Abstract
Aims
To investigate the effect of polyethylene manufacturing characteristics and irradiation dose on the survival of cemented and reverse hybrid total hip arthroplasties (THAs).
Methods
In this registry study, data from the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man (NJR) were linked with manufacturing data supplied by manufacturers. The primary endpoint was revision of any component. Cox proportional hazard regression was a primary analytic approach adjusting for competing risk of death, patient characteristics, head composition, and stem fixation.
Results
A total of 290,770 primary THAs were successfully linked with manufacturing characteristics. Overall 4,708 revisions were analyzed, 1,260 of which were due to aseptic loosening. Total radiation dose was identified as a risk factor and included in the Cox model. For statistical modelling of aseptic loosening, THAs were grouped into three categories: G1 (no radiation); G2 ( > 0 to < 5 Mrad); and G3 ( ≥ 5 Mrad). G1 had the worst survivorship. The Cox regression hazard ratio for revision due to aseptic loosening for G2 was 0.7 (95% confidence interval (CI) 0.58 to 0.83), and for G3 0.4 (95% CI 0.30 to 0.53). Male sex and uncemented stem fixation were associated with higher risk of revision and ceramic heads with lower risk.
Conclusion
Polyethylene irradiation was associated with reduced risk of revision for aseptic loosening. Radiation doses of ≥ 5 Mrad were associated with a further reduction in risk.
Cite this article: Bone Joint Res 2020;9(9):563–571.
Article focus
-
Crosslinked polyethylene (XLPE) has been associated with a significant reduction in revision surgery in total hip arthroplasty (THA) with uncemented acetabular components.
-
We investigated the effect of polyethylene irradiation on the survival of THAs with cemented acetabular components.
Key messages
-
Low-dose irradiated polyethylene (< 5 Mrad) was associated with a significant reduction in the risk of THA revision for aseptic loosening. Irradiation at ≥ 5 MRad, conventionally labelled XLPE, was associated with a further reduction in the risk of revision for aseptic loosening.
-
The use of non-irradiated polyethylene cups should be avoided with XLPE or low irradiation products used when possible.
Strengths and limitations
-
Our study was able to assess several manufacturing characteristics for their effect on the risk of revision using a large registry dataset.
-
The observational nature of registry data means that despite statistical modelling, there is potential for confounders that are not accounted for.
Introduction
Total hip arthroplasty (THA) surgery is a very popular and successful procedure.1 Cemented acetabular component fixation is common in the Nordic countries2 as well as in the UK, where it was used in 39% of THAs according to the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man (NJR).3 Excellent long-term survival has been reported using these components even in challenging cases.4,5
Polyethylene wear particles and the associated osteolysis is a recognized mechanism leading to aseptic loosening and subsequent failure of hip arthroplasty components.6,7 Modified polyethylene, commonly labelled crosslinked polyethylene (XLPE), was introduced in the late 1990s to reduce wear debris and associated loosening.8 The use of XLPE in uncemented THA has improved the survival of those joints in randomized controlled trials (RCTs) as well as registry data.9,10
The literature on cemented acetabular components has been less promising. Two RCTs and one registry study have failed to demonstrate a beneficial effect for XLPE in terms of loosening and osteolysis at mid-term of follow-up.11–13 Both RCTs reported reduced wear rates in the XLPE groups.11,13 These studies are limited by the follow-up duration and the low numbers that can reasonably be included in a RCT.
The aim of our study was to investigate the effect of polyethylene manufacturing modifications on the survival of THAs using cemented acetabular components.
Methods
Data sources
Two data sources were combined: primary THA survival outcomes from the NJR and manufacturing information on polyethylene acetabular components. Outcomes were obtained for all primary THAs using a cemented polyethylene cup between 1 January 2004 and 28 July 2016. For each cup, information was obtained on the manufacturing characteristics listed in Table I.
Table I.
Manufacturing characteristics of an acetabular component.
| Characteristic | Values |
|---|---|
| Resin type | 1020 |
| 1050 | |
| Radiation source | gamma irradiation |
| Ebeam | |
| None | |
| Multiple cross-linking treatments | Yes |
| No | |
| Cross-linking dose | In Mrad |
| Terminal sterilization method | γ |
| Ethylene oxide (EtO) | |
| Gas plasma | |
| Terminal sterilization radiation dose | In Mrad |
| Stabilization treatment (free radical scavenging) | None |
| Heated below melting point | |
| Heated above melting point | |
| Vitamin E infused | |
| Vitamin E blended | |
| Heated below + mechanical deformation | |
| Total radiation dose | Derived, cross-linking dose + terminal sterilization radiation dose in Mrad |
| Packaging | In air/air permeable |
| Inert gas/non-air permeable |
-
EtO, ethylene oxide; γ, gamma.
Data linkage
Linking between NJR data and cup manufacturing data was performed using the cup catalogue number and cup description. There were 309,057 records of primary THAs using a cemented all-polyethylene acetabulum. Manufacturing data were available and successfully linked for 301,680 (97.6%) primary THAs. A further 10,910 records were excluded for missing or erroneous key information. The final sample included 290,770 primary THAs.
Endpoints
The endpoint of interest was first revision, defined as the exchange of one or more implant components. If no revision occurred until the last follow-up date of 28 July 2016, the observation was censored. Participants who expired before undergoing revision were censored at the time of death. We investigated the risk of revision for any reason and for cause-specific revisions. The same revision can have multiple reasons reported. The following most common reasons were analyzed: infection (n = 1,040); aseptic loosening (n = 1,260); dislocation (n = 1,233); and periprosthetic fracture (n = 617).
Polyethylene groups
The total radiation dosage used in the polyethylene manufacturing process was classified into three categories: G1 is a non-irradiated polyethylene; in G2 (> 0 to < 5 Mrad) low dose radiation is used at the terminal sterilization phase; in G3 (≥ 5 Mrad) irradiation is delivered prior to the sterilization phase to generate cross-links followed by free radical scavenging. Translated into the clinical practice of dividing polyethylene into conventional polyethylene (CPE) and XLPE, G1 and G2 are CPE and G3 is XLPE. The effect of stabilization treatment was evaluated in G2 and G3; 95% confidence intervals (CIs) are presented.
Statistical analysis
Exploratory statistical analysis was based on Kaplan-Meier (K-M) product limit estimator. First, a series of exploratory K-M analyses were performed to identify manufacturing characteristics possibly associated with any reason and cause-specific revisions. These exploratory analyses were followed by Cox proportional hazard regression survival analyses. The Cox model is a semiparametric statistical approach to explain the effect of explanatory variables on survival hazards. It assumes that hazard ratios are constant and that the explanatory variables have an effect only on the baseline hazard and not on time to failure. There is no uniformly accepted approach to evaluate if these assumptions are met. Based on exploratory review of K-M cumulative incidence functions, a time-specific Cox regression model using an exploratory defined cut-off at four years was run in addition. A statistical sensitivity analysis was performed using a parametric life regression survival analysis (PROC LIFEREG) model. The best parametric model fit was achieved using γ distribution. All survival analyses were performed accounting for a competing risk of death in order to obtain cumulative incidence function estimates. Cause-specific analyses treated revisions for other causes as competing risk as well. A sensitivity K-M and Cox regression analyses were performed without adjustment for competing risk.
The full Cox proportional hazards regression and the life hazard models were built following exploration of individual candidate variables in age- and sex-adjusted models. The following variables were considered for inclusion: indication for implantation (osteoarthritis vs other); yearly cohort effect (2004, 2005, etc.); femoral head composition (metal, ceramic (including ceramicized metal)); type of stem fixation (cemented and cementless); acetabular/head size; and acetabular manufacturing characteristics (resin type, terminal sterilization method, packaging, and radiation dose).
All analyses were made using SAS/STAT software, Version 9.4 for PC, 2017 (SAS Institute, Cary, North Carolina, USA).
Results
Descriptive statistics by acetabular total radiation group are shown in Table II. XLPE (G3) was used in 43,078 cases associated with 386 revisions. Femoral heads of 32 mm or larger were mainly used in G3 (48.5%). Cementless stems (reverse hybrid) were used in 20.2% of cases in G3 and only 4.9% and 4.5% of cases in G1 and G2, respectively. Ceramic head use was more common in G3. Maximum follow-up was longer in G1 and G2 (13.3 years) than in G3 (7.8 years). There was a gradual increase in the use of G3 products. In 2010, G2 cups were used in 84.3% of cases and G3 cups in 9.1%. In 2015, G2 products accounted for 63% of cases, G3 cups for 36.8%, and the use of G1 reduced to 0.2%.
Table II.
Descriptive information by irradiation group.
| Variable | G1 | G2 | G3 |
|---|---|---|---|
| Radiation, Mrad | no radiation | < 5 | ≥ 5 |
| N | 12,449 | 232,562 | 45,759 |
| Sex (female), n (%) | 8,279 (66.5) | 154,886 (66.6) | 29,194 (63.8) |
| Mean age, yrs (SD) | 72.7 (9.4) | 73.8 (8.6) | 70.1 (10.7) |
| Age (years), n (%) | |||
| < 55 | 510 (4.1) | 5,349 (2.3) | 3,661 (8.0) |
| 55 to < 65 | 1,569 (12.6) | 24,884 (10.7) | 8,694 (19.0) |
| 65 to < 75 | 4,656 (37.4) | 86,280 (37.1) | 16,427 (35.9) |
| ≥ 75 | 5,714 (45.9) | 116,048 (49.9) | 16,977 (37.1) |
| Osteoarthritis indication, n (%) | 11,627 (93.4) | 217,213 (93.4) | 40,771 (89.1) |
| Acetabulum | |||
| Internal diameter size (mm), n (%) | |||
| 22.25 | 3,598 (28.9) | 29,768 (12.8) | 1,510 (3.3) |
| 26 | 523 (4.2) | 16,744 (7.2) | 0.0 (0) |
| 28 | 7,818 (62.8) | 155,584 (66.9) | 22,193 (48.5) |
| 32 | 510 (4.1) | 30,698 (13.2) | 18,624 (40.7) |
| 36 | 0.0 (0) | 0.0 (0) | 3,386 (7.4) |
| 40 | 0.0 (0) | 0.0 (0) | 46 (0.1) |
| Resin type = (1020), n (%) | 0.0 (0) | 14,186 (6.1) | 19,676 (43.0) |
| Terminal sterilization, n (%) | |||
| Gamma | 0.0 (0) | 232,562 (100) | 595 (1.3) |
| Ethylene oxide (EtO) | 12,449 (100) | 0.0 (0) | 2,059 (4.5) |
| Gas plasma | 0.0 (0) | 0.0 (0) | 43,105 (94.2) |
| Stabilization treatment (yes), n (%) | 0.0 (0) | 106,979 (46.0) | 45,759 (100) |
| Stabilization treatment typen (%) | |||
| None | 12,449 (100) | 125,583 (54.0) | 0.0 (0) |
| Heated below melting point | 0.0 | 106,979 (46.0) | 19,631 (42.9) |
| Heated above melting point | 0.0 | 0.0 (0) | 25,625 (56.0) |
| Vitamin E infused | 0.0 | 0.0 (0) | 503 (1.1) |
| Mean total radiation dose, Mrad (SD) | 0.0 (0.0) | 3.0 (0.1) | 7.0 (2.1) |
| Stem | |||
| Cementless stem, n (%) | 610 (4.9) | 10,465 (4.5) | 9,243 (20.2) |
| Head | |||
| Ceramic*, n (%) | 1,718 (13.8) | 20,465 (8.8) | 13,499 (29.5) |
| Metal, n (%) | 10,731 (86.2) | 212,097 (91.2) | 32,260 (70.5) |
| Outcomes | |||
| Mean person-time, yrs (SD) | 6.8 (3.0) | 5.6 (3.5) | 2.5 (1.7) |
| Maximum follow-up, yrs* | 13.3 | 13.3 | 7.8 |
| Outcome, n (%) | |||
| Not revised | 9,260 (74.4) | 182,757 (78.6) | 43,098 (94.2) |
| Revised | 309 (2.5) | 4,013 (1.7) | 386 (0.8) |
| Revised due to aseptic loosening (any component) | 132 | 1,376 | 68 |
| Revised due to aseptic loosening socket† | 110 | 1,107 | 41 |
| Revised due to aseptic loosening stem† | 59 | 665 | 35 |
| Expired | 2,880 (23.1) | 45,792 (19.7) | 2,275 (5.0) |
-
*
Includes 425 ceramicized metal (Oxinium) femoral heads.
-
†
Categories not mutually exclusive.
Revision for any reason
Exploratory K-M analyses identified that the largest magnitude of effect on the risk of revision is associated with the total radiation dose group. At 13 years post-THA, the cumulative incidence of revision for any reason was highest in G1 at 4.5 per 100 THAs (95% CI 3.9 to 5.2) compared to 3.2 per 100 THAs (95% CI 3.1 to 3.4) in G2. The follow-up time in G3 was shorter. The seven-year estimate of revision risk in G3 was 1.7 per 100 THAs (95% CI 1.4 to 2.1). K-M estimated cumulative incidence of revision for any reason differed among the groups (p = 0.0016; Gray's test) (Figure 1). A simple estimate based on our analysis indicates that 41% (approximately 640) revisions for aseptic loosening would have been avoided if all primary THAs used G3 polyethylene cups.
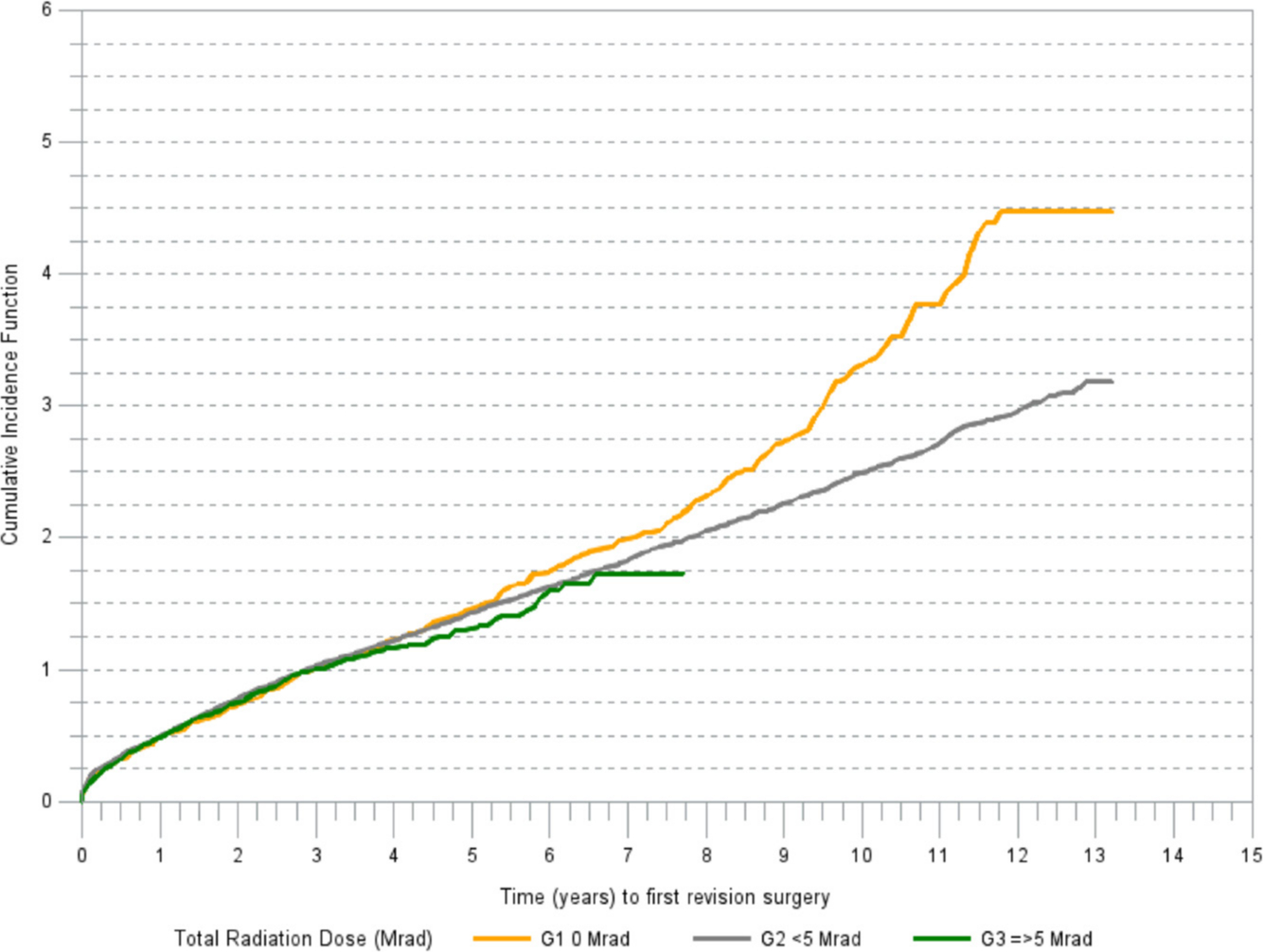
Fig. 1
Cumulative incidence of revision (any component) for any reason.
Cause-specific revisions
There was a marked difference in cumulative incidence of revision for aseptic loosening among the irradiation groups (Figure 2). The cumulative incidence of revision due to reasons other than aseptic loosening was increasing in a linear trend in all irradiation groups (Figure 3).
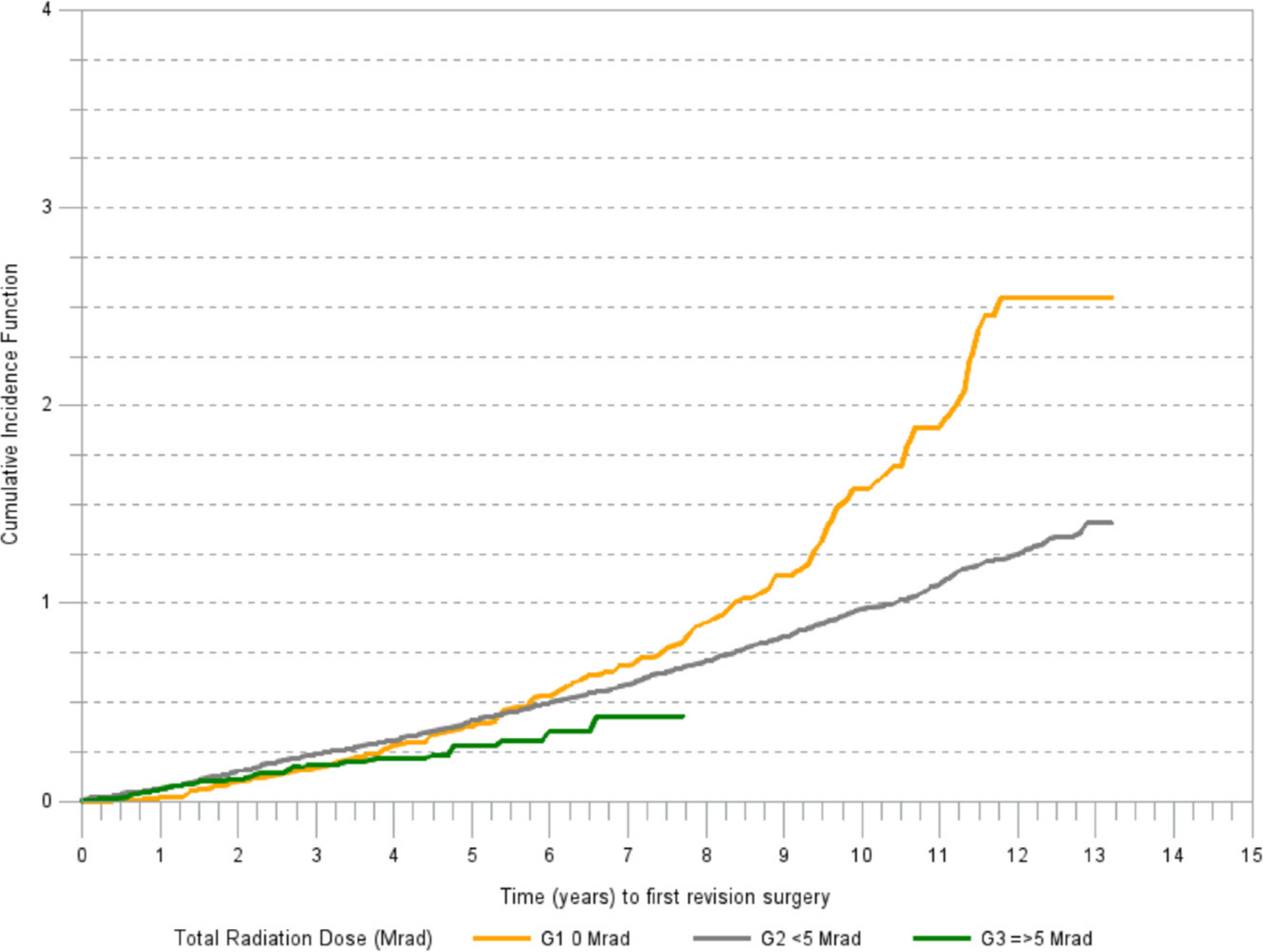
Fig. 2
Cumulative incidence of revision (any component) for aseptic loosening.
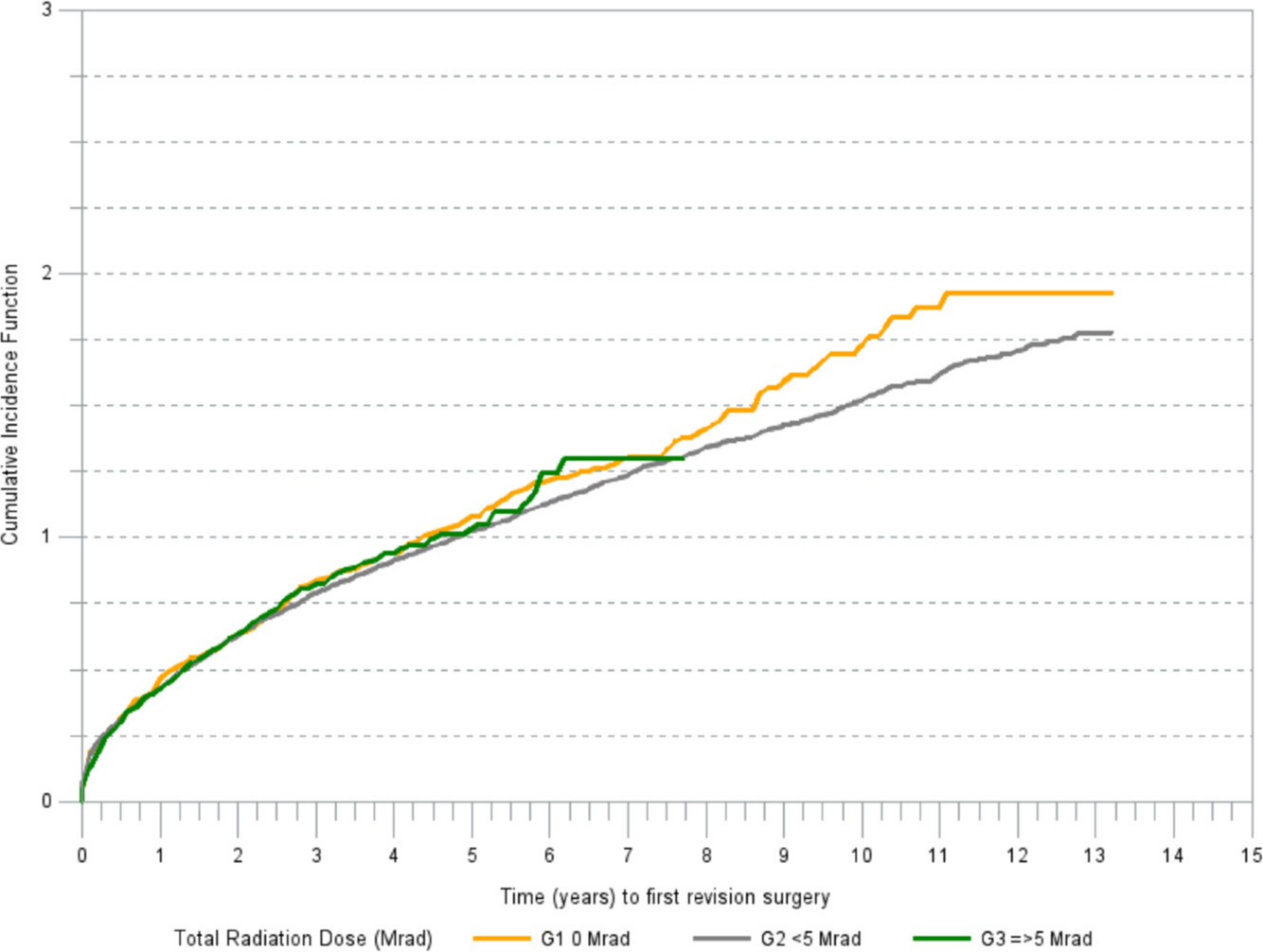
Fig. 3
Cumulative incidence of revision (any component) for reasons other than aseptic loosening.
Within G2, acetabular components stabilized with heating below the melting point and components without stabilization had a similar risk of revision due to aseptic loosening. There was no difference in cumulative risk of revision in G3 between acetabular components stabilized by heating above the melting point and those heated below the melting point (Figure 4).
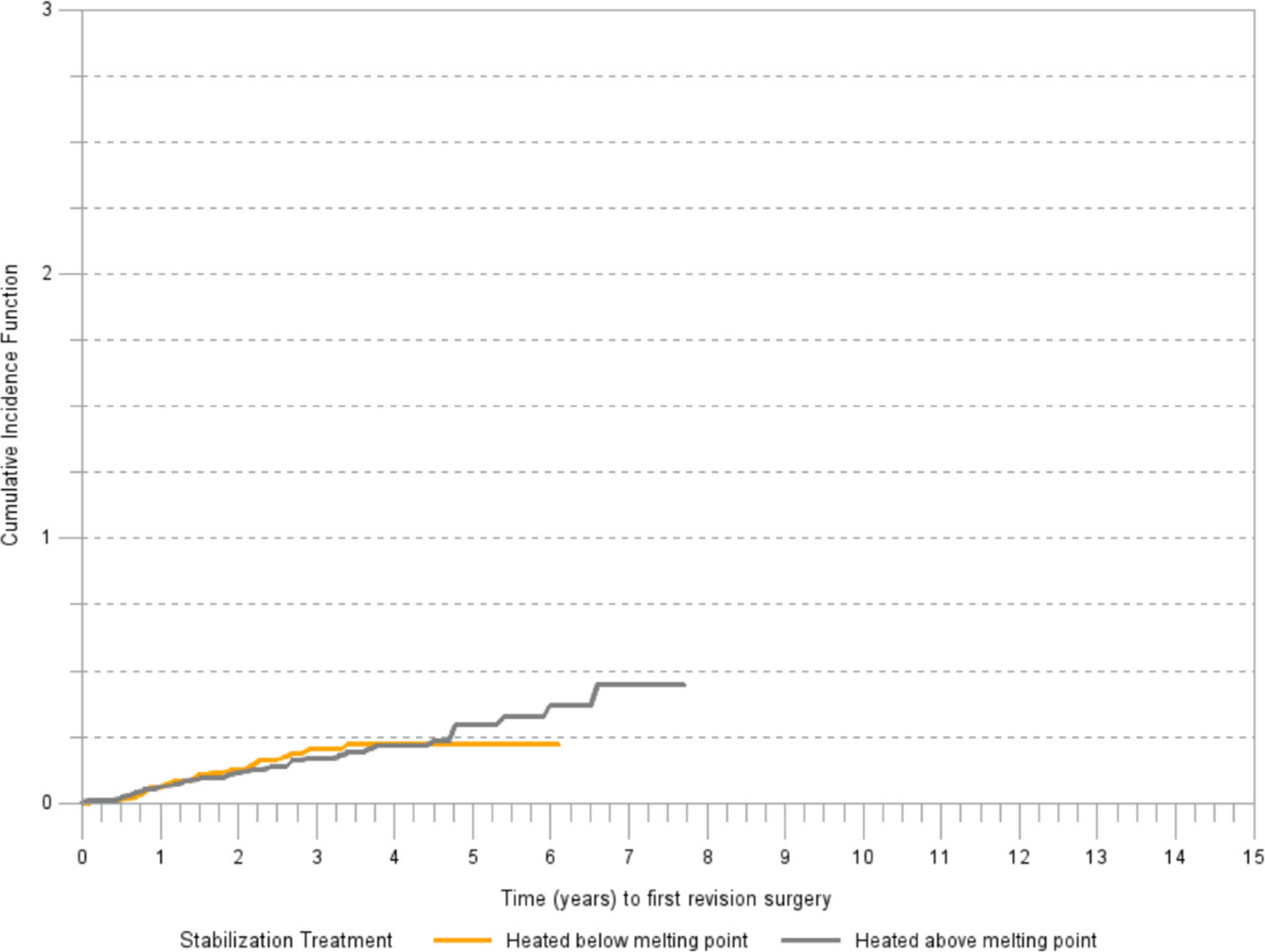
Fig. 4
Cumulative incidence of revision (any component) for aseptic loosening by type of stabilization treatment in G3 ( > 5 Mrad).
Cox regression
The following predictors were included in a final multivariate Cox regression proportional hazard model of revision due to aseptic loosening adjusted for competing risks of death and revision for other causes: age, sex, head composition (metal and ceramic/ceramicized metal, total irradiation (G1 to G3), and stem fixation method (cemented/cementless)). Distribution by head size across the irradiation groups did not allow for inclusion of head size into the model.
There was a difference in hazard ratios for revision due to aseptic loosening among the irradiation groups. The highest cumulative incidence function of revision due to aseptic loosening was in G1 (no radiation) followed by G2. Best survivorship over the entire period was in G3. Cemented stems had better survivorship than cementless stems. This was seen in the early post-implantation years (0 to 4) and was not seen in the late phase (after four years; Table III). Male sex and younger age were associated with a higher risk of revision due to aseptic loosening.
Table III.
Cox regression hazard ratios for revision due to aseptic loosening.
| Parameter | Hazard ratio (95% confidence Interval) | PROC LIFEREG | ||
|---|---|---|---|---|
| entire period | 0 to 4.0 yrs | > 4.0 yrs | ||
| Age | ||||
| < 55 yrs | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 55 to < 64 yrs | 0.70 (0.57 to 0.87) | 0.85 (0.59 to 1.23) | 0.61 (0.47 to 0.79) | 0.77 |
| 65 to < 75 yrs | 0.41 (0.33 to 0.50) | 0.59 (0.41 to 0.83) | 0.32 (0.25 to 0.42) | 0.51 |
| ≥ 75 yrs | 0.18 (0.14 to 0.22) | 0.33 (0.23 to 0.48) | 0.12 (0.09 to 0.16) | 0.30 |
| Sex | ||||
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Male | 1.19 (1.07 to 1.32) | 1.14 (0.98 to 1.32) | 1.26 (1.10 to 1.45) | 1.18 |
| Head composition | ||||
| Metal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Ceramic* | 0.57 (0.48 to 0.68) | 0.68 (0.53 to 0.87) | 0.49 (0.37 to 0.64) | 0.62 |
| Total radiation (Mrad) | ||||
| G1: no radiation | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| G2: < 5 | 0.70 (0.58 to 0.83) | 1.13 (0.80 to 1.59) | 0.55 (0.44 to 0.67) | 0.68 |
| G3: ≥ 5 and < 10 | 0.40 (0.30 to 0.53) | 0.67 (0.44 to 1.01) | 0.27 (0.12 to 0.59) | 0.39 |
| Stem implantation | ||||
| Cemented | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Cementless | 1.53 (1.26 to 1.85) | 2.01 (1.59 to 2.55) | 1.01 (0.70 to 1.44) | 1.43 |
-
*
Includes femoral heads made of ceramicized metal (Oxinium).
-
PROC LIFEREG, parametric life regression survival analysis.
A validation model using PROC LIFEREG and a γ model showed similar hazard ratios to the Cox regression model (Table III). A time-specific Cox regression model showed separation of risk of revision due to aseptic loosening among the groups after more than four years since primary surgery. A sensitivity K-M and Cox analyses without adjustment for competing risks, as expected, yielded higher K-M estimators and marginal numerical changes for Cox hazard ratios. There was no noticeable impact on observed associations between the polyethylene manufacturing characteristics and risk of revision.
Component-specific analysis
The effect of irradiation (G2 and G3) was noted in the risk of aseptic loosening for both femoral stems and acetabular components (Table IV). In this component-specific analysis, male sex was associated with a higher risk of loosening of the femoral stem but not the acetabulum.
Table IV.
Cox regression hazard ratios for revision due to aseptic loosening in socket and stem.
| Parameter | Hazard ratio (95% CI) socket | Hazard ratio (95% CI) stem |
|---|---|---|
| Age, yrs | ||
| < 55 | 1.00 (reference) | 1.00 (reference) |
| 55 to < 64 | 0.68 (0.53 to 0.87) | 0.91 (0.65 to 1.27) |
| 65 to < 75 | 0.40 (0.31 to 0.50) | 0.51 (0.37 to 0.70) |
| ≥ 75 | 0.17 (0.13 to 0.22) | 0.21 (0.15 to 0.29) |
| Sex | ||
| Female | 1.00 (reference) | 1.00 (reference) |
| Male | 0.99 (0.87 to 1.11) | 1.88 (1.63 to 2.17) |
| Head composition | ||
| Metal | 1.00 (reference) | 1.00 (reference) |
| Ceramic* | 0.57 (0.46 to 0.71) | 0.55 (0.42 to 0.71) |
| Total radiation (Mrad) | ||
| G1: no radiation | 1.00 (reference) | 1.00 (reference) |
| G2: < 5 | 0.62 (0.51 to 0.75) | 0.75 (0.58 to 0.98) |
| G3: ≥ 5 | 0.32 (0.22 to 0.45) | 0.46 (0.31 to 0.69) |
| Stem implantation | ||
| Cemented | 1.00 (reference) | 1.0 (reference) |
| Cementless | 1.16 | 1.76 (1.36 to 2.28) |
-
*
Includes femoral heads made of ceramicized metal (Oxinium).
-
CI, confidence interval.
Discussion
Our analysis has identified a lower risk of revision for aseptic loosening of THA when irradiated polyethylene (G2) was used versus the non-irradiated group (G1). G2 products, often labelled as conventional CPE, received irradiation and in some cases heat treatment during manufacturing. An increased dose of irradiation, often labelled highly crosslinked (XLPE, G3), was associated with a further reduction in the risk of revision for aseptic loosening. Other THA factors known to affect prosthesis survival were confirmed in our study and included in our statistical modelling. These included age, sex, head material, and stem fixation.
This finding is not in agreement with the results of a recent analysis of the Nordic Arthroplasty Register Association (NARA) dataset.12 In this study, the authors analyzed all cemented designs implanted from 2006 onwards with at least 7.5 years of follow-up. The polyethylene products were split between XLPE and CPE in a binary fashion. The combined all-design analysis revealed no difference between CPE and XLPE with regard to revision for any reason or aseptic loosening. The same study reported a product-specific analysis for three acetabular component products that had been manufactured and implanted with both CPE and XLPE. For two products, the XLPE version was found to have improved survival while the third product did not demonstrate this. One of these products was included in a similar analysis from the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) with concurring results.14
Our analysis differs from the analysis of the NARA dataset as polyethylene manufacturing modifications were assessed independently and the products were not split in a binary fashion. We grouped the polyethylene products further according to the radiation dose received to investigate the dose effect. Our study has identified a reduced risk of revision for aseptic loosening when G3 products were used compared to G1. G2 includes components irradiated at low doses, often as part of the sterilization process, which have therefore developed a degree of crosslinking. Some were subsequently heated below melting point.15 G2 was seen to have a lower risk of revision compared to G1 at more than four years from implantation. We believe that the inclusion of G2 products in the CPE group in the study using the NARA dataset is likely to contribute to the difference in results.12 Stabilization treatment was not associated with the risk of revision in our study. In clinical studies, both annealed16 and remelted17 XLPE acetabular components have demonstrated wear rates below the proposed osteolysis threshold.7 Our study did not include adequate numbers for analysis or antioxidant polyethylene. Promising results by clinical trials in uncemented cups18,19 and some concerns in cemented cups suggest that registry monitoring will be key for these components in the future.20
The reduced risk of revision in G3 acetabular components is consistent with the extensively reported beneficial effect of XLPE on aseptic loosening in uncemented acetabular components.21–25 Two RCTs have failed to identify a difference in prosthesis survival between XLPE and CPE components, despite improved wear performance at mid-term follow-up. Johanson et al11 compared survival and osteolysis between CPE and XLPE at ten years. They compared a product irradiated with a low dose for sterilization purposes (G2) with a product irradiated by 9.5 Mrad and remelted (G3) using 28 mm cobalt-chromium heads. They randomized 60 patients and 52 were available for analysis at follow-up. They reported reduced femoral head penetration in the XLPE (G3) group but not in radiolucencies or revision rate. Their findings are in agreement with an RCT by Langlois et al,13 who compared a mildly irradiated (3 Mrad) and annealed component (G2) to a highly irradiated (9.5 Mrad) and remelted one (G3) using 22.2 mm heads. They randomized 100 patients with 68 available for analysis at follow-up. The authors reported increased penetration rate in the low irradiation polyethylene, but this did not translate to any revisions for aseptic loosening at means of 8.7 years (highly irradiated group) and 9.1 years (low irradiation group). Our analysis identified a beneficial effect of low irradiation (G2) on the survival of cemented acetabular components when compared to the non-irradiated group. The difference between G2 and G3 was less marked, which may account for the lack of differences at this follow-up point in the RCTs. Furthermore, the authors used 28 mm and 22.2 mm heads, while a wider variation of head sizes was used in the NJR dataset. The effect of head size on volumetric wear in polyethylene is well described with smaller head sizes leading to lower volumetric wear.26
The effect of XLPE on survival of cemented polyethylene acetabular components has not been clear in the literature to date. The mode of failure of cemented and uncemented acetabular components may be different. Meticulous bone preparation and cementation technique is required to achieve the initial stability and bone cement interdigitation that are critical. The presence of radiolucency in the early postoperative period has been associated with an increased risk of acetabular migration and subsequent failure.27,28 Polyethylene wear debris-associated loosening requires a certain amount of wear prior to the effects being visible on the radiograph and leading to revision surgery.6,29 It is therefore possible that although XLPE has improved wear characteristics, this does not protect against all the failure modes of cemented acetabular components.
Our analysis was adjusted for competing risks of death and revision for other reasons. This approach better estimates true cumulative incidence of revision in a specific patient population.30 The unadjusted analysis would assume independence between competing risks and revision risk and would project censoring time beyond death or revision for a different reason. In practice, arguably both a selection of implants and propensity to revise is based upon patient characteristics. We performed a sensitivity statistical analysis using unadjusted approaches and, while the numerical risk estimates have increased as expected, the conclusions regarding the manufacturing characteristics remained the same.
Our statistical modelling was developed with a focus on polyethylene irradiation while controlling for factors that are known to affect THA survival and were confirmed in our analysis. Ceramic head use (including ceramicized metal) was associated with reduced risk of revision for aseptic loosening in our analysis. This finding was persistent when age-stratified analysis was run for patients over the age of 70 years (data not shown). We feel that this should not be considered a primary finding of this study as statistical modelling to appropriately control for confounding variables would be markedly different to the one used in this study. Cemented stem fixation was also associated with reduced risk of revision in the four years post-implantation (Table IV). Although controlling for this in our modelling was important, we do not feel that this is a primary finding of our study. Stems with design features such as the presence of a collar31 and geometry32 are likely to behave differently, and this cannot be controlled for within our statistical model and dataset.
Our study has several limitations. Registry data are observational in nature and there is potential for bias despite our statistical modelling, which was designed to control for confounding variables. The endpoint of revision surgery is a crude indicator of failure because a number of prosthetic joints might malfunction but not be revised. When analyzing polyethylene wear-related failure, follow-up duration is critical. Follow-up in G3 was shorter than for G1 and G2. Despite the shorter follow-up in G3, our analysis revealed a significantly lower cumulative revision risk at seven years of follow-up. Longer follow-up of G3 and a comparison to the other groups is likely to identify a larger difference because the effects of polyethylene wear are cumulative over time.6 Our dataset did not include adequate numbers of antioxidant stabilized products for inclusion in this analysis, and we can therefore not comment on this subtype of modified polyethylene. Under-reporting of revision surgery in the NJR dataset is a known issue, therefore the absolute revision numbers may be higher.3 However, the overall effect of under-reporting on the relative revision rates between groups would be small. We have attempted to control for variation in the revision capture rates between the years by adjusting the statistical model for the yearly cohort effect.
In conclusion, highly crosslinked polyethylene (G3) is associated with a marked reduction in the cumulative risk of revision for aseptic loosening when compared to non-irradiated polyethylene in cemented acetabular components. Irradiation of polyethylene at a low dose (G2, < 5 Mrad), often classed as conventional polyethylene, is also associated with a reduced risk of revision when compared to non-irradiated polyethylene.
References
1. Learmonth ID , Young C , Rorabeck C . The operation of the century: total hip replacement . Lancet . 2007 ; 370 ( 9597 ): 1508 – 1519 . Crossref PubMed Google Scholar
2. Fenstad AM , Pedersen AB . Nordic Arthroplasty Register Association (NARA) report . Nordic Arthroplasty Register Association (NARA) . 2016 . http://nrlweb.ihelse.net/NARA_2015_ORIG_ny.pdf (date last accessed 5 August 2020 ). Google Scholar
3. No authors listed . National Joint Registry 14th Annual Report 2017 . National Joint Registry . 2017 . https://www.hqip.org.uk/resource/national-joint-registry-14th-annual-report-2017/#.XywCMihKhPY (date last accessed 9 June 2018 ). Google Scholar
4. Colo E , Rijnen WHC , Gardeniers JWM , van Kampen A , Schreurs BW . Satisfying Results of Primary Hip Arthroplasty in Patients With Hip Dysplasia at a Mean Followup of 20 Years . Clin Orthop Relat Res . 2016 ; 474 ( 11 ): 2462 – 2468 . Crossref PubMed Google Scholar
5. Schreurs BW , Keurentjes JC , Gardeniers JWM , et al. Acetabular revision with impacted morsellised cancellous bone grafting and a cemented acetabular component: a 20- to 25-year follow-up . J Bone Joint Surg Br . 2009 ; 91-B ( 9 ): 1148 – 1153 . Crossref PubMed Google Scholar
6. Wilkinson JM , Hamer AJ , Stockley I , Eastell R . Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship . J Orthop Res . 2005 ; 23 ( 3 ): 520 – 525 . Crossref PubMed Google Scholar
7. Dumbleton JH , Manley MT , Edidin AA . A literature review of the association between wear rate and osteolysis in total hip arthroplasty . J Arthroplasty . 2002 ; 17 ( 5 ): 649 – 661 . Crossref PubMed Google Scholar
8. Kurtz SM , Gawel HA , Patel JD . History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene . Clin Orthop Relat Res . 2011 ; 469 ( 8 ): 2262 – 2277 . Crossref PubMed Google Scholar
9. Hopper RH , Ho H , Sritulanondha S , Williams AC , Engh CA . Otto Aufranc Award: crosslinking reduces THA wear, osteolysis, and revision rates at 15-year followup compared with noncrosslinked polyethylene . Clin Orthop Relat Res . 2018 ; 476 ( 2 ): 279 – 290 . Crossref PubMed Google Scholar
10. de Steiger R , Lorimer M , Graves SE . Cross-Linked polyethylene for total hip arthroplasty markedly reduces revision surgery at 16 years . J Bone Joint Surg Am . 2018 ; 100-A ( 15 ): 1281 – 1288 . Crossref PubMed Google Scholar
11. Johanson PE , Digas G , Herberts P , Thanner J , Kärrholm J . Highly crosslinked polyethylene does not reduce aseptic loosening in cemented THA 10-year findings of a randomized study . Clin Orthop Relat Res . 2012 ; 470 ( 11 ): 3083 – 3093 . Google Scholar
12. Johanson PE , Furnes O , Ivar Havelin L , et al. Outcome in design-specific comparisons between highly crosslinked and conventional polyethylene in total hip arthroplasty . Acta Orthop . 2017 ; 88 ( 4 ): 363 – 369 . Crossref PubMed Google Scholar
13. Langlois J , Atlan F , Scemama C , Courpied JP , Hamadouche M . A randomised controlled trial comparing highly cross-linked and contemporary annealed polyethylene after a minimal eight-year follow-up in total hip arthroplasty using cemented acetabular components . Bone Joint J . 2015 ; 97-B ( 11 ): 1458 – 1462 . Crossref PubMed Google Scholar
14. No authors listed . Hip, Knee & Shoulder Arthroplasty: 2017 Annual Report . Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) . 2017 . https://aoanjrr.sahmri.com/documents/10180/397736/Hip%2C%20Knee%20%26%20Shoulder%20Arthroplasty (date last accessed 6 August 2020 ). Google Scholar
15. Kurtz SM . UHMWPE Biomaterials Handbook . Third ed . Waltham, Massachusetts : Elsevier , 2016 . Google Scholar
16. Röhrl SM , Nivbrant B , Nilsson KG . No adverse effects of submelt-annealed highly crosslinked polyethylene in cemented CUPS: an RSA study of 8 patients 10 yaers after surgery . Acta Orthop . 2012 ; 83 ( 2 ): 148 – 152 . Crossref PubMed Google Scholar
17. Kadar T , Hallan G , Aamodt A , et al. Wear and migration of highly cross-linked and conventional cemented polyethylene CUPS with cobalt chrome or Oxinium femoral heads: a randomized radiostereometric study of 150 patients . J Orthop Res . 2011 ; 29 ( 8 ): 1222 – 1229 . Crossref PubMed Google Scholar
18. Galea VP , Rojanasopondist P , Laursen M , et al. Evaluation of vitamin E-diffused highly crosslinked polyethylene wear and porous titanium-coated shell stability: a seven-year randomized control trial using radiostereometric analysis . Bone Joint J . 2019 ; 101-B ( 7 ): 760 – 767 . Crossref PubMed Google Scholar
19. Galea VP , Connelly JW , Shareghi B , et al. Evaluation of in vivo wear of vitamin E-diffused highly crosslinked polyethylene at five years: a multicentre radiostereometric analysis study . Bone Joint J . 2018 ; 100-B ( 12 ): 1592 – 1599 . Crossref PubMed Google Scholar
20. Sköldenberg OG , Rysinska AD , Chammout G , et al. A randomized double-blind noninferiority trial, evaluating migration of a cemented vitamin E-stabilized highly crosslinked component compared with a standard polyethylene component in reverse hybrid total hip arthroplasty . Bone Joint J . 2019 ; 101-B ( 10 ): 1192 – 1198 . Crossref PubMed Google Scholar
21. Glyn-Jones S , Thomas GER , Garfjeld-Roberts P , et al. The John Charnley Award: highly crosslinked polyethylene in total hip arthroplasty decreases long-term wear: a double-blind randomized trial . Clin Orthop Relat Res . 2015 ; 473 ( 2 ): 432 – 438 . Crossref PubMed Google Scholar
22. Teeter MG , Yuan X , Somerville LE , et al. Thirteen-year wear rate comparison of highly crosslinked and conventional polyethylene in total hip arthroplasty: long-term follow-up of a prospective randomized controlled trial . Can J Surg . 2017 ; 60 ( 3 ): 212 – 216 . Crossref PubMed Google Scholar
23. Davis ET , Pagkalos J , Kopjar B . Polyethylene manufacturing characteristics have a major effect on the risk of revision surgery in cementless and hybrid total hip arthroplasties . Bone Joint J . 2020 ; 102-B ( 1 ): 90 – 101 . Crossref PubMed Google Scholar
24. Teeter MG , Lanting BA , Naudie DD , et al. Highly crosslinked polyethylene wear rates and acetabular component orientation: a minimum ten-year follow-up . Bone Joint J . 2018 ; 100-B ( 7 ): 891 – 897 . Crossref PubMed Google Scholar
25. Clement ND , Bardgett M , Merrie K , et al. Cemented Exeter total hip arthroplasty with a 32 MM head on highly crosslinked polyethylene: does age influence functional outcome, satisfaction, activity, stem migration, and periprosthetic bone mineral density? Bone Joint Res . 2019 ; 8 ( 6 ): 275 – 287 . Crossref PubMed Google Scholar
26. Lachiewicz PF , Soileau ES , Martell JM . Wear and Osteolysis of Highly Crosslinked Polyethylene at 10 to 14 Years: The Effect of Femoral Head Size . Clin Orthop Relat Res . 2016 ; 474 ( 2 ): 365 – 371 . Crossref PubMed Google Scholar
27. Ranawat CS , Deshmukh RG , Peters LE , Umlas ME . Prediction of the long-term durability of all-polyethylene cemented sockets . Clin Orthop Relat Res . 1995 ; 317 : 89 – 105 . PubMed Google Scholar
28. Flivik G , Kristiansson I , Kesteris U , Ryd L . Is removal of subchondral bone plate advantageous in cemented cup fixation? A randomized RSA study . Clin Orthop Relat Res . 2006 ; 448 : 164 – 172 . Crossref PubMed Google Scholar
29. Orishimo KF , Claus AM , Sychterz CJ , Engh CA . Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up . J Bone Joint Surg Am . 2003 ; 85-A ( 6 ): 1095 – 1099 . Crossref PubMed Google Scholar
30. Sayers A , Evans JT , Whitehouse MR , Blom AW . Are competing risks models appropriate to describe implant failure? Acta Orthop . 2018 ; 89 ( 3 ): 256 – 258 . Crossref PubMed Google Scholar
31. Lamb JN , Baetz J , Messer-Hannemann P , et al. A calcar collar is protective against early periprosthetic femoral fracture around cementless femoral components in primary total hip arthroplasty: a registry study with biomechanical validation . Bone Joint J . 2019 ; 101-B ( 7 ): 779 – 786 . Crossref PubMed Google Scholar
32. Palan J , Smith MC , Gregg P , et al. The influence of cemented femoral stem choice on the incidence of revision for periprosthetic fracture after primary total hip arthroplasty: an analysis of national joint registry data . Bone Joint J . 2016 ; 98-B ( 10 ): 1347 – 1354 . Crossref PubMed Google Scholar
Author contributions
E. T. Davis: Conceptualized and designed the study, Acquired and interpreted the data, Drafted the manuscript.
J. Pagkalos: Conceptualized and designed the study, Acquired and interpreted the data, Drafted the manuscript.
B. Kopjar: Conceptualized and designed the study, Acquired and interpreted the data, Drafted the manuscript.
Funding statement
This study was supported by an unrestricted research grant from Smith & Nephew, Inc.The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
Professor Davis reports grants from Smith & Nephew during the conduct of the study, speaker fees from Smith & Nephew, paid travel from Stryker and Corin, and research grants from Zimmer Biomet. Mr. Pagkalos reports grants from Smith & Nephew during the conduct of the study. Dr. Kopjar reports grants from Smith & Nephew during the conduct of the study, other support from Hip Innovation Technology, and grants and other support from Smith & Nephew outside the submitted work.
Acknowledgements
The authors would like to thank the patients and staff of all the hospitals in England, Wales, Northern Ireland, and the Isle of Man who have contributed data to the National Joint Registry (NJR). The authors are also grateful to the Healthcare Quality Improvement Partnership (HQIP), the NJR Research Sub-committee, and NJR Centre staff for facilitating this work. The authors would also like to thank Keith Tucker (Spire Hospital Norwich, Norwich, UK), Martin Pickford (Craneswater Consulting, Locks Heath, UK), and Claire Newell (Northgate Information Solutions Ltd, Hemel Hempstead, UK). The authors have conformed to the standard protocol of NJR for data access and publication. The views expressed represent those of the authors and do not necessarily reflect those of the NJR Steering Committee or the HQIP, who did not vouch for how the information was presented. In addition, the authors are grateful for the support of the implant manufacturers in supplying the details on their polyethylene manufacturing. Additionally, the authors thank Karen K. Anderson, BS, employed by Nor Consult, LLC (Tukwila, Washington, USA), for her assistance with the preparation and editing of this manuscript.
Ethical review statement
An application for the study was approved by the National Joint Registry (NJR) Research Committee and the Trust clinical governance department.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









