Abstract
Aims
The aticularis genu (AG) is the least substantial and deepest muscle of the anterior compartment of the thigh and of uncertain significance. The aim of the study was to describe the anatomy of AG in cadaveric specimens, to characterize the relevance of AG in pathological distal femur specimens, and to correlate the anatomy and pathology with preoperative magnetic resonance imaging (MRI) of AG.
Methods
In 24 cadaveric specimens, AG was identified, photographed, measured, and dissected including neurovascular supply. In all, 35 resected distal femur specimens were examined. AG was photographed and measured and its utility as a surgical margin examined. Preoperative MRIs of these cases were retrospectively analyzed and assessed and its utility assessed as an anterior soft tissue margin in surgery. In all cadaveric specimens, AG was identified as a substantial structure, deep and separate to vastus itermedius (VI) and separated by a clear fascial plane with a discrete neurovascular supply. Mean length of AG was 16.1 cm ( ± 1.6 cm) origin anterior aspect distal third femur and insertion into suprapatellar bursa. In 32 of 35 pathological specimens, AG was identified (mean length 12.8 cm ( ± 0.6 cm)). Where AG was used as anterior cover in pathological specimens all surgical margins were clear of disease. Of these cases, preoperative MRI identified AG in 34 of 35 cases (mean length 8.8 cm ( ± 0.4 cm)).
Results
AG was best visualized with T1-weighted axial images providing sufficient cover in 25 cases confirmed by pathological findings.These results demonstrate AG as a discrete and substantial muscle of the anterior compartment of the thigh, deep to VI and useful in providing anterior soft tissue margin in distal femoral resection in bone tumours.
Conclusion
Preoperative assessment of cover by AG may be useful in predicting cases where AG can be dissected, sparing the remaining quadriceps muscle, and therefore function.
Cite this article: Bone Joint Open 2020;1-9:585–593.
Take home message
Articularis genu is a substantial and discrete muscle of the anterior compartment of the thigh which should be regarded as the “fifth quadriceps muscle”.
Articularis genu provides a safe surgical margin, which may be pre-determined by MRI.
With a clear anatomical dissection it can provide a safe anterior soft tissue margin in the resection of bone tumours in the distal one-half of the femur.
Introduction
Limb salvage with resection of the distal femur for primary and metastatic bony tumours is a standard procedure in orthopaedic oncology. It is mandatory that a complete resection with a clear margin is obtained so as to prevent recurrence of disease while allowing limb and extensor mechanism preservation. Stevens et al1 reviewed the surgical approach to distal femur resection, and noted that, depending on tumour characteristics and biopsy site the choice of midline, medial, or lateral extensile approach is used in exposure of the extensor compartment and tumour mass.
The standard medial approach for distal femoral resection of a bone tumour requires a medial incision through skin and subcutaneous fat (including the biopsy tract); this is followed by incision and reflection of the deep fascia to expose the quadriceps muscle. Rectus femoris (RF) is divided longitudinally in the midline through a relatively avascular tendinous plane to expose the deeper quadriceps muscles below. The vastus medialis (VM) and vastus lateralis (VL) are retracted medially and laterally respectively with the split RF. This exposes the fourth quadriceps muscle, vastus intermedius (VI), which may be divided midline to expose the bone below. Depending on the tumour site, size, and soft tissue extension, VI may also be resected with the underlying distal femur as part of the anterior soft tissue margin. If the tumour is contained within bone, the overlying muscle may be spared, but if there is soft tissue extension of the tumour then adequate soft tissue cover, including VI, VM, and VL, is required to obtain a clear surgical margin.
With careful dissection, a fifth muscle of the quadriceps compartment, the articularis genu (AG) can be identified deep to VI.2-5 The classical anatomical description of the AG in Gray’s Anatomy6 indicates that the AG is a small vestigial structure of uncertain purpose. In a cadaveric anatomical study in 1981 of 48 knees, the morphology of AG was described as “highly variable” and “flat and wispy”.3 When present, the AG had a width ranging from 1.5 cm to 3 cm; the muscles were found to arise from the anterior aspect of the supracondylar portion of the femur and to insert into the joint capsule at the level of the suprapatellar pouch.3 Kimura and Takahashi in 1987 reported similar findings but also noted the presence of discrete superficial and deep bundles of AG.2 This study commented on the difficulty in distinguishing AG from overlying VI solely on the basis of its origin and surrounding fatty tissue, and noted that AG and VI innervation shared a common origin.2 Woodley et al5 used magnetic resonance imaging (MRI) and dissection to analyze 22 cadaveric lower limbs from 11 donors. They noted that there were superficial and deep bundles of AG, reporting an average of seven bundles (4 to 10) on dissection, and four bundles on MRI.5 This study again recognized that distinguishing AG from VI with MRI was difficult.5 Mean cross-section area of AG was 1.5 cm2 ± 0.7 cm2, and fascicular length 5.9 cm ± 1.0 cm. Schuma et al also analyzed 40 joints from 22 cadaveric donors, reporting fewer bundles (mean 2.7 ± 0.5), though noting that their mean length was similar (5.4 cm ± 1.3 cm); the mean cross-sectional area of bundles was 5.5 cm ± 2.6 cm.2,4
The status and nature of the AG muscle is uncertain. Our aim in this study was to determine whether the AG could provide suitable anterior soft tissue cover of expansile bony tumours of the distal femur. We have done this by characterizing the anatomy of AG by dissection in cadaveric specimens, conducting gross examination and histopathological analysis of distal femoral resection specimens for primary and metastatic bone tumours, and evaluating preoperative MRI of AG in patients who had undergone distal femoral resections for these tumours.
Methods
Cadaveric study
Overall, 24 thighs from 12 whole, embalmed human cadavers (six females and 18 males) were obtained from the mortuary of the Medical Sciences Teaching Centre, Department of Physiology, Anatomy, and Genetics, University of Oxford. As per HTA guidelines, full consent was obtained from all cadaveric donors. The thigh of each cadaver was inspected before dissection, to ensure there were no scars or other evidence indicating previous surgery of the anterior thigh compartment.
An adapted extensile medial parapatellar approach was used to dissect the cadaveric thighs. This approach has been described for use in sarcoma surgery for resection of distal femoral osteosarcoma (Stevens et al1). After incision through the skin, subcutaneous fat, superficial, and deep fascia, the quadriceps were visualized. At this stage, an extended medial flap was dissected to expose fully the quadriceps mechanism, patella, and patella tendon (Figure 1).
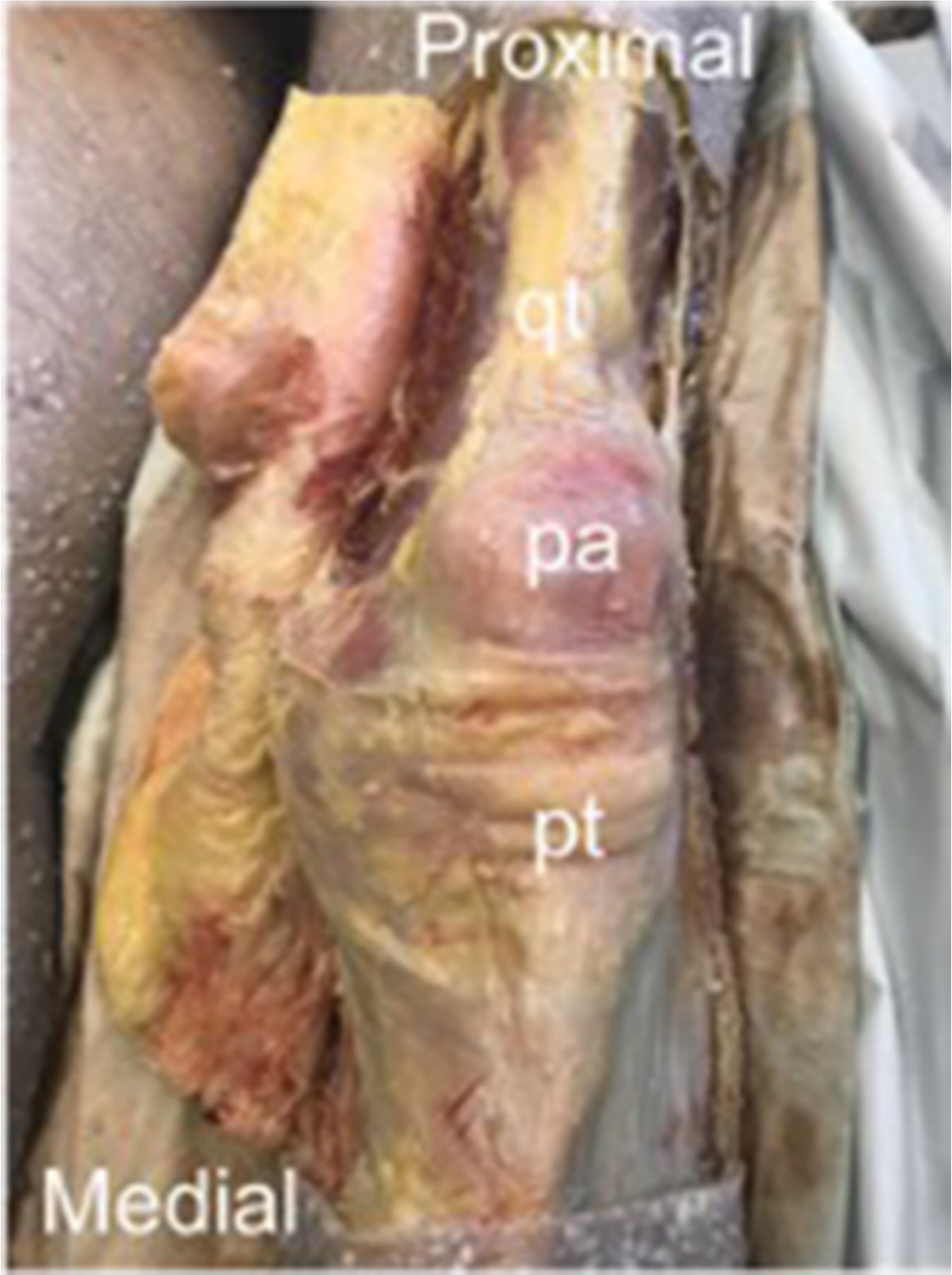
Fig. 1
Extended medial flap exposing quadriceps tendon (qt), patella (pa), and patella tendon (pt).
RF was identified distally from the quadriceps tendon, and isolated distal to proximal, lateral to medial, and cut at the distal tendon-muscle border, and the tendon reflected laterally; likewise, VM was identified and dissected distal to proximal, medial to lateral, and reflected medially. This created a large window to identify and dissect out the VI starting at the quadriceps tendon, the tendon of VI was dissected distal to proximal. As the dissection was carefully continued proximally, a clear tissue plane deep to VI was identified in which there is a distinct layer of fascia covering the deep VI muscle. Deep to this discrete fascial layer, a composite muscle, the AG was identified (Figure 2). The dissection of VI was continued so that the entirety of the deeper muscle could be visualized from insertion to its uppermost origin proximally. The patella and patella tendon were dissected and reflected to visualize the medial to lateral suprapatellar bursal insertion (Figure 2). Photographs were taken of the isolated AG muscle. The length of the muscle from the superior pole of the patella to its most proximal femoral origin was measured. Blood vessels and nerves entering the muscle bulk were dissected and documented and their course followed proximally to identify their source.
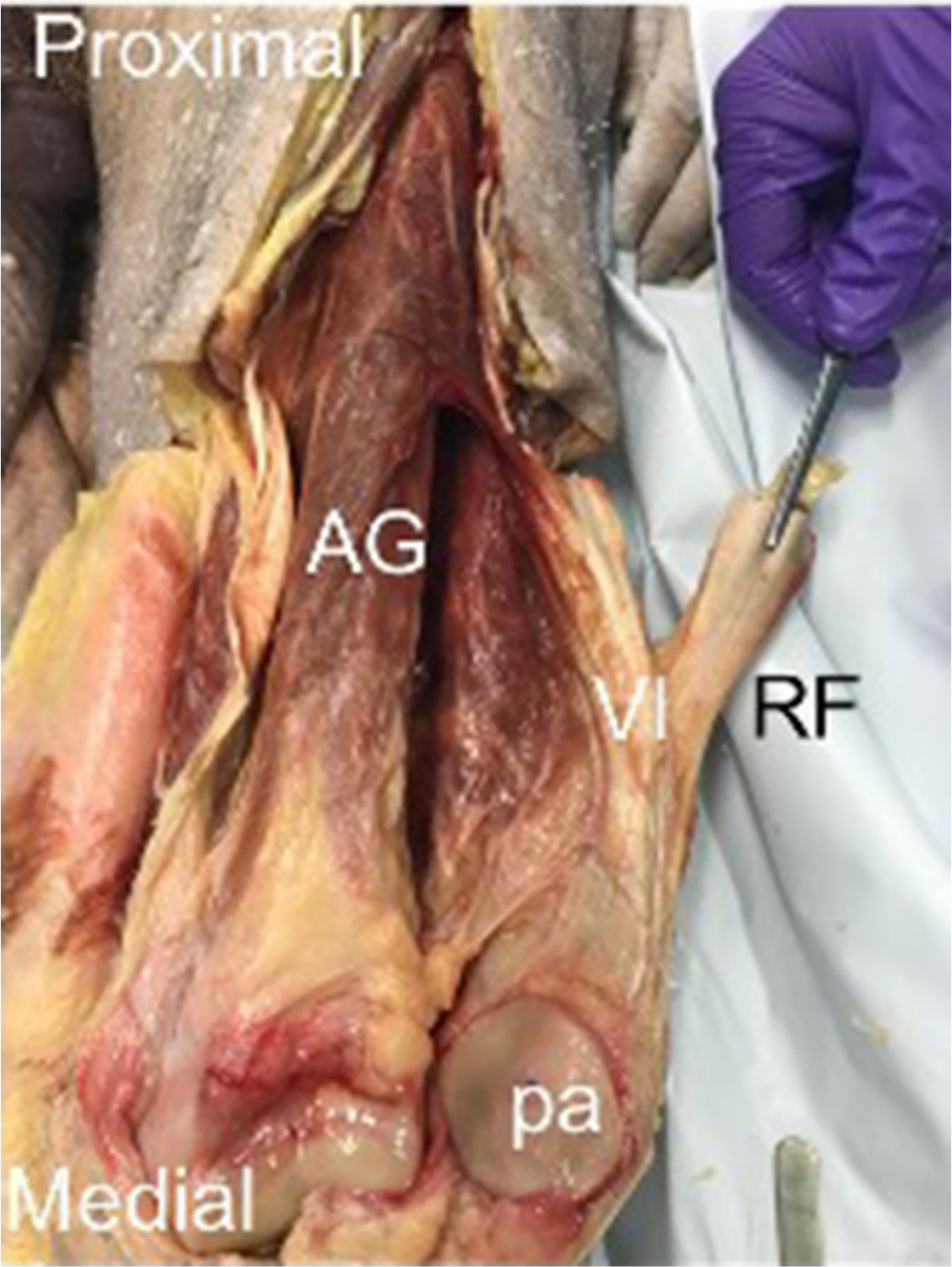
Fig. 2
Exposed articularis genu – reflection of rectus femoris (RF) and vastus intermediua (VI) reveals an additional muscle bulk deep to VI, articularis genu (AG). Reflection of the patella (pa) shows insertion of AG into the suprapatellar bursa (sb).
Macroscopic and histopathological study of distal femoral resection
In all, 35 distal femoral resection specimens from operative patients (48.5% male, 51.5% female, mean age 38.7 years (8 to 85) were identified between the dates 1 June 2009 and 30 June 2117 and studied. All cases had a biopsy proven primary malignant bone tumour or solitary bone metastasis in the distal femur (Table I).
Table I.
List of tumour types identified in study.
| Tumour type | n (%) |
|---|---|
| Primary bone tumour | 29 (82.9) |
| Osteosarcoma | 19 (54.3) |
| Chondrosarcoma | 5 (14.3) |
| Ewing’s sarcoma | 2 (5.7) |
| GCT | 1 (2.9) |
| Leiomyosarcoma | 1 (2.9) |
| Haemangioendothelioma | 1 (2.9) |
| Solitary metastatic bone disease | 6 (17.1) |
| Total | 52 (100) |
-
GCT, giant cell tumour.
In distal femur resection surgery, an extensile/medial/midline/lateral approach incorporating biopsy tract was used in all cases. Following an incision through the skin and sub-cutaneous fat the deep fascia was incised in line to expose the quadriceps. The quadriceps were then mobilized either midline or subvastus medially/laterally. With careful dissection at this stage, the AG could be identified overlying the distal femur in most cases. The distal femur was then resected proximally and distally with macroscopic soft tissue cover (AG). If preoperative imaging indicated extensive soft tissue involvement by the tumour then a resection of VI and associated quadriceps was carried out to ensure tumour-free macroscopic margin. Reconstruction with an endoprosthesis and standard closure of the soft tissues was undertaken.
Following distal femoral resection, the AG was identified by gross examination and photographed in each specimen. The AG was measured distally to proximally, i.e. the length of AG from the superior pole of the suprapatellar bursa to the uppermost origin on the femur. Further specimen dissection and histopathological analysis was carried out to characterize the nature of the tumour and to determine whether AG provided sufficient cover of tumour with clear surgical margins.
MRI evaluation
The preoperative MRI of all patients in the study was retrospectively evaluated by MSK radiologist blinded to the femoral resection histopathological findings. All MRI studies were performed on a 1.5 T magnet; however, MRI sequences were variable given the retrospective nature of the study and that imaging had been performed both locally and at external referral centres.
MRI analysis included whether the AG could be identified on MRI and which sequences most optimally depicted the muscle. The maximum anteroposterior dimension of the anterior tumour soft tissue mass breaching the anterior femoral cortex was documented. MRI assessment of whether the AG adequately covered and contained the distal femoral tumour was made. Where the tumour had not significantly distorted or breached the AG, the length of the AG on sagittal images was measured from femoral origin to the superior patellar pole. Even though it is recognized that this patient group with an anterior femoral tumour mass is not ideal to assess detailed AG anatomy, the number of layers and bundles making up the AG on axial images was also documented.
Statistics
All statistical analysis was conducted in GraphPad Prism8 (GraphPad, San Diego, California, USA). Data for length and width of muscle bulk, and follow-up, were assessed for normality using the Shapiro-Wilk test. In the presence of non-normal distribution, we elected to use non-parametric methods. No data were transformed. For survival analysis, the Kaplan-Meier method was utilized. Subjects alive at the end of the study period or lost to follow-up were censored. Statistical significance was defined as p < 0.05.
Results
Cadaveric study
In all 24 thighs, a discrete and substantial muscle, the AG, was identified deep to the fascia of vastus intermedius (Figure 3). The discrete muscle was separated by a clear fascial plane from the overlying VI (Figure 3). The fibres of AG ran exactly parallel to the long axis of the femur, in contrast to those of VI, which ran more obliquely at 12° medial to lateral in keeping with AG representing discrete muscle (Figure 3). The origin and insertion of AG was clearly identified in all specimens. The origin reliably extended from and involved the distal anterior one-half to one-third of the femur with insertion into the suprapatellar bursa. The mean length of AG was 16.1 cm ( ± 1.6 cm). The maximum length measured from the superior patellar pole was 20.5 cm. No specimen length was less than 12.0 cm (Figure 4).
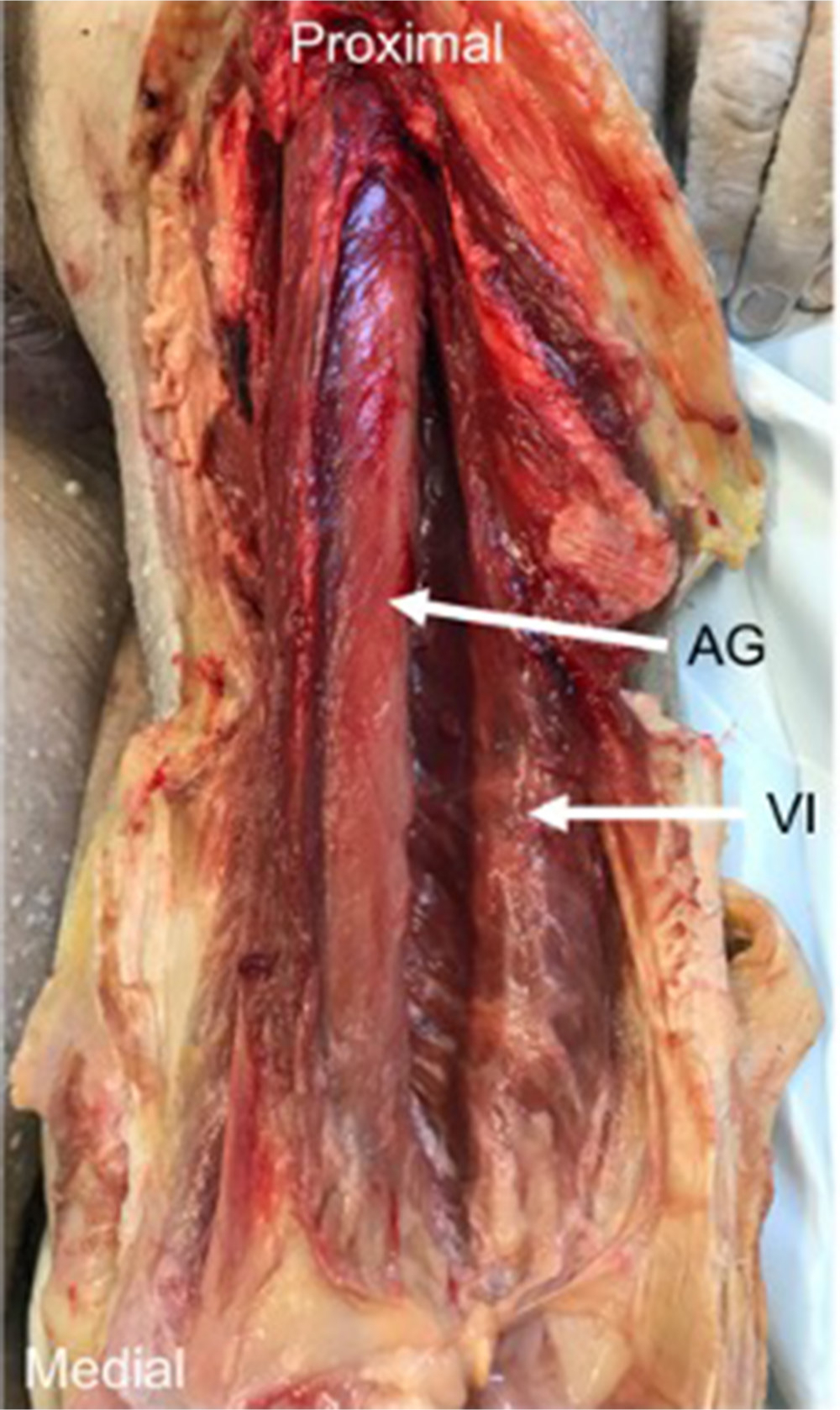
Fig. 3
The articularis genu (AG) muscle – dissection of cadavers demonstrated that deep to vastus intermedius (VI), a deeper muscle, and AG could be identified. The two muscles were separated by a fascial plane, and their fibres ran in different directions.
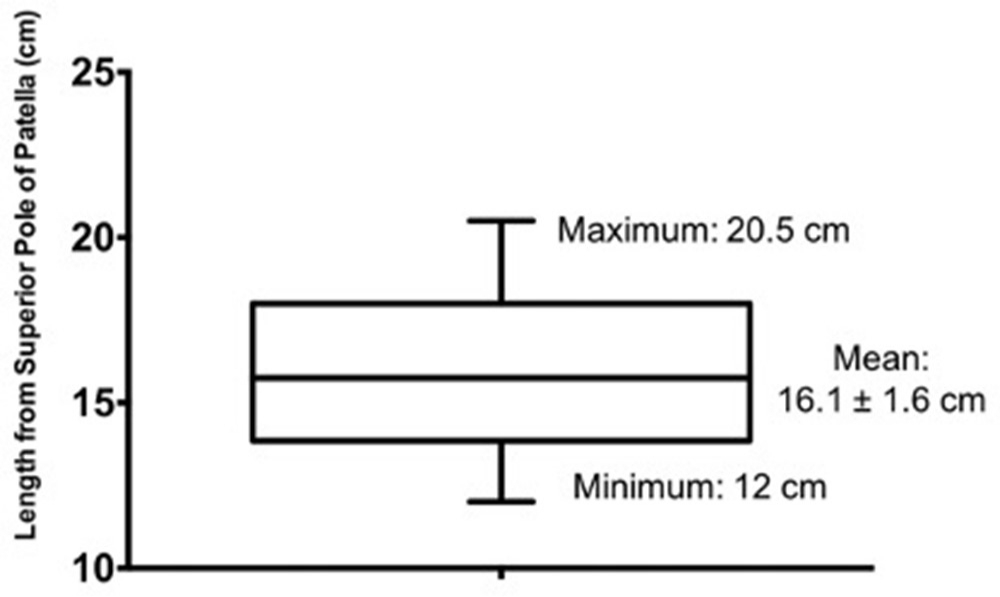
Fig. 4
Length of articularis genu in cadaveric specimens.
Neurovascular supply of the AG was identified, isolated, and dissected in all of the specimens; following the neurovascular structures proximally, it was noted that the origin of discrete blood supply was from the deep circumflex branch of the femoral artery and innervation from deep intermuscular branches of the femoral nerve (Figure 5).
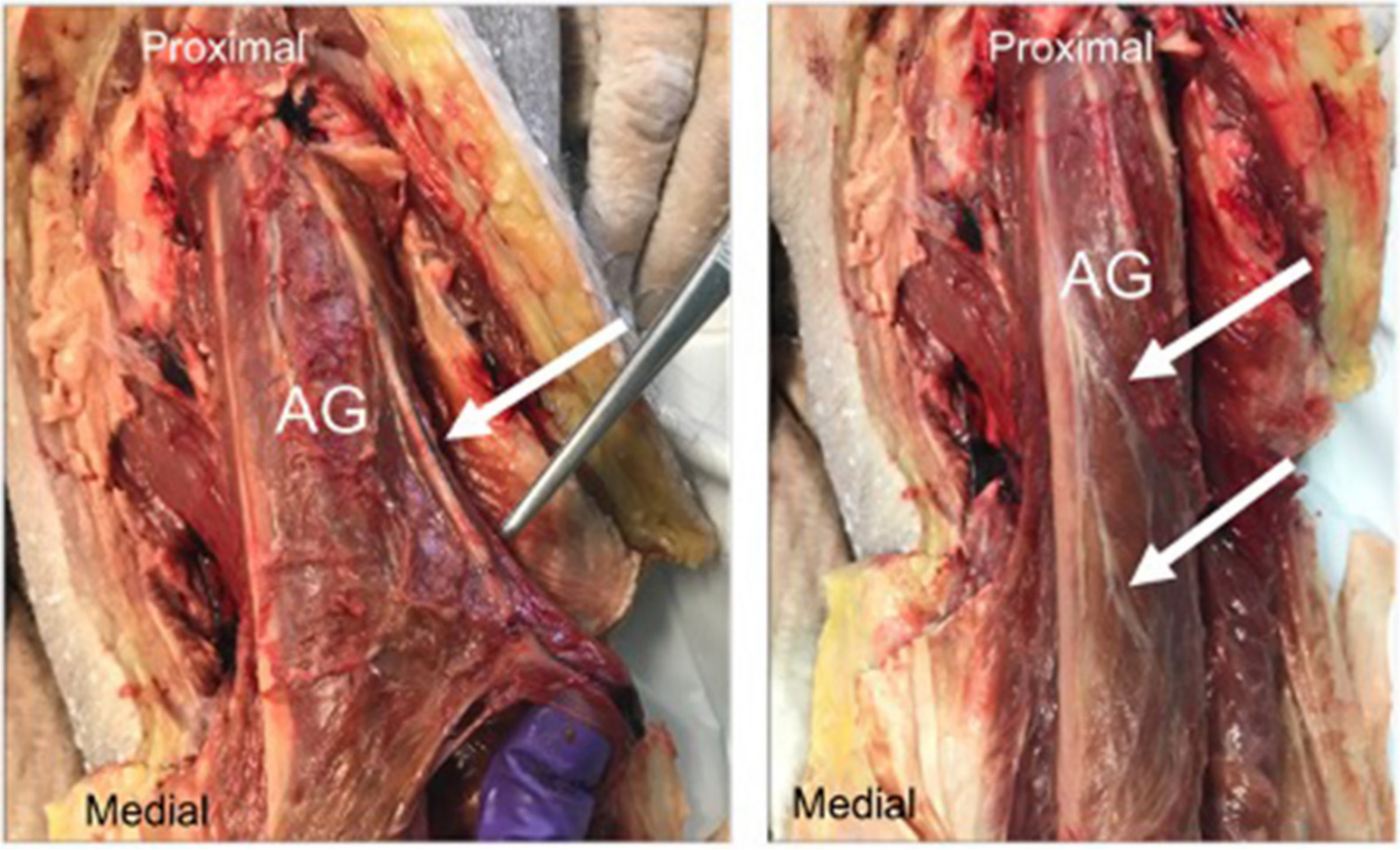
Fig. 5
(a) Blood supply to articularis genu (AG) (white arrow); (b) Nerve supply to the AG (white arrows).
Macroscopic and histopathological study
Of the 35 cases, 60% of distal femoral resections were of the right distal femur. Osteosarcoma was the most common resection (54.3%), followed by metastatic disease (17.1%). The remainder were chondrosarcoma (14.3%), giant cell tumour (5.7%), Ewing’s sarcoma (2.9%), leiomyosarcoma (2.9%), and haemangioendothelioma (2.9%) (Table I). 25% of cases presented as pathological fractures.
In 32 of the 35 cases, the AG was clearly identified in the distal femoral resection specimens (Figure 6). The mean length of AG was 12.8 cm ± 0.6 cm in intact specimens of muscle; some specimens were cut and thus the full length of the AG could not be determined. The AG was not identified in two of the three cases as there was solely interosseous disease, necessitating only extra-periosteal dissection and resection, thus preserving AG. In the remaining case, tumour extended wider into soft tissues, necessitating a wider resection margin involving AG, VI, and VM.
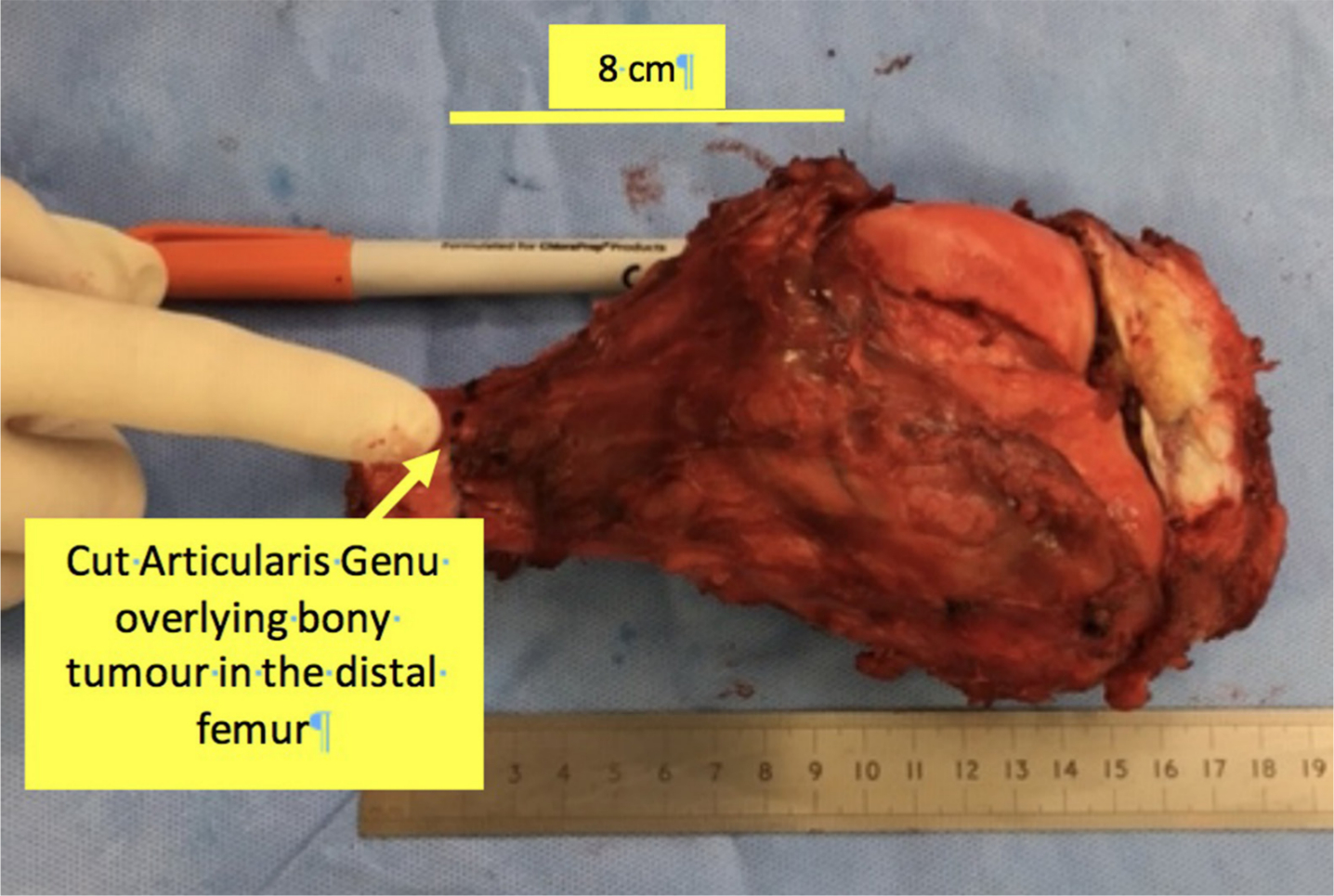
Fig. 6
Gross pathological specimen showing articularis genu covering a resected distal femur osteosarcoma.
Macroscopic surgical assessment of all specimens found coverage to be sufficient in all cases. In 17 of the 35 specimens, AG was identified, dissected from VI along a clear avascular plane, and resected in its entirety with the distal femur. In 16 of 35 cases additional anterior quadriceps (VI, VM, or VL) was resected to provide sufficient cover and a macroscopically clear resection. In the remaining 2 of 35 cases, AG was identified, and partially resected for contained intraosseous disease with no periosteal or soft tissue spread. Of 35 cases, there were 5 local recurrence. There was no local recurrence anteriorly where AG provided all or part of the tumour cover. In three cases, recurrence was attributed to haematological spread. In two cases, there was local recurrence posteriorly despite clear margin which may reflect microvascular or nodal spread of disease.
Median follow up time was 2.93 years (0.03 to 9.37). During the study period, 13 patients died, five-year survival was 63.2%, and ten-year survival was 58.7% (Figure 7).
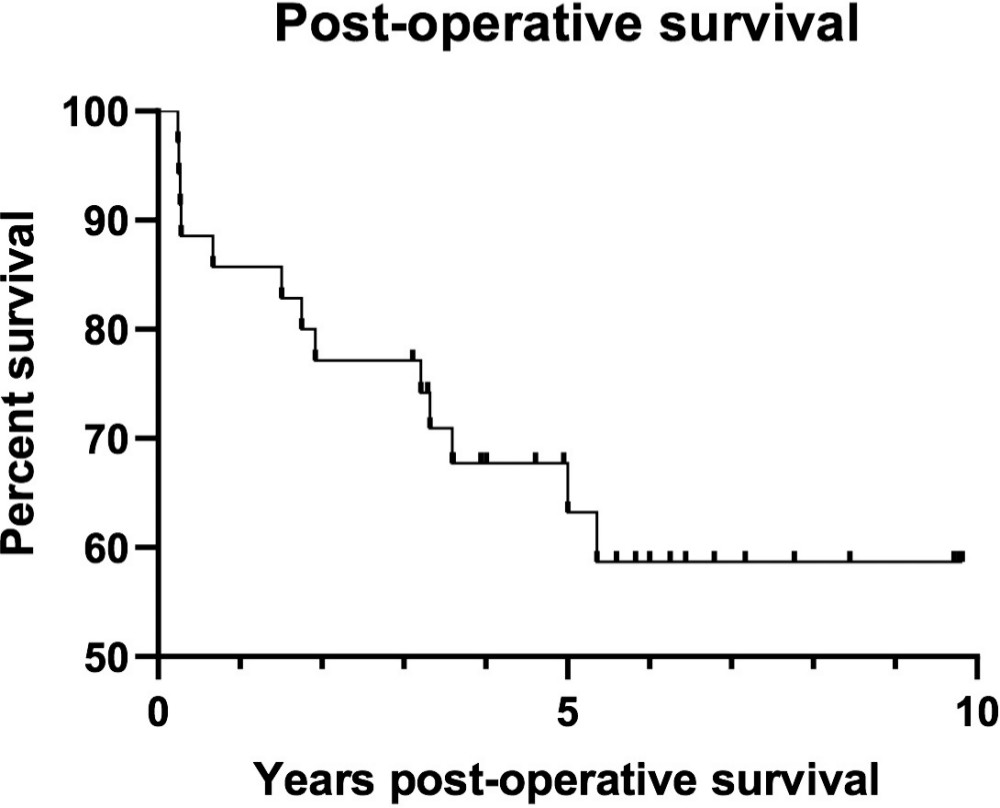
Fig. 7
Postoperative survival following distal femur resection was assessed using the Kaplan-Meier method. Patients lost to follow-up or still alive at the end of the study period were censored.
MRI findings
The average time between preoperative MRI and surgical resection was two months. Despite the variable MRI sequences, all studies had an axial T1-weighted and fluid sensitive (STIR, T2 or proton density with fat saturation) sequence. Long axis sequences included sagittal (n = 11), coronal (n = 8), or both (n = 16) planes. All patients who received intravenous gadolinium (n = 10) had an axial T1-weighted fat saturated sequence post injection.
In 34 of the 35 cases, the AG could be identified on MRI imaging as one or more discrete muscle bundles arising from the anterior surface of the femur and coursing distally to insert on the superior or posterior surface of the suprapatellar bursa. The AG was separated, at least along part of its length, from the vastus muscles by a fat plane. The AG could be identified on MRI in the three cases where it was not visualized in the distal femur resection specimens. A massive tumour mass invading the soft tissues prevented visualization of the AG in one case.
MRI images showed that the AG originated from the anterior surface of the femur. In some cases, superficial fibres also appeared to originate from the deep surface of VI. Insertion was into the superior suprapatellar bursa, with some cases showing deeper fibres inserting into the posterior surface of the suprapatellar bursa and posterior joint capsule.
The mean length of the AG measured from sagittal imaging was 8.8 cm ± 0.4 cm. The median number of AG muscle bundles identified on axial T1-weighted images was 5 (interquartile range (IQR) 3 to 7). The bundles could be divided into medial, central and lateral bundles. The number of layers of AG muscle bundles identified using a combination of axial and sagittal T1-weighted images was 0 (n = 1), 1 (n = 8), 2 (superficial and deep; n = 14) or 3 (superficial, intermediate and deep; n = 12). Fat between the AG layers was most noticeable between the distal intermediate and deep layers.
In 33 of the 35 cases, tumour breached the anterior femoral cortex with an anterior soft tissue tumour mass. The average anteroposterior dimension of the anterior soft tissue tumour was 1.6 cm.
The AG was thought to adequately cover and contain the tumour in 25 patients. This assessment was made on the basis of the following MRI features; either, the pre-femoral fat plane was preserved with a cleavage plane between the anterior tumour and the AG or, part of the pre-femoral fat plane was lost by tumour just contacting the deep AG layer, but there was no muscle displacement or invasion and one (superficial) or two (superficial and intermediate) overlying muscle layers could be clearly identified.
In six patients, the pre-femoral fat plane was lost, with tumour displacing the AG resulting in loss of definition of the deep and intermediate layers, but the superficial layer of muscle bundles could still be identified without evidence of tumour invasion. In these cases, it was difficult to determine whether the deep AG layer had been infiltrated by tumour but MRI indicated that there was no tumour extension into the quadriceps or knee joint. Confidence was increased when a thin layer of suprapatellar fat was present between the AG and VI.
In four patients, tumour displaced and distorted at least part of the AG where individual muscle layers could not be clearly delineated or separated from the VI. It was not possible to determine whether tumour was confined by the AG or infiltrated the deep surface of VI. One of these patients had a knee joint effusion attributed to intra-articular extension of a pathological fracture; however, tumour extension into the joint could not be excluded.
Discussion
The results of the cadaveric part of this study demonstrate that the AG is a discrete and substantial structure in the anterior compartment of the thigh. In the clinical component of this study, AG was clearly identified on preoperative MRI, and in postoperative distal femur resection specimens. This supports the previously anecdotal evidence that AG facilitates anterior soft tissue cover for safe and successful distal femur resections of bone tumours.
In contrast to previous studies,2-5 in which the AG is described as “wispy and “highly variable”, this study describes the AG as an identifiable and discrete structure in all specimens, covering one-third to one-half of the femur distally and anteriorly. In addition, the length of AG is greater than has been previously described.2-5 The shortest length of AG measured, 12.0 cm, was much greater than recent study findings, where mean bundle length was found to be 5.9 cm ± 1.0 cm and 5.4 cm ± 1.3 cm.4,5
Previous studies reported difficulty in distinguishing AG from VI, particularly at its origin.2,3 Apart from a few MRI cases this was not observed in any arm of our study where with careful dissection of the fascia deep to VI, it was possible to delineate the full extent of AG. In addition, AG could be distinguished by its different fibre orientation which is parallel to the long axis of the femur in contrast to VI. This has not been recognized previously, and may be important in distinguishing the AG and VI in dissection. The cadaveric study is consistent with previous evidence,2 in that the blood supply and innervation to AG shares a common origin with VI. These findings would be consistent with a common embryological origin. Taken together, this supports classification of AG as the “fifth quadriceps muscle”.
From the MRI component of this study, despite an anterior femoral soft tissue tumour mass, in line with dissection observations, the AG was seen, at least in part, as a discrete structure in 97% of cases.
The AG is separated from the anterior femoral cortex by pre-femoral fat and from the deep surface of vastus intermedius by suprapatellar fat. In addition, the individual layers and bundles of the AG are separated from each other by fat. The high signal of fat on T1-weighted sequences allows better definition of AG anatomy and fat planes. T1-weighted images were however less useful to determine whether tumour invaded adjacent soft tissues as tumour was often isointense to muscle (same signal intensity). Axial imaging should therefore include a fluid sensitive sequence in which the higher signal of tumour can better define the tumour-muscle margin. Sagittal and axial planes were the most useful. MR imaging protocol for distal femoral tumours should therefore include, at the very minimum, sagittal and axial T1 and fluid sensitive sequences.
The length measured from MRI found the AG to be shortened compared with cadaveric specimens. This may be because the point where the AG is seen to be inseparable from the anterior femoral cortex represents where the muscle is closely applied and inseparable from the femur and not its actual origin.
It is well recognized that this patient group with an anterior femoral tumour mass is not ideal to assess detailed AG anatomy. Despite an anterior tumour mass, up to three layers of muscle bundles could be identified in some cases. This corresponds to a previous cadaver studies in which the AG consisted of multiple muscle bundles that were organized into three main layers, a superficial, intermediate and deep layer.5,7 An average of five AG muscle bundles were identified in this study. Woodley et al5 found that significantly fewer muscle bundles were observed with MRI compared with dissection. They found the AG consisted of a mean of 7 ± 1.8 (4 to 10) muscle bundles at dissection, but they only identified a mean of 3.8 ± 0.8 (2 to 5) muscle bundles at MRI. Puig et al8 documented a mean of 2.4 ± 0.7 (1 to 4) AG muscles bundles in their MRI study. The wide variation among studies concerning the number of AG muscle layers and bundles may reflect differences in methodology and definition of what actually constitutes the AG muscle between studies.
MRI assessment was able to predict adequate tumour coverage by the AG in 71% of cases. Even with an anterior tumour mass displacing the AG and resulting in loss of definition of the deeper layers, the superficial layers could be identified without evidence of macroscopic tumour invasion suggesting that tumour was contained by the AG with no quadriceps or intra-articular tumour extension. Postoperative histopathological analysis confirmed clear margins in all these cases. Preoperative MRI assessment could help surgeons plan surgical resection by indicating whether the AG is a potential anterior soft tissue cover for tumours to provide a safe anterior margin.
AG alone provided sufficient coverage for clear marginal resection in approximately 50% of cases. In the remaining cases, either: additional quadriceps resection was required for more invasive disease, or minimal resection of AG was indicated in cases of contained intraosseous disease. Across all cases, we document local recurrence in five of 35. The European Osteosarcoma Group, in 2002, report an association between limb salvage and local disease recurrence in a multicentre study of patients with osteosarcoma of an extremity.9 The authors state a local recurrence rate of 13.3% from a centre with 85% limb salvage. Extrapolating this figure, given 100% of cases in this study were a primary limb salvage procedure, we would argue that our local recurrence rate of 14.3% is acceptable, and possibly below expected. Furthermore, of the five local recurrences in this study, it could be argued that this was progression of nodal or microvascular disease rather than local recurrence due to inadequate resection.
Overall, this evidence is significant when determining an adequate anterior soft tissue cover for resection of distal femur with primary or metastatic bony tumour. In all cases, margins were negative with histological analysis. Where appropriate, the AG may offer surgeons the opportunity to spare the majority of quadriceps during resection where these muscles have not been compromised by tumour and the AG has not been breached by the tumour. As a result, bulk quadriceps function can be spared, giving patients better function and reduced morbidity post-operatively.
Conclusion
This study clearly demonstrates that articularis genu is a substantial and discrete muscle of the anterior compartment of the thigh, identifiable in cadaveric dissection, MR imaging, and on gross pathological specimens of bone tumour. From observations in cadaveric specimens, it has a different morphology from the overlying VI. However, it has the same neurovascular supply; consistent with a shared embryological origin and so should be regarded as the “fifth quadriceps muscle”. In addition, findings suggest that it provides a safe surgical margin, which may be pre-determined by MRI. With a clear anatomical dissection it can provide a safe anterior soft tissue margin in the resection of bone tumours in the distal one-half of the femur.
References
1. Stevens J , Clement N , Patton J . The extensile medial parapatellar approach to the distal femur and knee: anatomic landmarks and surgical technique . Techniques in Orthopaedics . 2019 ; 34 ( 2 ): 129 – 133 . Google Scholar
2. Kimura K , Takahashi Y . M. articularis genus. observations on arrangement and consideration of function . Surg Radiol Anat . 1987 ; 9 ( 3 ): 231 – 239 . Crossref PubMed Google Scholar
3. Reider B , Marshall JL , Koslin B , Ring B , Girgis FG . The anterior aspect of the knee joint . J Bone Joint Surg Am . 1981 ; 63 ( 3 ): 351 – 356 . PubMed Google Scholar
4. Sakuma E , Sasaki Y , Yamada N , Wada I , Soji T . Morphological characteristics of the deep layer of articularis genus muscle . Folia Morphol . 2014 ; 73 ( 3 ): 309 – 313 . Crossref PubMed Google Scholar
5. Woodley SJ , Latimer CP , Meikle GR , Stringer MD . Articularis genus: an anatomic and MRI study in cadavers . J Bone Joint Surg Am . 2012 ; 94 ( 1 ): 59 – 67 . Crossref PubMed Google Scholar
6. Standring S . Gray’s Anatomy : Elsevier , 2015 . Google Scholar
7. Grob K , Gilbey H , Manestar M , Ackland T , Kuster MS . The anatomy of the Articularis genus muscle and its relation to the extensor apparatus of the knee . JB JS Open Access . 2017 ; 2 ( 4 ): e0034 . Crossref PubMed Google Scholar
8. Puig S , Dupuy DE , Sarmiento A , Boland GW , Grigoris P , Greene R . Articular muscle of the knee: a muscle seldom recognized on MR imaging . AJR Am J Roentgenol . 1996 ; 166 ( 5 ): 1057 – 1060 . Crossref PubMed Google Scholar
9. Grimer RJ , Taminiau AM , Cannon SR . Surgical Subcommittee of the Europen osteosarcoma intergroup. surgical outcomes in osteosarcoma . J Bone Joint Surg Br . 2012 ; 84 ( 3 ): 395 – 400 . Google Scholar
Author contributions
J. Caterson: Designed the study, Collected the data, Prepared the draft manuscript.
M. A. Williams: Designed the study, Analyzed the data, Critically revised and submitted the manuscript.
C. McCarthy: Collected the data, Prepared the manuscript.
N. Athanasou: Collected the data, Prepared the manuscript.
H. T. Temple: Revised the manuscript.
T. Cosker: Designed the study, Critically revised and submitted the manuscript.
M. Gibbons: Designed the study, Critically revised and submitted the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
H. T. Temple reports consultancy, royalties, and stock/stock options from Vivex Biologics, employment by HCA Healthcare, grants/grants pending from Nova Southeastern University, and payment for lectures (including service on speakers bureaus), and for development of educational presentations from Stryker Orthopedics, all of which are unrelated to this article.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC-ND), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









