Abstract
Aims
To establish the survivorship, function, and metal ion levels in an unselected series of metal-on-metal hip resurfacing arthroplasties (HRAs) performed by a non-designer surgeon.
Methods
We reviewed 105 consecutive HRAs in 83 patients, performed by a single surgeon, at a mean follow-up of 14.9 years (9.3 to 19.1). The cohort included 45 male and 38 female patients, with a mean age of 49.5 years (SD 12.5)
Results
At the time of review 13 patients with 15 hips had died from causes unrelated to the hip operation, and 14 hips had undergone revision surgery, giving an overall survival rate of rate of 86.7% (95% confidence interval (CI) 84.2 to 89.1). The survival rate in men was 97.7% (95% CI 96.3 to 98.9) and in women was 73.4% (95% CI 70.6 to 75.1). The median head size of the failed group was 42 mm (interquartile range (IQR) 42 to 44), and in the surviving group was 50 mm (IQR 46 to 50). In all, 13 of the 14 revised hips had a femoral component measuring ≤ 46 mm. The mean blood levels of cobalt and chromium ions were 26.6 nmol/l (SD 24.5) and 30.6 nmol/l (SD 15.3), respectively. No metal ion levels exceeded the safe limit. The mean Oxford Hip Score was 41.5 (SD 8.9) and Harris Hip Score was 89.9 (14.8). In the surviving group, four patients had radiolucent lines around the stem of the femoral component, and one had lysis around the acetabular component; eight hips demonstrated heterotopic ossification.
Conclusion
Our results confirm the existing understanding that HRA provides good long-term survival and function in patients with adequate-sized femoral heads. This is evidenced by a 97.7% survival rate among men (larger heads) in our series at a mean follow-up of 14.9 years. Failure is closely related to head sizes ≤ 46 cm.
Cite this article: Bone Jt Open 2022;3(1):68–76.
Take home message
Hip resurfacing arthroplasty demonstrates excellent long-term survival if the the size of the femoral component is 48 mm or larger.
Since this is more often realized in male patients, resurfacing remains an attractive option for hip reconstruction in the younger male, with preservation of bone stock for a future revision if required.
Introduction
Hip resurfacing arthroplasty (HRA) was introduced as an alternative to conventional total hip arthroplasty (THA) in the 1970s, with the intention of providing a bone-conserving operation for the younger patient. The initial designs were metal-on-polyethylene but due to osteolysis and early failure, these fell from favour.1 Improvements in metallurgy and tribology led to the development of metal-on-metal (MoM) HRA, allowing the manufacture of thin acetabular components accepting large-diameter femoral resurfacing.2-4 Thus the McMinn HRA (Corin Medical, UK) was introduced in 1991.5 Proposed advantages of HRA over traditional THA include the reduced dislocation risk due to large head diameter, more accurate restoration of hip biomechanics, less likelihood of limb length discrepancy, higher rate of return to full activity including sports in the younger patient, conservation of femoral bone stock, and thus a simpler revision operation if subsequently required.6 The surgeon designer McMinn developed the Birmingham Hip Resurfacing (BHR, initially MMT, UK; subsequently Smith & Nephew, UK), which was met with good early success and marked a renewed interest in HRA in the UK.7-9 This success was replicated by other non-designer surgeons, and others using prostheses from different manufacturers.3,10 However, in the last decade, the procedure came under scrutiny due to reports of complications specific to the MoM articulation, and high rates of failure from some designs which were eventually withdrawn from the market.11,12 Consequently, there has been an overall decline in the use of HRA globally.
In 2006, HRA accounted for over 10% of primary hip arthroplasties on the National Joint Registry (NJR); today the proportion is 3.3%, with the number implanted in 2019 accounting for only 0.6% of all primary hip replacements in the year.13 Over the last decade, it has been established that HRA outcomes are poorer in females and those with smaller femoral head sizes, with the procedure being restricted to a selected group of younger active male patients with arthritis.6,14 Nonetheless, at the beginning of the 21st century, this association had not been established, and HRA was offered to both men and women with end-stage arthritis, This was also the practice in our unit. Given current guidance by the UK Medical and Healthcare Regulatory Authority (MHRA) on the need for surveillance for patients who received these implantations, we performed a formal service evaluation of our patients to see how their outcomes compared to contemporary reports.15
Methods
All patients who underwent HRA in our hospital between February 2001 and September 2010 by the senior author (GS) were retrospectively reviewed. This was a service evaluation project for which ethical approval was not mandatory. The decision to perform HRA at the patients’ request was taken by the surgeon at the time, based on the clinical presentation and extent of the arthritic changes making the intervention feasible. The target group consisted of patients of either sex, with end-stage arthritis of any aetiology, but who were otherwise physically active individuals, and had exhausted conservative measures. Other than infection, all diagnoses were accepted. Beyond the patients’ personal desire to receive a resurfacing arthroplasty, there was a specific surgical exclusion when the femoral head was damaged or collapsed beyond the point where templating revealed there was insufficient femoral head available which could be fashioned to accept the resurfacing component. No patient was rejected intraoperatively once they had consented for resurfacing. All operations were done through a modified anterolateral approach.16 Patients were allowed full weightbearing postoperatively with two crutches as necessary, and then discharged when medically fit. The prostheses used were BHR (from MMT; 2001 to 2005) and ADEPT (Finsbury Orthopaedics; subsequently MatOrtho, UK; 2005 onwards). All implants used were manufactured by Finsbury/MatOrtho for both distributors and produced with the same metallurgy (high carbon cobalt chrome as cast) and bearing clearance. Minor differences existed between the designs in the acetabular introducers and external texturing of the acetabular components, as well as the internal, nonarticular geometry of the femoral head. The ADEPT design offered a wide range of sizes when first introduced. There was no difference in our surgical technique for the two implant types.
All patients should have been attending for periodic review in accordance with Medicines and Healthcare products Regulatory Agency (MHRA) guidelines but some had defaulted.15 Accordingly, all patients were initially contacted by telephone and invited to attend a review clinic. Those who consented were seen in the clinics, where they were examined physically, and up-to-date anteroposterior (AP) pelvic radiographs and lateral radiographs of the hip and blood tests for cobalt and chromium ion levels were obtained. Those patients who declined a clinical review, either because they had moved or because they were well and considered attendance unnecessary, were administered functional hip scoring questionnaires remotely and their last radiographs were used for analysis. Informed consent was obtained from all patients for use of the information for research and publication. Details of surgery and the implants used were retrieved from records. Details on outcome of deceased patients were established from their records.
A total of 105 HRAs were performed in 83 patients (22 bilateral). There were 45 males (60 hips) and 38 females (45 hips); the mean age at surgery was 49.5 yrs (18 to 91). The most common indication for surgery was primary osteoarthritis (OA) in 70 hips (66.7%), followed by inflammatory arthritis in 16 hips (15.2%). Roughly one-third of the hips received BHRs and rest received ADEPT implants. (Table I)
Table I.
Summary of the study cohort.
| Variable | Total |
|---|---|
| Total patients/hips | 83/105 |
| Male hips, n (%) | 60 (57.1) |
| Female hips, n (%) | 45 (42.9) |
| Mean age at surgery, yrs (SD) | 49.5 (12.5) |
| Indication for HRA, n (%) | |
| Primary OA | 70 (66.7) |
| Avascular necrosis | 8 (7.6) |
| Developmental dysplasia | 5 (4.8) |
| Post-traumatic OA | 3 (2.8) |
| Other noninflammatory aetiologies | 3 (2.8) |
| Inflammatory arthritis | 16 (15.2) |
| Prosthesis used, n (%) | |
| BHR | 36 (34.3) |
| ADEPT | 69 (65.7) |
| Median femoral head size, mm (IQR) | |
| Males | 50 (48 to 52) |
| Females | 42 (42 to 46) |
| Mean acetabular component inclination angle, ° (SD) | 41.8 (8.7) |
| Males | 41.3 (8.7) |
| Females | 42 (9) |
| Mean ion levels, nmol/l (SD) | |
| Co | 26.6 (24.5) |
| Cr | 30.6 (15.3) |
| Functional scores | |
| Mean OHS (SD) | 41.5 (8.9) |
| Mean HHS (SD) | 89.9 (14.8) |
| Mean follow-up duration, yrs (range) | 14.9 (9.3 to 19.1) |
| Total failures/revisions, n hips (%) | 14/105 (13.3) |
| Males | 2/60 (2.3) |
| Females | 12/45 (26.6) |
-
HHS, Harris Hip Score; HRA, hip resurfacing arthroplasty; IQR, interquartile range; OA, osteoarthritis; OHS, Oxford Hip Score; SD, standard deviation.
Radiographs were analyzed using the picture archiving and communication system (PACS) tools (Sectra IDS ver.7, Sectra AB, Sweden). Quantitative radiological assessment was done by two observers (MHG and UZ) with acetabular component abduction angle; version of the components was not measured because patient positioning for the lateral views could not be reliably standardized. Qualitative evaluation for radiolucent lines, osteolysis around the components, and heterotopic ossification was carried out by a single observer (MHG) using previously described methods and definitions.17-19
Ion levels were examined by reference to the published acceptable ranges (≥ 7 ppb; 119 nmol/l Co or 134.5 nmol/l Cr).15 Functional scoring was undertaken with Oxford Hip Score (OHS) and Harris Hip Score (HHS).20-22 Patients with pain in the hip(s) and low functional scores were investigated with MRI scans for evidence of adverse local tissue reaction (ALTR) and/or pseudotumour formation.23-25 All complications were recorded. Failure was defined as revision for any reason.
Statistical analysis
Statistical analysis was performed by an independent statistician, using SPSS v 22.0 (IBM, USA). Quantitative variables were presented in terms of range, mean and standard deviation, (SD) or medians with interquartile ranges (IQRs) as appropriate. For qualitative variables, absolute and relative frequencies were determined. Confidence intervals (CIs) were set at 95%. Mann-Whitney U and paired t-tests was used to analyze differences in functional scores between subgroups. Two-tailed chi-squared testing was performed to determine the relative effect of two confounding variables on outcome. Intraclass correlation coefficient (ICC) was used to ensure consistency in radiological measurements. The Kaplan-Meier method was used to illustrate survivorship of the prosthesis, and subgroup comparison of survivorship was performed using the log-rank test. Statistical significance was defined as a p-value ≤ 0.05.
Results
At the time of review, 13 patients with 15 hips had died with the implants in situ. A total of 14 hips (in 13 patients) had been revised for various reasons. In all, 47 patients with 63 implanted hips attended for follow-up in person; all of them had up-to-date radiographs and blood Cr and Co ion level measurements in addition to completing the HHS and OHS. Additionally, 11 patients (12 hips) completed the OHS by telephone. A breakdown flowchart is presented in Figure 1. One patient with unilateral hip resurfacing was bed-bound with end-stage multiple sclerosis, but with a pain-free prosthesis in situ. The mean follow-up duration was 14.9 years (9.3 to 19.1).
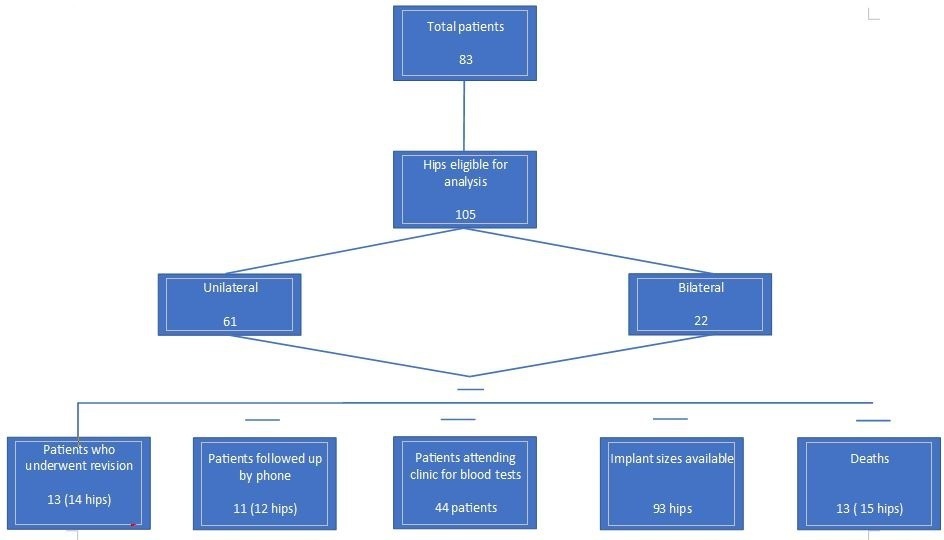
Fig. 1
Flowchart showing the breakdown of the analyzed hips.
In the whole series, the implant sizes used were available for 93 hips. The median femoral component size was 50 mm in males (IQR 48 to 52 mm) and 42 mm in females (IQR 42 to 46 mm) this was statistically significant (p < 0.001, paired t-test). The mean HHS and OHS for males were 91.6 points (SD 4.3) and 42.7 (SD 2.2), respectively. In females these mean scores were both lower at 86.9 (SD 6.4) and 39.7 (SD 3.9), respectively. However, this difference was not significant (p = 0.227 and 0.164, respectively; paired t-tests). The ICC for acetabular component inclination angle was 0.911 (95% CI 0.836 to 0.951) suggesting excellent agreement between the two scorers. The mean acetabular component inclination angle was 41.8° (SD 8.7o) and this was not significantly different in any subgroups analyzed (Table I). The mean inclination in the revision group was 43.7° (SD 8°), and in the surviving group was 41.6° (SD 9°) (p = 0.420, paired t-test).
The mean Co ion level was 26.6 nmol/l (SD 24.5) and that of Cr was 30.6 nmol/l (SD 15.3). There was no significant difference in either ion levels between males and females or unilateral and bilateral HRAs. There was a statistically significant difference in the levels of Cr ions in patients with femoral components ≤ 46 mm (mean 36.1 nmol/l, SD 17.2) and those with femoral components measuring 48 mm or larger (mean 23.5 nmol/l, SD 10.4 nmol/l) (p = 0.005, paired t-test). There was no association of mean ion levels with the mean OHS (p = 0.211, Mann-Whitney U test). No patient had a metal ion value at the MRHA published limit (7 ppb; 118 nmol/l for Co; 134 nmol/l for Cr)15 (Figure 2 and Figure 3, Table II).
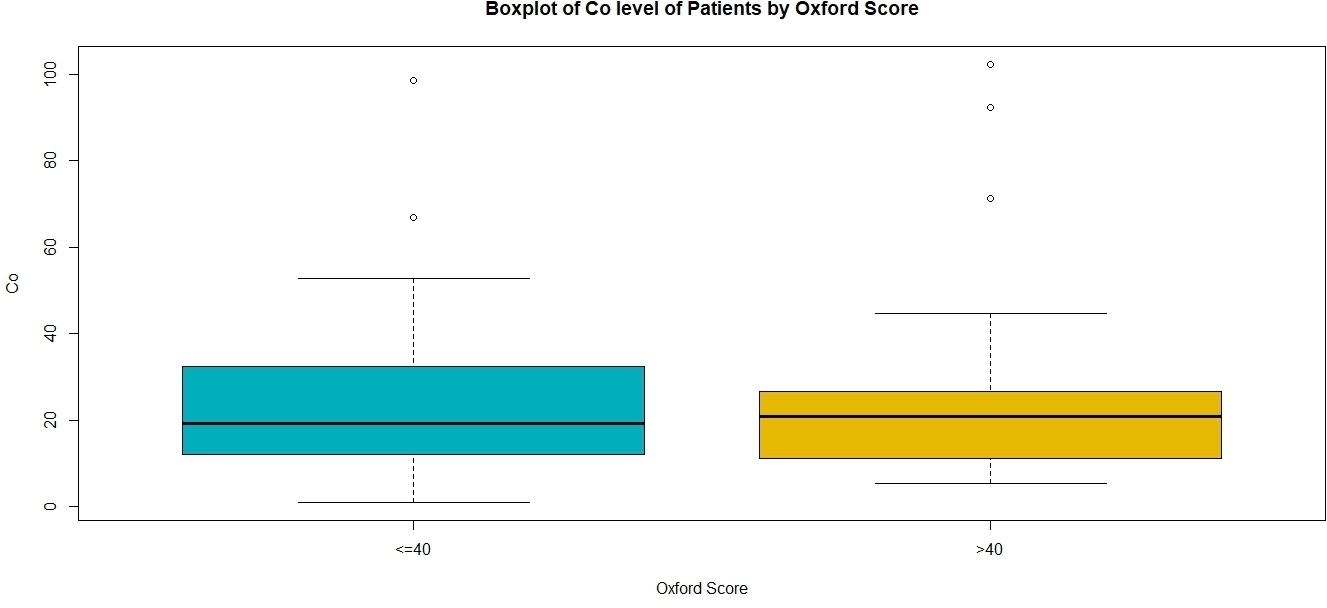
Fig. 2
Box plot showing the distribution of cobalt ion levels in patients with Oxford Hip Score ≤ 40 and > 40. The box denotes the interquartile range and the whiskers the 95% confidence interval, the thick transverse black line in the box denotes the median. "o" denotes outlier values. No patient reached the critical value of 118 nmol/l (7 ppb).

Fig. 3
Box plot showing chromium ion levels in patients with Oxford Hip Score ≤ 40 and > 40. The box denotes the interquartile range, the whiskers the 95% confidence interval, the thick black line in the box denotes the median. "o" denotes outlier values. No patient reached the critical value of 134 nmol/l (7 ppb).
Table II.
Mean (standard deviation) cobalt and chromium ion levels by subgroups; only mean chromium level differences between the smaller and larger head size components were significantly different.
| Variable | Cr (normal < 134 nmol/l) | Co (normal < 118 nmol/l) |
|---|---|---|
| Sex | ||
| Male | 27.1 (12.3) | 25.3 (24.8) |
| Female | 36.2 (18.2) | 28.7 (24.9) |
| p-value* | 0.082 | 0.659 |
| Size of femoral component, mm | ||
| ≤ 46 | 36.1 (17.2) | 28.5 (22.8) |
| ≥ 48 | 23.5 (10.4) | 23.2 (26.2) |
| p-value* | 0.014 | 0.514 |
| Unilateral or bilateral | ||
| Unilateral | 28.1 (14.8) | 26.8 (30) |
| Bilateral | 35 (15.7) | 26.3 (10) |
| p-value* | 0.162 | 0.932 |
-
*
Paired t-test.
In all, 14 hips (13 patients) of the total of 105 implanted were revised, giving an overall failure rate of 13.33%. The revision rate in females (12/45 hips; 26.6%) was significantly more than that of males (2/60; 2.3%) (p < 0.005, paired t-test). The median head size of the failed group was 42 mm (IQR 42 to 44), while that of the group with intact prosthesis was 50 mm (IQR 46 to 50); this was statistically significant (p = 0.001, paired t-test). There was no significant difference between the mean patient age at the time of HRA in the failure (50.7 yrs (SD 14.5)) and successful (49.4 yrs (SD 12.2)) groups (p = 0.703, paired t-test). The rate of revision was also significantly higher for hips with inflammatory arthritis (5/16; 31.2%) than that in other aetiologies (9/89; 10.1%) (p = 0.022, paired t-test). However, the median femoral size component in the inflammatory arthritis group (42 mm; IQR 40 to 46) was also significantly smaller than the OA group (50 mm; IQR 46 to 52) (p < 0.001, Mann-Whitney U test). Examination of the confounding between aetiology and size, revealed that the effect of femoral component size was significant (p = 0.010, two-sided chi-squared test) but not the effect of inflammatory aetiology (p = 0.241, two-sided chi-squared). Out of five hips with developmental dysplasia, one required revision (Table III).
Table III.
Patient and procedure characteristics of those hips that underwent revision.
| Patient | Sex | Age, yrs | Diagnosis | Implant used | Head size, mm | Acetabular component inclination, ° | Time to revision, yrs | Reason for revision |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 25 | AS | A | 42 | 44 | 2 | AVN |
| 2 | M | 59 | RA | A | 52 | 37 | 4.92 | Femoral neck fracture |
| 3 | F | 59 | OA | A | 42 | 40 | 1.75 | Acetabular component loosening |
| 4 | F | 47 | AS | A | 46 | 44 | 7.92 | Femoral neck fracture |
| 5 | F | 25 | RA | A | 38 | 52 | 2.58 | AVN |
| 6 | F | 55 | DDH | A | 42 | 65 | 6.25 | Femoral component loosening |
| 7 | F | 71 | OA | A | 44 | Missing | 2.08 | Femoral neck fracture |
| 8 | F | 65 | OA | A | 44 | 39 | 1.42 | Femoral neck fracture |
| 9 | F | 53 | OA | A | 42 | 41 | 3.5 | Femoral component loosening |
| 10 | F | 35 | RA | A | 42 | 37 | 4.75 | AVN |
| 11 | F | 63 | OA | B | 42 | 48 | 9 | Femoral component loosening |
| 12 | F | 62 | OA | B | 42 | 39 | 4.67 | Femoral neck fracture |
| 13 | F | 52 | OA | B | 38 | 47 | 13.83 | Femoral component loosening |
| 14 | F | 40 | OA | B | 38 | 44 | 2.33 | Femoral neck fracture |
-
A, ADEPT; AS, ankylosing spondylitis; AVN, avascular necrosis of femoral head; B, BHR; DDH, developmental dysplasia of hip; OA, osteoarthritis; RA, rheumatoid arthritis.
The mean time to revision was 4.8 years (1.4 to 14.0). Three patients (hips) underwent revision after developing avascular necrosis beneath the femoral component, six for femoral neck fracture, one for acetabular component loosening, and four for loosening of the femoral component which were revised without macroscopic evidence of avascularity of the femoral head. One of the femoral neck fractures was extracapsular sustained in a fall, when the prosthesis was well-fixed. (Figure 4 and Figure 5). The failure rate did not appear to bear a relationship with the learning experience of the operating surgeon (GS, who had been undertaking HRA since 1996), with revisions distributed proportionately to the number of HRAs performed in a given period (Figure 6).
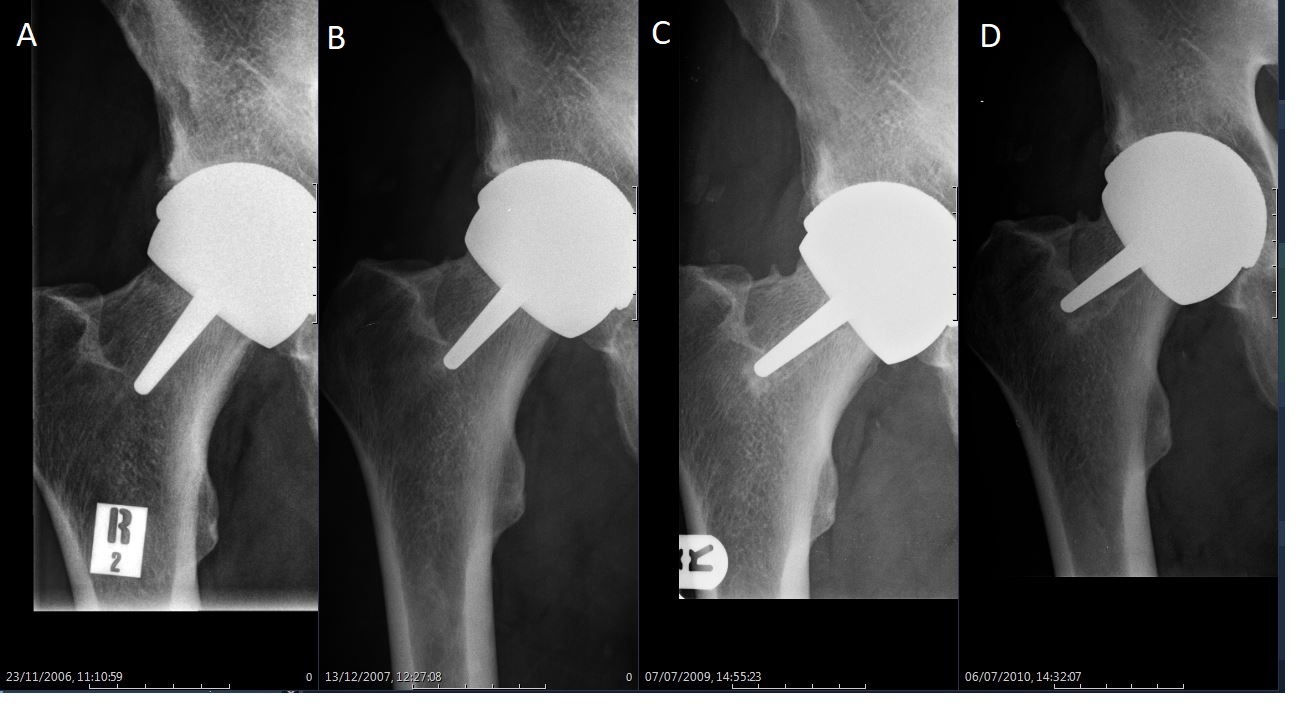
Fig. 4
Serial radiographs in a 59-year-old man who underwent hip resurfacing arthroplasty for rheumatoid arthritis. From the left to right: a) initial postoperative view; b) at one year post-surgery; c) a ‘divot sign’ is obvious on the superior aspect of the femoral neck at three years.
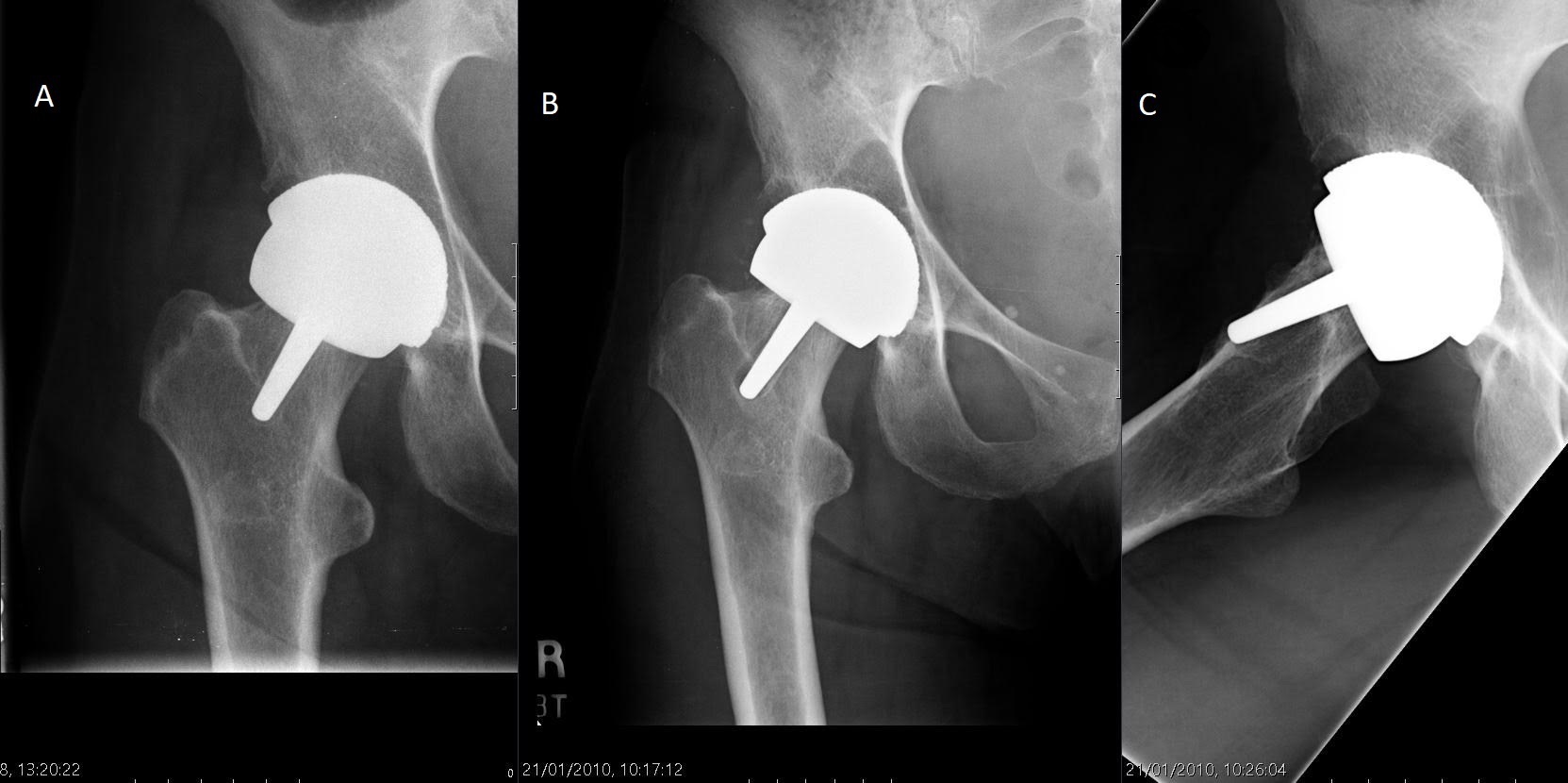
Fig. 5
Hip radiographs in a 56-year-old woman. From left to right: a) immediate postoperative view; b) two years following index surgery extensive lysis had formed behind the acetabular component and radiolucency was observed around the femoral peg; c) lateral view at two years showing resorption of bone beneath the femoral component causing loosening and migration of the femoral component. Revision was undertaken.
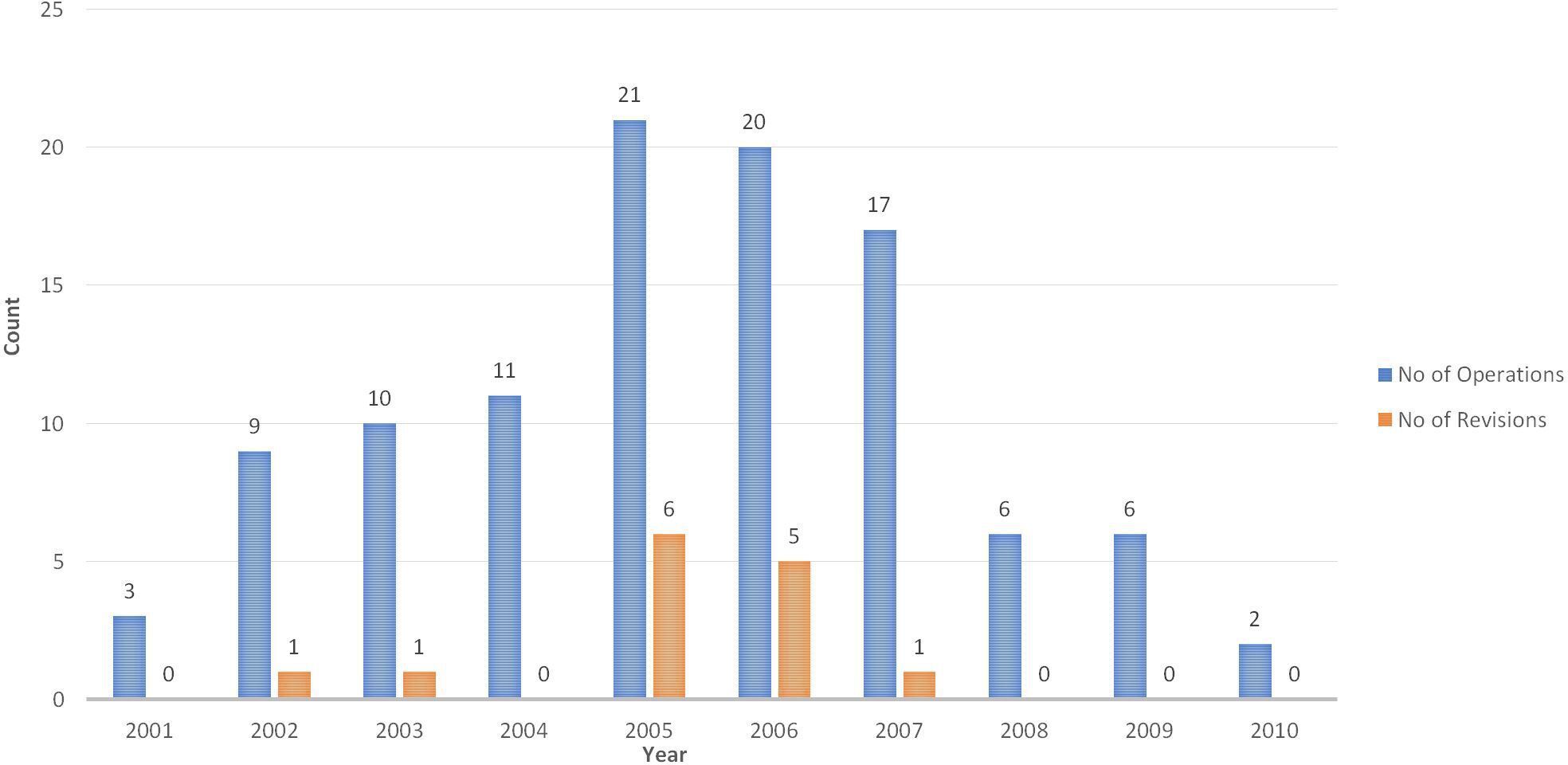
Fig. 6
Distribution of revisions relative to number of operations carried conducted from 2001 to 2010. Blue bars show the number of hip resurfacing arthroplasties performed in that year; orange bars denote the number subsequently needing revision.
In the surviving group, four patients had radiolucent lines around the stem of the femoral component, and one had lysis around the acetabular component. The latter patient had lower functional scores but no pseudotumour or joint effusion on MRI and normal blood metal ion levels; she is under close follow-up, and potentially for revision in case of continued symptoms or changes in interval scans. No patient exhibited progressive narrowing of the femoral neck. Four patients with low hip scores were investigated further with MRI scans; none showed any features of pseudotumour. Heterotopic ossification, according to the Brooker classification,19 was identified in eight hips (three grade 1, four grade 2, and one grade 3). Two patients (hips) complained of clicking arising from the HRA hip. One patient with bilateral HRAs had below-knee amputation because of Thiemann disease. One patient was bed-bound with advanced multiple sclerosis. In all, 13 patients (15 hips) died with the prosthesis in situ from causes unrelated to the HRA.
At a mean follow-up of 14.9 years (9.3 to 19.1), the cumulative survival rate was 86.7% (95% CI 84.2 to 89.1) with 30 hips at risk. The survival rate in men was 97.7% (95% CI 96.3 to 98.9) and that in women was 73.4% (95% CI 70.6 to 75.1), and this difference was statistically significant (p = 0.001; log-rank/Mantel-Cox). There was also a significant difference of survivorship in the subgroup with femoral component (head) size ≥ 48 mm (97.8% (95% CI 96.9 to 99.5)) compared with those with size ≤ 46 mm (71.1% (95% CI 69.6 to 73)) (p = 0.001, log-rank/Mantel-Cox) (Figures 7 to 9).
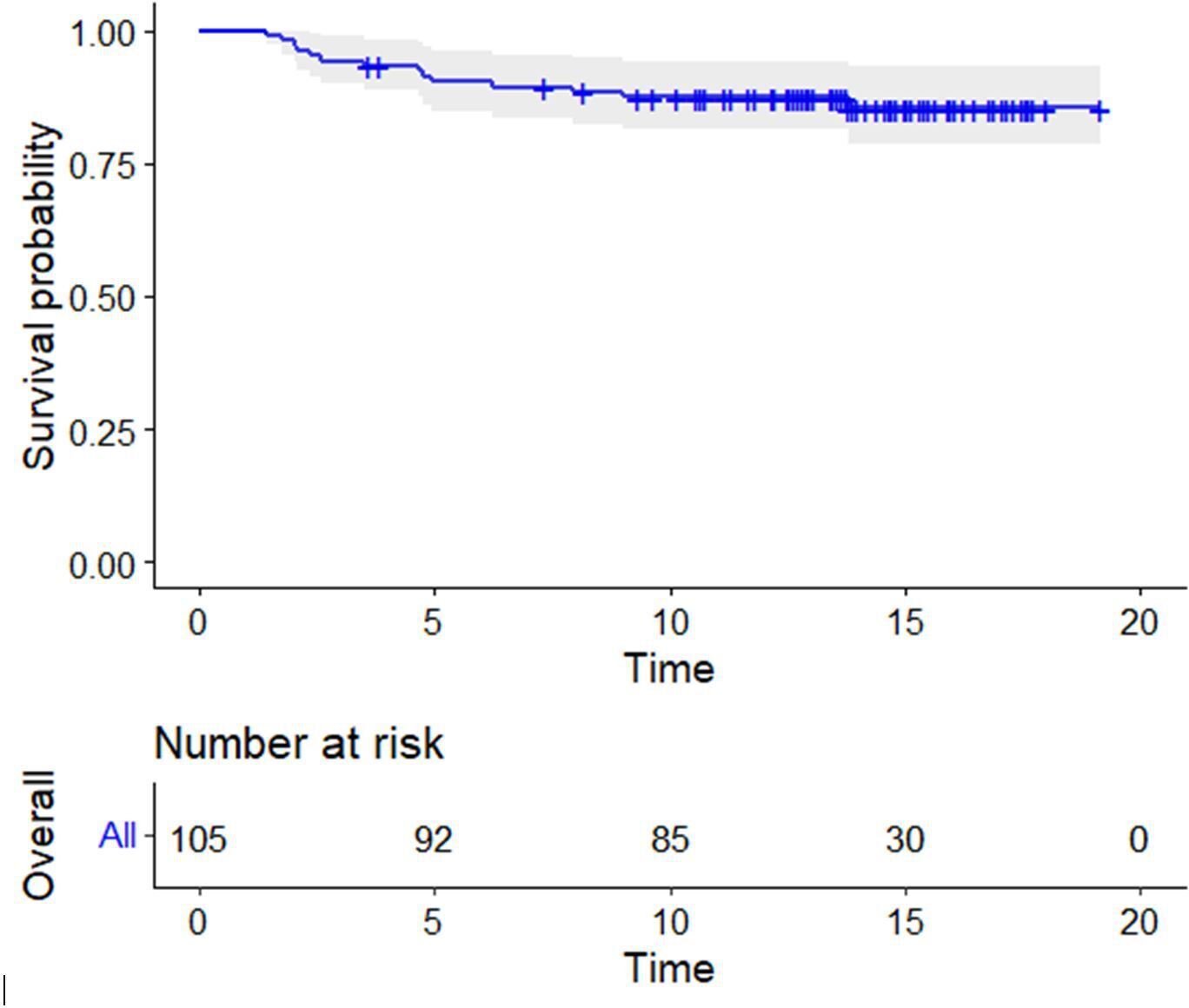
Fig. 7
Kaplan-Meier survival curve of the whole cohort, with numbers at risk below the x-axis; censored for revision for any reason. The shaded area represents 95% confidence intervals.
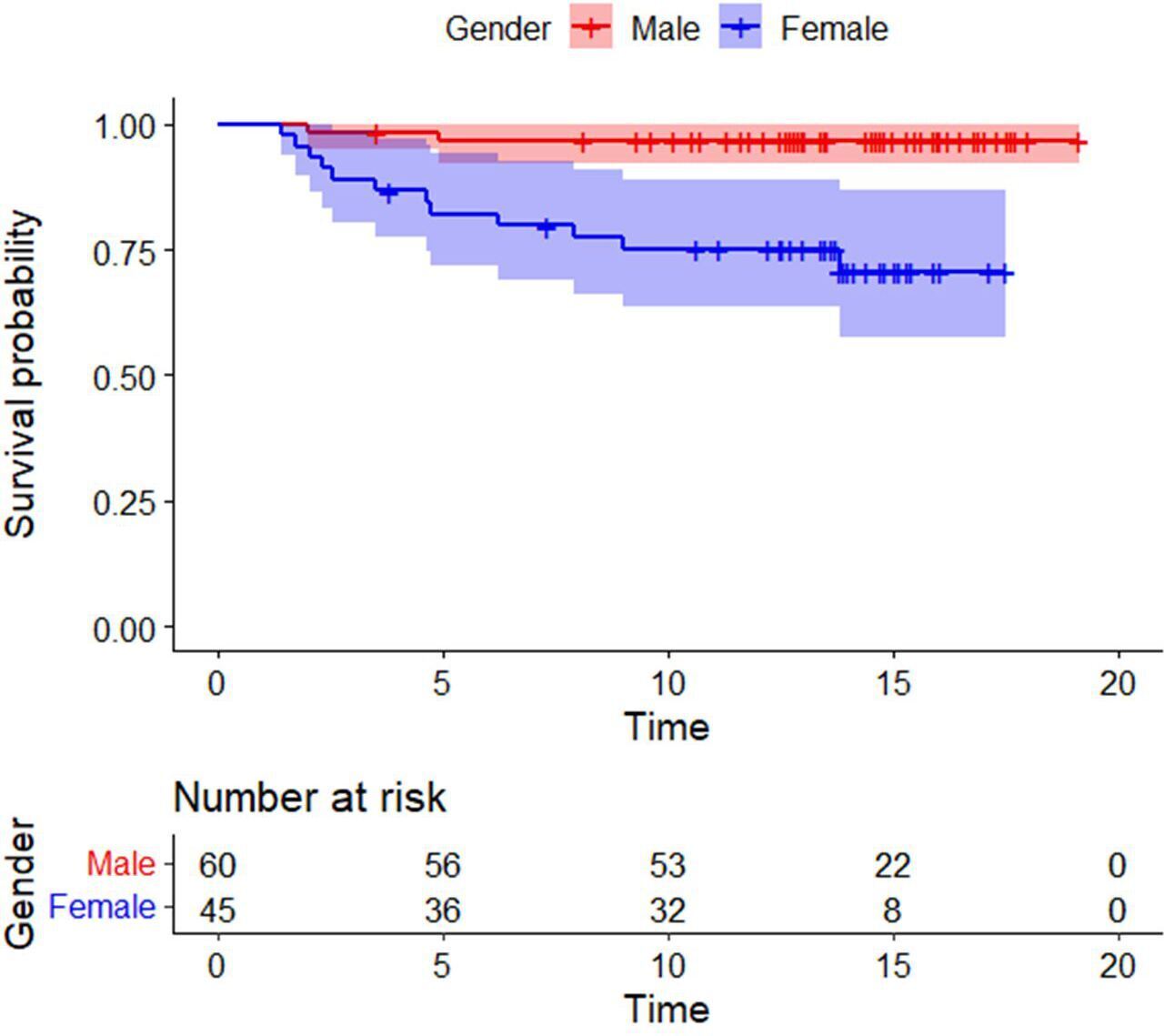
Fig. 8
Kaplan-Meier survival curve comparing males and females shows superior survivorship amongst men. The shaded areas represent 95% confidence intervals.

Fig. 9
Kaplan-Meier survival curve comparing head sizes; shows superior survivorship when the femoral component is 48 mm or larger in size. The shaded areas represent 95% confidence intervals.
Discussion
HRA remains an attractive reconstructive option as it offers the capacity to replace the arthritic hip while conserving femoral bone. MoM HRA was reintroduced in the 1990s as an improvement over previous metal-on-polyethylene designs that had met with high rates of failure.26 Over the subsequent 15 years several devices were available and results from these have contributed to the present understanding of the place of this procedure in modern hip surgery.3 The advantages of modern MoM HRA in young, active patients are established.10,27 However, after a peak in the mid 2000s, the numbers have dropped drastically following concerns regarding adverse reaction to metal debris (ARMD) and pseudotumour formation. The high failure rates associated with the ASR HRA (DePuy, UK) and its withdrawal have created uncertainty about the role of the procedure.28
Designer and non-designer surgeons have reported satisfactory outcomes of HRA in general, but for selected patient groups in particular. The NJR lists the 15-year cumulative survivorship of MoM HRA at 85.16%.13 Hunter et al29 reported ten-year cumulative survivorship of 91% (males 97%; females 80%) in 121 hips (111 patients) with BHR. Daniel et al8 showed 95.8% cumulative survival at 15 years (95% CI 95.1 to 96.5), again with males performing better with a 98% survival (95% CI 97.4 to 98.6). Our results with cumulative 15-year survivorship of 86.7% are comparable with the NJR figures published in the latest report.13 The superior male survivorship of 97.7% (95% CI 96.3 to 98.9) in our series is also in keeping with existing literature. Coulter et al10 used Poisson regression to establish that female sex and small head size were both risk factors for failure after HRA. They concluded that women were 1.4 times more likely to fail than men, and with every 1 mm increase in size of the femoral component, the risk of failure decreased by 19%. The association between head size and failure has been strongly established, with a femoral component diameter > 46 mm considered to be protective against failure.4,30 In our series, there was a significant difference in the mean femoral component size of the revision and survival groups (p = 0.001). In fact, 13 out of the 14 revised hips had a femoral component of ≤ 46 mm implanted at index surgery. This influence of head size also confounded the results in inflammatory arthritis, which had a significantly lower survivorship than the OA group. However, a two-sided chi-squared test showed that the effect was associated with the lower mean head size used in the inflammatory arthritis group. In 12 of 14 hips with inflammatory arthritis for which the implant size was known, the femoral component was ≤ 46 mm. Aulakh et al31 reported a 96.3% 11-year survival of HRA done in 47 patients (54 hips) for rheumatoid arthritis, which was not significantly different from the survival in the OA group. None of the patients in our series showed metal ion levels above the accepted safe limits. Cr ion levels were significantly higher in the subgroup with femoral component size ≤ 46 mm than in the subgroup with larger femoral components. There was no significant difference in the metal ion levels between males and females, unilateral and bilateral HRAs, or those with an OHS less than or more than 40. Van der Straeten et al32 reported that Co and Cr ion levels decline significantly in well-functioning MoM HRAs at ten years, and do not have any significant correlation with sex, laterality of the operation, or size of the components used. They did however find that patients with relatively higher ion levels had lower functional scores, which has not been our experience.
We acknowledge the limitations of our study, in particular the retrospective design, small sample size, and absence of any comparator group. The qualitative analysis of radiographs was done by a single observer who was not blinded to the results, which may be a source of bias. Also, with some patients refusing to follow-up in person because they felt completely well, there exists the theoretical possibility of under-diagnosing ARMD. However, our results demonstrate that MoM HRA can offer good long-term survival for male patients with end-stage arthritis, as long as the femoral component is above the threshold size of 46 mm.
Our results are in keeping with existing understanding of the long-term outcomes of MoM HRA. The procedure appears to work best in male patients as evidenced by our > 97% 15-year survival in men. Female patients are less well served by HRA, although it is argued that the underlying problem has to do more with size of the femur than sex.25,27,33 ARMD or pseudotumour formation have not been a problem in our series, but with a reported incidence under 1% in males, our series might have been underpowered to detect this complication. However, we are continuing to monitor patients with low functional scores, despite their MRI scans and metal ion levels being within normal range.
References
1. Cotella L , Railton GT , Nunn D , Freeman MAR , Revell PA . ICLH double-cup arthroplasty, 1980-1987 . J Arthroplasty . 1990 ; 5 ( 4 ): 349 – 357 . Crossref PubMed Google Scholar
2. Amstutz HC , Clarke IC , Christie J , Graff-Radford A . Total hip articular replacement by internal eccentric shells: The “tharies” Approach to total surface replacement arthroplasty . Clin Orthop Relat Res . 1977 ; 128 : 261 . Google Scholar
3. Amstutz HC , Le Duff MJ . Hip resurfacing: A 40-year perspective . HSS J . 2012 ; 8 ( 3 ): 275 – 282 . Crossref PubMed Google Scholar
4. Grigoris P , Roberts P , Panousis K , Jin Z . Hip resurfacing arthroplasty: the evolution of contemporary designs . Proc Inst Mech Eng H . 2006 ; 220 ( 2 ): 95 – 105 . Crossref PubMed Google Scholar
5. McMinn D , Daniel J . History and modern concepts in surface replacement . Proc Inst Mech Eng H . 2006 ; 220 ( 2 ): 239 – 251 . Crossref PubMed Google Scholar
6. Girard J . Hip Resurfacing: International Perspectives: Review Article . HSS J . 2017 ; 13 ( 1 ): 7 – 11 . Crossref PubMed Google Scholar
7. McMinn D , Treacy R , Lin K , Pynsent P . Metal on metal surface replacement of the hip. Experience of the Mcminn prothesis . Clin Orthop Relat Res . 1996 ; 329 ( Suppl ): S98S89 . Crossref PubMed Google Scholar
8. Daniel J , Pradhan C , Ziaee H , Pynsent PB , McMinn DJW . Results of Birmingham hip resurfacing at 12 to 15 years: A single-surgeon series . Bone Joint J . 2014 ; 96-B ( 10 ): 1298 – 1306 . Crossref PubMed Google Scholar
9. Schuh R , Neumann D , Rauf R , Hofstaetter J , Boehler N , Labek G . Revision rate of Birmingham Hip Resurfacing arthroplasty: comparison of published literature and arthroplasty register data . Int Orthop . 2012 ; 36 ( 7 ): 1349 – 1354 . Crossref PubMed Google Scholar
10. Coulter G , Young DA , Dalziel RE , Shimmin AJ . Birmingham hip resurfacing at a mean of ten years: results from an independent centre . J Bone Joint Surg Br . 2012 ; 94-B ( 3 ): 315 – 321 . Crossref PubMed Google Scholar
11. Borgwardt A , Borgwardt L , Borgwardt L , Zerahn B , Fabricius SD , Ribel-Madsen S . Clinical Performance of the ASR and Re-Cap Resurfacing Implants—7 Years Follow-Up . J Arthroplasty . 2015 ; 30 ( 6 ): 993 – 997 . Google Scholar
12. Kohan L , Field CJ , Kerr DR . Early complications of hip resurfacing . J Arthroplasty . 2012 ; 27 ( 6 ): 997 – 1002 . Crossref PubMed Google Scholar
13. No authors listed . National Joint Registry 17th Annual Report 2020 . National Joint Registry . https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2017th%20Annual%20Report%202020.pdf ( date last accessed 23 December 2021 ). Google Scholar
14. Matharu GS , McBryde CW , Pynsent WB , Pynsent PB , Treacy RB . The outcome of the birmingham hip resurfacing in patients aged < 50 years up to 14 years post-operatively . Bone Joint J . 2013 ; 95-B ( 9 ): 1172 – 1177 . Crossref PubMed Google Scholar
15. No authors listed . All metal-on-metal (MoM) hip replacements: updated advice for follow-up of patients . https://www.gov.uk/drug-device-alerts/all-metal-on-metal-mom-hip-replacements-updated-advice-for-follow-up-of-patients ( date last accessed 5 January 2022 ). Google Scholar
16. Stephenson PK , Freeman MA . Exposure of the hip using a modified anterolateral approach . J Arthroplasty . 1991 ; 6 ( 2 ): 137 – 145 . Crossref PubMed Google Scholar
17. Amstutz HC , Beaulé PE , Dorey FJ , Le Duff MJ , Campbell PA , Gruen TA . Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study . J Bone Joint Surg Am . 2004 ; 86-A ( 1 ): 28 – 39 . PubMed Google Scholar
18. DeLee JG , Charnley J . Radiological demarcation of cemented sockets in total hip replacement . Clin Orthop Relat Res . 1976 ( 121 ): 20 – 32 . PubMed Google Scholar
19. Brooker AF , Bowerman JW , Robinson RA , Riley LH Jr . Ectopic ossification following total hip replacement . J Bone Joint Surg Am . 1973 ; 55-A ( 8 ): 1629 – 1632 . PubMed Google Scholar
20. Dawson J , Fitzpatrick R , Carr A , Murray D . Questionnaire on the perceptions of patients about total hip replacement surgery . J Bone Joint Surg Br . 1996 ; 78-B : 185 – 190 . Google Scholar
21. Murray DW , Fitzpatrick R , Rogers K , et al. The use of the Oxford hip and knee scores . J Bone Joint Surg Br . 2007 ; 89-B ( 8 ): 1010 – 1014 . Google Scholar
22. Mahomed NN , Arndt DC , McGrory BJ , Harris WH . The Harris hip score: comparison of patient self-report with surgeon assessment . J Arthroplasty . 2001 ; 16 ( 5 ): 575 – 580 . Crossref PubMed Google Scholar
23. Harris WH , Schiller AL , Scholler JM , Freiberg RA , Scott R . Extensive localized bone resorption in the femur following total hip replacement . J Bone Joint Surg Am . 1976 ; 58-A ( 5 ): 612 – 618 . PubMed Google Scholar
24. Pandit H , Glyn-Jones S , McLardy-Smith P , et al. Pseudotumours associated with metal-on-metal hip resurfacings . J Bone Joint Surg Br . 2008 ; 90-B ( 7 ): 847 – 851 . Crossref PubMed Google Scholar
25. Langton DJ , Jameson SS , Joyce TJ , Hallab NJ , Natu S , Nargol AVF . Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement . J Bone Joint Surg Br . 2010 ; 92-B ( 1 ): 46 : 38 . Crossref PubMed Google Scholar
26. Freeman MA , Bradley GW . ICLH surface replacement of the hip. An analysis of the first 10 years . J Bone Joint Surg Br . 1983 ; 65-B ( 4 ): 405 – 411 . Crossref PubMed Google Scholar
27. Amstutz HC , Le Duff MJ , Campbell PA , Gruen TA , Wisk LE . Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up . J Bone Joint Surg Am . 2010 ; 92-A : 2663 . Crossref PubMed Google Scholar
28. Maurer-Ertl W , Friesenbichler J , Holzer LA , et al. Recall of the ASR XL head and Hip resurfacing systems . Orthopedics . 2017 ; 40 ( 2 ): e340 – e347 . Crossref PubMed Google Scholar
29. Hunter TJA , Moores TS , Morley D , Manoharan G , Collier SG , Shaylor PJ . 10-year results of the Birmingham Hip Resurfacing: a non-designer case series . Hip Int . 2018 ; 28 ( 1 ): 50 – 52 . Crossref PubMed Google Scholar
30. McBryde CW , Theivendran K , Thomas AMC , Treacy RBC , Pynsent PB . The influence of head size and sex on the outcome of Birmingham hip resurfacing . J Bone Joint Surg Am . 2010 ; 92-A ( 1 ): 112 – 105 . Crossref PubMed Google Scholar
31. Aulakh TS , Kuiper JH , Dixey J , Richardson JB . Hip resurfacing for rheumatoid arthritis: Independent assessment of 11-year results from an international register . Int Orthop . 2011 ; 35 ( 6 ): 803 – 808 . Crossref PubMed Google Scholar
32. Van Der Straeten C , Van Quickenborne D , De Roest B , Calistri A , Victor J , De Smet K . Metal ion levels from well-functioning Birmingham hip resurfacings decline significantly at ten years . Bone Joint J . 2013 ; 95-B ( 10 ): 1332 – 1338 . Crossref PubMed Google Scholar
33. Langton DJ , Joyce TJ , Jameson SS , et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear . J Bone Joint Surg Br . 2011 ; 93-B : 164 . Crossref PubMed Google Scholar
Author contributions
M. Haseeb Gani: Formal analysis, Investigation, Writing – original draft.
U. Zahoor: Investigation, Software.
S. A. Hanna: Methodology, Writing – review & editing.
G. Scott: Conceptualization, Methodology, Writing – review & editing.
Funding statement
The author(s) received no financial or material support for the research, authorship, and/or publication of this article.
Acknowledgements
Dr. Bilal A Para, Assistant Professor of Statistics, Islamic University of Science and Technology, Awantipora, Kashmir, for his help with statistical analysis. Mr. Prabu Balasubramanian, Orthopaedic Registrar at the Royal London Hospital, for his help with data collection.
Open access funding
The authors confirm that the open access fee for this study was self-funded.
Follow M. Haseeb Gani @bonesmith_
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









