results of an extensively screened prospective cohort of large-diameter MoM THA with a surprisingly high rate of soft-tissue reactions.4,5 This protocol was continued over the following years, with repeated annual follow-up and cross-sectional imaging in all patients. In this study, we present the results of the previously reported patient group in a concise follow-up.13
Methods
Between January 2005 and November 2007, 116 patients (117 hips) were treated with large-head MoM THA for primary osteoarthritis (OA). Local ethics board approval and written informed consent were obtained. In this prospective study, patients were scheduled for an extensive screening protocol. The protocol consisted of an annual follow-up (including a physical examination and radiographs of the hip and pelvis), and CT scanning of the hip in all patients in 2010 (three to five years postoperatively), followed by subsequent CT scans at five (if applicable) and ten years postoperatively. Blood samples for metal-ion analysis were collected according to accepted guidelines.14
We defined a pseudotumour as a solid/semi-solid or cystic periprosthetic soft-tissue mass eccentric to the joint with a minimum diameter of 2 cm that could not be attributed to infection, malignancy, bursa, or scar tissue.4 One radiologist evaluated all CT scans,15 and used a CT-grading system for postoperative CT findings in MoM THA.15
Cobalt ranges were set according to the recent guidelines: normal < 2 µg/l, intermediate 2 to 5 µg/l, elevated 5 to 10 µg/l, and extremely elevated > 10 µg/l.9,10,16,17 We used the bimetric porous-coated uncemented stem with a MoM M2a-Magnum femoral head and Recap acetabular component (Zimmer Biomet, USA). The system is modular, with increasing head size and concomitant larger shell size, plus the option to adapt the neck length using different length tapers. The main components of the head and acetabular component are produced from a cobalt-chromium alloy containing a small proportion of molybdenum and carbon. The stem and taper adapter are made of a titanium, aluminium, and vanadium alloy.4
Statistical analysis
Descriptive statistics (mean, median, and standard deviation (SD)) were used to analyze patient demographics and metal ions levels. Metal ion data distributions were asymmetric, and therefore expressed as a group median and range. Differences between groups were determined by independent-samples t-test for variables with normal distribution, the Mann-Whitney test for variables without normal distribution, and the Pearson chi-squared test for categorical variables. Kaplan-Meier procedure was used to estimate survival curves using any revision as an endpoint. Receiver operating characteristics (ROC) were constructed to define the best cut-off point for cobalt levels. A two-sided p-value < 0.05 was considered significant. Statistical software (SPSS Statistics, v. 23.0; IBM, USA) was used for all statistical analyses.
Results
The trial profile is listed in Figure 1. Initially, 116 patients (117) hips were included. Ultimately, 94 patients (95 hips) completed the ten-year follow-up period: however, 11 patients were lost to follow-up, one was revised prior the screening in 2010 for an infection, four refused further follow-up, and six patients died in the follow-up period due to unrelated reasons.

Fig. 1
Flowchart showing the trail profile at ten-years follow-up. MoM, metal-on-metal; *Three pseudotumours; **Two pseudotumours.
Patients and demographics
The cohort contained 54 males (55 hips) and 62 females (62 hips) with a mean age at index surgery of 60.1 years (38 to 72). All patients were diagnosed with primary OA. The characteristics of the 116 patients are shown in Table I. Mean follow-up after the index operation was 9.6 years (3.9 to 12.2).
Table I.
Patient characteristics.
| Variable | All patients | Patients who completed ten-year follow-up | Revisions | Soft-tissue reaction, no revision | No revision, no soft-tissue reaction |
|---|---|---|---|---|---|
| Total patients, n (hips) | 116 (117) | 94 (95) | 19 (19) | 26 (26) | 49 (50) |
| Sex, n (%) | |||||
| Males | 54 (47) | 44 (47) | 5 (26) | 14 (54) | 26 (53) |
| Females | 62 (53) | 50 (53) | 14 (74) | 12 (46) | 24 (47) |
| Mean age at follow-up, yrs (range) | 70.2 (50 to 83) | 71.2 (50 to 83) | 67.8 (50 to 80) | 72.2 (50 to 82) | 71.9 (55 to 83) |
| Follow-up after index operation, yrs (range) | 9.6 (3.9 to 12.2) | 10.9 (10.1 to 12.2) | 11.0 (10.0 to 11.8) | 10.9 (9.9 to 11.8) | 10.9 (9.9 to 11.8) |
| Side, n (%) | |||||
| Left | 41 (35.0) | 32 (33.7) | 4 (21) | 8 (30.8) | 20 (40.0) |
| Right | 76 (64.9) | 63 (66.3) | 15 (78.9) | 18 (69.2) | 30 (60.0) |
| Pseudotumour, n (%) | 44 (37.9) | 39 (41.5) | 13 (68.4) | 26 (100.0) | 0 (0.0) |
CT scan evaluation
In 2010, all eligible patients (116 patients/117 hips) were evaluated with an initial (baseline) CT scan. As 24 patients were operated on between 2005 and 2006, the initial CT scan was the five-year follow-up scan; they were also scanned at ten years follow-up.
The remaining hips received the first CT scan in 2010, the second scan five years after the index operation, and a third scan ten years after the index operation. One patient was revised for an infection before 2010 and therefore did not received a CT scan in 2010. Another 18 hips were revised between the five- and ten-year follow-up. Ultimately, the ten-year CT scan was performed in 77 hips.
Soft-tissue reaction
A total of 42 (39%) soft-tissue reactions met the criteria of a pseudotumour and were diagnosed in our previous study evaluating the initial CT scan of 2010.4 Of the 22 patients that were lost to follow-up, five (23%) had a pseudotumour and were excluded from further statistical analysis (Figure 1).
After comparing the CT scans at five and ten years, we found in four patients that there was regression of the pseudotumour, and in 19 patients that the pseudotumour remained unchanged. In one patient there was a progression of the pseudotumour. In two patients, there was a new symptomatic (painful) pseudotumour and one of them was subsequently revised. Between five and tenyears of follow-up, 13 patients were revised for a pseudotumour. At ten years follow-up, we found 39 pseudotumours in 95 hips (41%).
Revisions
A total of 19 hips (16%) were revised during the ten-year follow-up: 13 for a symptomatic pseudotumour, three for pain without a pseudotumour, one for aseptic loosening, one for infection, and one for asymptomatic extensive progressive osteolysis. Mean time to revision was 4.7 years (2.1 to 7.7) after index operation.
Implant survival
In all, 13 hips were revised within three years after starting the screening protocol in 2010. Revision-free survival was 82.4% after ten years. Revision-free survival after ten years with a pseudotumour was 66.7% and without a pseudotumour 92.4% (p = 0.005, log-rank test) (Figure 2).
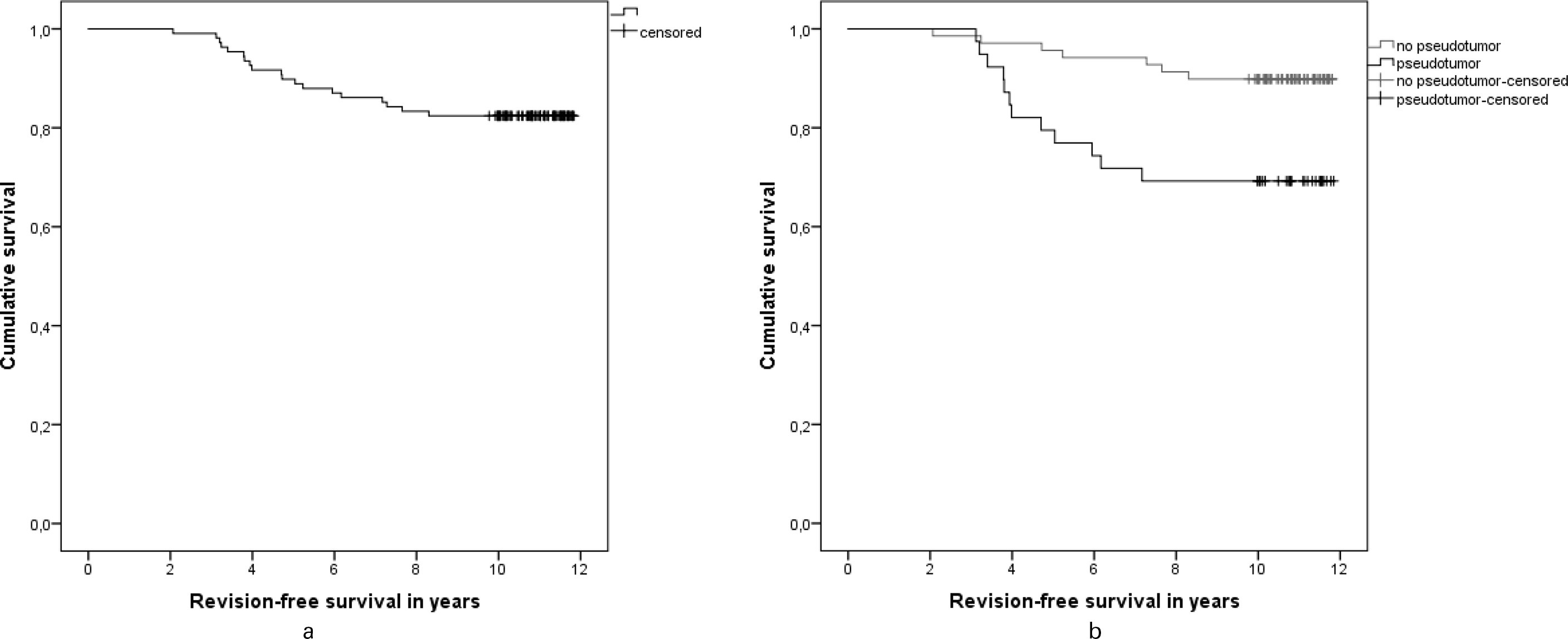
Fig. 2
a) Kaplan-Meier implant survival estimate for revision-free survival (all revision causes).b) Kaplan-Meier implant survival analysis for pseudotumour and without pseudotumour (p = 0.005, log-rank test).
Metal ions analysis
A total of 546 blood samples were analyzed, with a median of five samples per patient. The development of cobalt levels during the ten-year follow-up period is shown in Figure 3. Median level of cobalt at ten years follow-up was 5.3 µg/l (0.7 to 38.8; n = 48) in the group without a pseudotumour, 12.7 µg/l (0.6 to 105.9; n = 19) in the group with an unchanged pseudotumour, 7.2 µg/l (1.3 to 17.7; n = 2) in the group with progression of the pseudotumour, and 2.0 µg/l (0.9 to 4.9; n = 4) in the group with regression of the pseudotumour. ROC curve showed poor discriminatory ability for cobalt in predicting pseudotumour formation (Figure 4).

Fig. 3
a) Median cobalt levels for men and women without a revision. b) Median cobalt levels of patients with and without a pseudotumour. c) Four groups of cobalt levels, normal < 2 µg/L (group 1 (n = 20)), normal to high, 2 to 5 µg/L (group 2 (n = 41)), elevated 5 to 10 µg/L (group 3 (n = 15)) and extremely elevated > 10 µg/L (group 4 (n = 19)) at follow-up; the first measurement in 2010 allocated patients to a specific group.

Fig. 4
Receiver operating characteristic curve for blood cobalt.
Discussion
In this study, we evaluated ten years of extensive monitoring of a prospectively followed cohort of patients with large-head MoM THA. Initial CT scan revealed a high incidence of pseudotumours. This resulted in an unacceptably high revision rate of 17.6% after ten years.
Sequential CT scans reveal progression of a soft tissue mass to be rare. Patients who did have a progressive lesion were found to have a symptomatic (painful) hip. Detectable pseudotumour formation therefore seems to develop early (within the first five years) after implantation.
There were only minor fluctuations in cobalt levels in patients with a level < 10 µg/l; fluctuations at a level > 10 µg/l were modest. Cobalt is a poor predictor for the presence of a pathological soft-tissue mass, and in our experience contributed little to the decision of whether or not to perform a hip revision. Different studies report on the survival of the M2a-Magnum articulation we used in this study, albeit with different stems.4,5,18-21 In our previous study, survival was 88% after 3.6 years.5 In a similar study conducted by Boland et al,22 a comparable survival of 92.4% was found after five years.
This study shows a revision-free survival of 82.4% after ten years. This survival rate is comparable to that reported by the four largest implant registries of MoM THA.6,12,23-25 Other studies report that in asymptomatic patients with normal blood metal ion levels (< 2 μg/l) and normal ultrasound imaging, the risk of progression of ultrasound findings is small within five years of initial assessment.26,27
Besides the local effect of metal debris, there are concerns about possible systemic toxic effects of elevated levels of metal ions found in large-diameter MoM THA.28,29 Risk factors for elevated blood levels are malposition, taper issues, and prosthesis design.30,31 Systemic metal ion levels decline after revision.
The MoM wear trend is biphasic. Run-in wear with a maximum of two years is followed by steady-state wear.32,33 The patients in our group were assumed to be in the steady-state wear because the extensive screening protocol started after a minimum of three years after the index operation. Our study adds evidence to the hypothesis that elevated blood metal ion levels, as reported by several institutions, have poor discriminant ability in predicting pseudotumour formation.26,27
In a study on MoM hip resurfacing, it was noted that the discriminatory capacity of cobalt alone was similar to cobalt and chromium combined, suggesting the limited utility of chromium ion levels.34 It was recommended that the decision for revision surgery cannot be made based on blood metal ion values. Most current guidelines advice against the use of systemic metal ions alone for the decision to revise a MoM hip prosthesis.10,11
We believe that the decision for revision should be based on the clinical scenario of symptoms, radiological findings, and, to a lesser extent, systemic metal ions. We trust the results of this study to be valid because we report on a unique prospective cohort of MoM THAs with an extensive screening protocol with clinical follow-up, elaborate imaging, and metal ion measurements during ten years of follow-up.
Limitations
Our study has several limitations. The number of included patients is small. Furthermore, the use of CT scan to detect soft-tissue masses around metal hip articulations is a subject of debate.34,35 Similarly to MRI, the usefulness of CT scan is largely dependent on the quality of the scanner and the implementation of metal artefact reduction algorithms. CT scan has the advantages of being more readily available than MRI, is fast and low-cost, and with the introduction of low-dose protocols the radiation dose is strongly reduced. Measuring implant orientation is much more reliable, and CT is superior to all other imaging methods in detecting osteolysis.35,36
In conclusion, this study reveals a high revision rate of large-head MoM THA after ten-year concise follow-up. Time to onset of symptoms, as well as development of soft-tissue reactions and raised metal ion levels, rarely exceeds four years post-implantation. This suggests that abnormalities occur in the early years after implantation and subsequently develop into a steady state. We therefore advise screening patients with cross-sectional imaging and metal ion levels within five years of implantation of a MoM hip prosthesis. In the absence of significant abnormalities on initial screening, we advise against the use of routine repeated cross-sectional imaging or measurement of systemic metal ions in asymptomatic patients.
References
1. Smith AJ , Dieppe P , Vernon K , Porter M , Blom AW , National Joint Registry of England and Wales . Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales . Lancet . 2012 ; 379 ( 9822 ): 1199 – 1204 . Crossref PubMed Google Scholar
2. Smith AJ , Dieppe P , Howard PW , Blom AW , National Joint Registry for England and Wales . Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales . Lancet . 2012 ; 380 ( 9855 ): 1759 – 1766 : S0140-6736(12)60989-1 . Crossref PubMed Google Scholar
3. Pandit H , Glyn-Jones S , McLardy-Smith P , et al. Pseudotumours associated with metal-on-metal hip resurfacings . J Bone Joint Surg Br . 2008 ; 90-B ( 7 ): 847 – 851 . Crossref PubMed Google Scholar
4. Bosker BH , Ettema HB , Boomsma MF , Kollen BJ , Maas M , Verheyen C . High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study . J Bone Joint Surg Br . 2012 ; 94-B ( 6 ): 755 – 761 . Crossref PubMed Google Scholar
5. Bosker BH , Ettema HB , van Rossum M , et al. Pseudotumor formation and serum ions after large head metal-on-metal stemmed total hip replacement. Risk factors, time course and revisions in 706 hips . Arch Orthop Trauma Surg . 2015 ; 135 ( 3 ): 417 – 425 . Crossref PubMed Google Scholar
6. Australian Orthopaedic Association National Joint Replacement Registry . Hip and knee arthroplasty annual report . 2016 . https://aoanjrr.sahmri.com/annual-reports-2016 ( date last accessed 15 December 2021 ). Google Scholar
7. Matharu GS , Judge A , Murray DW , Pandit HG . Prevalence of and risk factors for hip resurfacing revision: a cohort study into the second decade after the operation . J Bone Joint Surg Am . 2016 ; 98-A ( 17 ): 1444 – 1452 . Crossref PubMed Google Scholar
8. Verheyen C , Verhaar JAN . Failure rates of stemmed metal-on-metal hip replacements . Lancet . 2012 ; 380 ( 9837 ): 105 – 106 . Crossref PubMed Google Scholar
9. Medicines & Healthcare products Regulatory Agency . All metal-on-metal (MoM) hip replacements: updated advice for follow-up of patients . https://assets.publishing.service.gov.uk/media/5954ca1ded915d0baa00009b/MDA-2017-018_Final.pdf ( date last accessed 15 December 2021 ). Google Scholar
10. US Food and Drug Administration . Medical devices: general recommendations for orthopaedic surgeons before metal-on-metal hip resurfacing surgery . https://www.fda.gov/medical-devices/metal-metal-hip-implants/information-orthopaedic-surgeons ( date last accessed 15 December 2021 ). Google Scholar
11. Hannemann F , Hartmann A , Schmitt J , et al. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing . Orthop Traumatol Surg Res . 2013 ; 99 ( 3 ): 263 – 271 : S1877-0568(13)00027-3 . Crossref PubMed Google Scholar
12. van Lingen CP , Zagra LM , Ettema HB , Verheyen CC . Sequelae of large-head metal-on-metal hip arthroplasties: Current status and future prospects . EFORT Open Rev . 2016 ; 1 ( 10 ): 345 – 353 . Crossref PubMed Google Scholar
13. Goosen JHM , Kollen BJ , Castelein RM , Kuipers BM , Verheyen CC . Minimally invasive versus classic procedures in total hip arthroplasty: a double-blind randomized controlled trial . Clin Orthop Relat Res . 2011 ; 469 ( 1 ): 200 – 208 . Crossref PubMed Google Scholar
14. MacDonald SJ , Brodner W , Jacobs JJ . A consensus paper on metal ions in metal-on-metal hip arthroplasties . J Arthroplasty . 2004 ; 19 ( 8 Suppl 3 ): 12 – 16 . Crossref PubMed Google Scholar
15. Boomsma MF , Edens MA , Van Lingen CP , et al. Development and first validation of a simplified CT-based classification system of soft tissue changes in large-head metal-on-metal total hip replacement: intra- and interrater reliability and association with revision rates in a uniform cohort of 664 arthroplasties . Skeletal Radiol . 2015 ; 44 ( 8 ): 1141 – 1149 . Crossref PubMed Google Scholar
16. Government of Canada . https://recalls-rappels.canada.ca/en/alert-recall/metal-metal-hip-implants-information-orthopaedic-surgeons-regarding-patient-0 ( date last accessed 15 December 2021 ). Google Scholar
17. Dutch Orthopaedic Society . Advice on metal on metal THA . https://www.orthopeden.org/downloads/80/advies-mom-per-01-08-2015.pdf ( date last accessed 15 December 2021 ). Google Scholar
18. Koper MC , Mathijssen NMC , Witt F , Morlock MM , Vehmeijer SBW , Nazarian A . Clinical and wear analyses of 9 large metal-on-metal total hip prostheses . PLoS One . 2016 ; 11 ( 10 ): e0163438 . Crossref PubMed Google Scholar
19. Koper MC , Mathijssen NMC , Vehmeijer SBW . A 5-year survival analysis of 160 Biomet Magnum M2 metal-on-metal total hip prostheses . Hip Int . 2016 ; 26 ( 1 ): 50 – 56 . Crossref PubMed Google Scholar
20. Borgwardt A , Borgwardt L , Zerahn B , Daugaard H , Borgwardt L , Ribel-Madsen S . A randomized seven-year study on performance of the stemmed metal M2a-magnum and ceramic C2a-taper, and the resurfacing ReCap hip implants . J Arthroplasty . 2018 ; 33 ( 5 ): 1412 – 1420 . Crossref PubMed Google Scholar
21. Lombardi AV , Berend KR , Morris MJ , Adams JB , Sneller MA . Large-diameter metal-on-metal total hip arthroplasty: dislocation infrequent but survivorship poor . Clin Orthop Relat Res . 2015 ; 473 ( 2 ): 509 – 520 . Crossref PubMed Google Scholar
22. Bolland B , Culliford DJ , Langton DJ , Millington JPS , Arden NK , Latham JM . High failure rates with a large-diameter hybrid metal-on-metal total hip replacement: clinical, radiological and retrieval analysis . J Bone Joint Surg Br . 2011 ; 93-B ( 5 ): 608 – 615 . Crossref PubMed Google Scholar
23. No authors listed . The NJR editorial board. National joint registry, 16th annual report 2019 . https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf ( date last accessed 15 December 2021 ). Google Scholar
24. EFORT . European Commission issued final opinion on the safety of metal-on-metal joint replacements with a particular focus on hip implants . https://www.efort.org/eu-initiatives/metal-on-metal/ ( date last accessed 15 December 2021 ). Google Scholar
25. Danish Hip Arthroplasty Register . Annual report 2019 . http://danskhoftealloplastikregister.dk/wp-content/uploads/2019/09/DHR-%C3%A5rsrapport-2019_til-offentligg%C3%B8relse-1.pdf ( date last accessed 15 December 2021 ). Google Scholar
26. Low AK , Matharu GS , Ostlere SJ , Murray DW , Pandit HG . How should we follow-up asymptomatic metal-on-metal hip resurfacing patients? A prospective longitudinal cohort study . J Arthroplasty . 2016 ; 31 ( 1 ): 146 – 151 . Crossref PubMed Google Scholar
27. van der Weegen W , Hoekstra H , Brakel K , Sijbesma T . Limited need for screening of metal-on-metal hip resurfacing patients beyond 10 years of follow-up . Epub ahead of print . Hip Int . 2020 ; 1120700020917939 . Crossref PubMed Google Scholar
28. Brodner W , Bitzan P , Meisinger V , Kaider A , Gottsauner-Wolf F , Kotz R . Serum cobalt levels after metal-on-metal total hip arthroplasty . J Bone Joint Surg Am . 2003 ; 85-A ( 11 ): 2168 – 2173 . Crossref PubMed Google Scholar
29. Daniel J , Ziaee H , Pradhan C , McMinn DJW . Systemic metal exposure in large- and small-diameter metal-on-metal total hip replacements . Orthopedics . 2008 ; 31 ( 12 Suppl 2 ). PubMed Google Scholar
30. Glyn-Jones S , Pandit H , Kwon YM , Doll H , Gill HS , Murray DW . Risk factors for inflammatory pseudotumour formation following hip resurfacing . J Bone Joint Surg Br . 2009 ; 91-B ( 12 ): 1566 – 1574 . Crossref PubMed Google Scholar
31. Kwon Y-M , Glyn-Jones S , Simpson DJ , et al. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours . J Bone Joint Surg Br . 2010 ; 92-B ( 3 ): 356 – 361 . Crossref PubMed Google Scholar
32. Heisel C , Streich N , Krachler M , Jakubowitz E , Kretzer JP . Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis . J Bone Joint Surg Am . 2008 ; 90-A ( Suppl 3 ): 125 – 133 . Crossref PubMed Google Scholar
33. Lee R , Essner A , Wang A . Tribological considerations in primary and revision metal-on-metal arthroplasty . J Bone Joint Surg Am . 2008 ; 90-A ( Suppl 3 ): 118 – 124 . Crossref PubMed Google Scholar
34. Hart AJ , Sabah S , Henckel J , et al. The painful metal-on-metal hip resurfacing . J Bone Joint Surg Br . 2009 ; 91-B ( 6 ): 738 – 744 . Crossref PubMed Google Scholar
35. Wellenberg RHH , Ettema HB , Verheyen CCPM , Maas M , Boomsma MF . Cross sectional imaging of metal-on-metal hip arthroplasties . Acta Orthop . 2015 ; 86 ( 2 ): 272 – 273 . PubMed Google Scholar
36. Roth TD , Maertz NA , Parr JA , Buckwalter KA , Choplin RH . CT of the hip prosthesis: appearance of components, fixation, and complications . Radiographics . 2012 ; 32 ( 4 ): 1089 – 1107 . Crossref PubMed Google Scholar
Author contributions
C. P. van Lingen: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Data curation, Visualization, Writing – original draft, Writing – review & editing.
H. B. Ettema: Conceptualization, Writing – review & editing.
B. H. Bosker: Data curation, Writing – review & editing.
C. C. P. M. Verheyen: Conceptualization, Supervision, Writing – review & editing.
Funding statement
The author(s) received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement
B. H. Bosker reports a fee from Zimmer Bioment for being a speaker at an arthroplasty congress in 2019, which is unrelated to this work. H. B. Ettema declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Stryker, Link Nederland, and Smith & Nephew, all of which are also unrelated.
Ethical review statement
Local ethics board approval was obtained.
Open access funding
The authors confirm that the open access funding for this manuscript was self-funded.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









