Abstract
A balanced inflammatory response is important for successful fracture healing. The response of osteoporotic fracture healing is deranged and an altered inflammatory response can be one underlying cause. The objectives of this review were to compare the inflammatory responses between normal and osteoporotic fractures and to examine the potential effects on different healing outcomes. A systematic literature search was conducted with relevant keywords in PubMed, Embase, and Web of Science independently. Original preclinical studies and clinical studies involving the investigation of inflammatory response in fracture healing in ovariectomized (OVX) animals or osteoporotic/elderly patients with available full text and written in English were included. In total, 14 articles were selected. Various inflammatory factors were reported; of those tumour necrosis factor-α (TNF-α) and interleukin (IL)-6 are two commonly studied markers. Preclinical studies showed that OVX animals generally demonstrated higher systemic inflammatory response and poorer healing outcomes compared to normal controls (SHAM). However, it is inconclusive if the local inflammatory response is higher or lower in OVX animals. As for clinical studies, they mainly examine the temporal changes of the inflammatory stage or perform comparison between osteoporotic/fragility fracture patients and normal subjects without fracture. Our review of these studies emphasizes the lack of understanding that inflammation plays in the altered fracture healing response of osteoporotic/elderly patients. Taken together, it is clear that additional studies, preclinical and clinical, are required to dissect the regulatory role of inflammatory response in osteoporotic fracture healing.
Cite this article: Bone Joint Res 2020;9(7):368–385.
Article focus
-
The early inflammatory response in osteoporotic/aged fracture healing is poorly understood.
-
This systematic review aimed to analyze the difference in inflammatory responses in normal and osteoporotic fractures.
Key messages
-
The inflammatory response in osteoporotic/aged fracture healing is altered and this could imply poorer healing in ovariectomized (OVX) subjects or postmenopausal osteoporotic or aged individuals with fragility fracture.
-
Further studies should be performed to explore the role of inflammatory response in the healing cascade for potential immunomodulation to enhance osteoporotic fracture healing.
Strengths and limitations
-
This study systematically reviewed both the local and systemic inflammatory responses in ovariectomy-induced osteoporotic fractures or fragility fractures and the potential effects of inflammatory response on differential healing outcomes.
-
There is no clinical study investigating the difference in the inflammatory response between osteoporotic and non-osteoporotic patients.
Introduction
The process of fracture healing is highly complex. The success of fracture healing relies on the well-coordinated stages of inflammation, callus formation, and remodelling. When a bone fracture occurs, an inflammatory (innate immune) response immediately occurs to trigger and coordinate all the subsequent healing processes such as recruitment of reparative mesenchymal stem cells (MSCs),1,2 neoangiogenesis at the callus,3,4 endochondral ossification,5 and callus remodelling.6,7 Different immune cells infiltrate the fracture site and multiple inflammatory factors are secreted. This intense inflammatory interaction has been shown to initiate the repair phase8 and the cytokines, signalling molecules, receptors, and transcription factors involved enabling a wide range of dynamic cross-talk between cells of monocyte-macrophage-osteoclast and MSC-osteoblast lineages, which are essential to bone remodelling.9,10 Thus, the inflammatory stage is important to ensure normal fracture healing through angiogenesis, repair of injured tissues, and eventually remodelling.11,12 Any disruption of this inflammatory response could result in impaired or delayed fracture healing.13-16
Previous studies reported that ageing could affect the inflammatory response during fracture healing in terms of ageing of the immune response known as immunosenescence17,18 and increased levels of circulating proinflammatory cytokines.19 Some important cells in fracture healing such as macrophages,20,21 T cells,22 and MSCs23,24 have all been demonstrated to undergo intrinsic age-related changes that could have a negative impact on fracture healing. In addition, bone healing in aged individuals has a higher risk of failure, resulting in an increased incidence of fracture nonunions.25
Postmenopausal osteoporosis has been considered as a chronic inflammatory disease.26 Postmenopausal osteoporotic women have been shown to exhibit loss of bone-protective role of oestrogen and increased levels of proinflammatory cytokines.27-30 Oestrogen has been shown to have both immunosuppressive and proinflammatory effects.31,32 Tumour necrosis factor-α (TNF-α) and interleukin (IL)-6 are both mediated by the level of oestrogen indirectly through Jun or nuclear factor kappa B (NF-κB) pathways, respectively.27 Moreover, oestrogen was reported to modulate inflammatory responses through oestrogen receptors (ERs), and ERs were found to demonstrate different expression patterns in osteoporotic fractures.7 Following fracture injury in wild-type rats, ER expression increased during the earliest stage of repair. This response was abrogated in ovariectomized (OVX) animals, suggesting that oestrogen may function in coordination with inflammation for the altered repair response in these animals. The local inflammatory response to injury was lowered in OVX animal models mimicking oestrogen decline after menopause,33 while there are other studies demonstrating that oestrogen deficiency increased the early inflammatory response after injury as shown by increased numbers of neutrophils and expression of proinflammatory cytokines, midkine, and IL-6 in the fracture callus in oestrogen-deficient mice.34,35 Taken together, these studies provide evidence that oestrogen deficiency, such as in post-menopausal women, alters the inflammatory response following fracture injury, but the mechanisms of this response are still vaguely understood.
Despite some evidence on the altered inflammatory response observed in postmenopausal osteoporotic patients or OVX animals after injury, still little is known about the early inflammatory response in fragility (osteoporotic) fracture healing, which could be a potential target stage for accelerating osteoporotic fractures accompanied usually with delayed healing or even nonunions. The purpose of this systematic review was to analyze the difference in inflammatory responses in normal and osteoporotic fractures and explore the possible influence of inflammatory response on healing outcomes. This could serve as a reference for the planning of potential inflammation-related therapeutic approaches for osteoporotic fracture patients.
Methods
Search strategy
Literature search was performed on PubMed, Embase, and Web of Science (last access date on 8 January 2020). The keywords included were “osteoporo*”, “inflammat*”, and “fracture*”. We combined the keywords as “osteoporo* AND inflammat* AND fracture*” and searched in all fields. This search strategy was used in the three databases.
Search criteria
Inclusion criteria were: 1) preclinical or clinical studies that involved osteoporotic or fragility fracture and assessments examining the inflammatory response after fracture; 2) involves the comparison between the inflammatory response in OVX-induced osteoporotic and normal control (SHAM) subjects in preclinical studies; 3) involves the examination of inflammatory response in osteoporotic or fragility fracture in clinical studies; and 4) literature published in English with full text.
Exclusion criteria were: 1) non-English papers; 2) non-fracture-related; 3) non-inflammatory response-related; 4) involves other diseases; 5) review articles; 6) reports published as conference abstracts; and 7) in vitro studies.
Selection of studies
Study selection was conducted by two reviewers (YNC and VMHC) independently. Primary screening of titles and abstracts was performed to exclude obviously irrelevant papers. Potentially relevant articles were then retrieved and reviewed according to the inclusion and exclusion criteria. Disagreements were resolved by discussion and consensus.
Data extraction
The following information was extracted by reviewers: preclinical studies including methodology, fracture models used, sample size, species, fracture location, fracture type, fixation type, inflammatory factor findings, and fracture healing outcomes; and clinical studies including subject age, sample size, fracture type, fracture site, inflammatory factor findings, and fracture healing outcomes.
Statistical analysis
Both clinical and preclinical studies were included in this review and there was variability in terms of human subjects, animal species, and methodological and statistical heterogeneity. Outcome measures of human subjects and animal species were not comparable. Thus, only qualitative systematic review was performed.
Results
Results of the search
A total of 1,028, 811, and 1,195 studies were identified from PubMed, Embase, and Web of Science, respectively. After reviewing titles and abstracts of the 3,034 studies, 644 studies were excluded due to overlap and 2,352 studies were excluded based on the selection criteria, leaving 38 studies for further examination. After further screening, 24 more studies were excluded, out of which nine were conference reports, three did not involve osteoporosis, two did not involve fracture, and one was not inflammatory response-related. Eight studies only involved baseline data taken before fracture occurred but not data after fracture. One used a drill-hole injury model. Hence, in total, 14 studies were finally analyzed in this systematic review. The literature screening process is illustrated in the flow diagram in Figure 1.

Fig. 1
Flow diagram showing the literature search process.
Characteristics of the papers
The 14 studies were published between 2004 and 2019. Six of these studies were preclinical studies and seven were clinical studies. One paper included both preclinical and clinical studies.
The seven (including the one with preclinical and clinical studies) preclinical studies included four mouse studies and three rat studies. All these studies used female animals, which received OVX surgery for induction of osteoporosis. Fractures were created in the animals. Two studies performed open osteotomy with external fixator, two studies performed open osteotomy with intramedullary pinning, and three used closed fracture with intramedullary pinning. Four studies involved fracture on the femur of the animals and three used tibia fracture model (Table I).
Table I.
Summary of the study characteristics.
| Included studies | Type | Study design | Species | Strain | Age | Fracture site | Fracture type | Fixation type | Timepoint | Inflammatory factor |
|---|---|---|---|---|---|---|---|---|---|---|
| Chan et al37 (2015) | PC | Day 14: OVX + 1 ng TNF (n = 6); OVX + PBS (n = 6), Day 28: OVX + 1 ng TNF (n = 7); OVX + PBS (n = 7) | Mouse (Female) | C57BL/6 J | 12 to 14 wks old | Tibia | Osteotomy | Intramedullary fixation pin | Days 14, 28 | Local administration of rhTNF |
| Chow et al38 (2019) | PC | SHAM (n = 6); OVX (n = 6) | Rat (Female) | SD | 9 mths old | Femur | Closed femoral fracture | Intramedullary fixation pin | Wks 1, 2, 4, and 8 | TNF, IL-6, IL-10 |
| Fischer et al34 (2018) | PC | SHAM & OVX (6 hrs: SHAM n = 5, OVX n = 7; Day 1: SHAM n = 8, OVX n = 6; Day 2: SHAM n = 5, OVX n = 5; Day 3: SHAM n = 6, OVX n = 5) | Mouse (Female) | C57BL/6 J | 5 to 6 mths old | Femur mid-shaft | Osteotomy | External fixator with screws | 6 hrs, Days 1, 2, 3 | IL-6, IL-1β, IL-13, IL-4, CXCL1, MCP-1, MIP-1α |
| Haffner-Luntzer et al35 (2017) | PC | SHAM & OVX | Mouse (Female) | C57BL/6 J | 5 to 6 mths old | Femur mid-shaft | Osteotomy | External fixator with screws | Days 1, 3, 23 | Mdk, IL-6, CCL2, CXCL1, macrophages, neutrophils, monocytes, B-lymphocytes, T-lymphocytes (cytotoxic T- lymphocytes and T-helper-lymphocytes) |
| Wang F et al39 (2018) | PC | SHAM (n = 54); OVX (n = 48) | Mouse (Female) | C57BL/6 J | 21 wks old | Proximal third of the right tibia diaphysis | Transverse osteotomy | Intramedullary pinning | Days 3, 5, 11 | TNF-α, IL-1β |
| Wang J et al41 (2018) | PC | SHAM (n = 15); OVX (n = 15) | Rat (Female) | SD | 24 wks old | Femur | Closed femoral fracture | Intramedullary pinning | 6 wks post fracture | TNF-α, IL-6 |
| Zhang et al40 (2019) | PC | SHAM (n = 10); OVX (n = 10) | Rat (Female) | Wistar | Adult | Tibia | Unilateral cross-tibial fracture | Intramedullary pinning | 5 wks post fracture | TNF-α, IL-1β |
| Caetano-Lopes et al63 (2011) | C | Patients suffering a hip fragility fracture (those who had surgery less than 3 days after fracture (n = 13); between 4 and 7 days (n = 33); 8 or more days (n = 10) | N/A | N/A | Mean 80 yrs old (SD 7) | Hip | Fragility fracture | N/A | Received hip arthroplasty surgery less than 3 days after fracture; 4 to 7 days; 8 or more days | TNF, IL-6, IL-1β |
| Fischer et al34 (2018) | C | Patients with metaphyseal/diaphyseal fractures of long bones (Day 0: n = 26; Day 14: n = 7; Day 42: n = 4); Healthy controls (n = 20); Subgroup Day 0: Male fracture patients (n = 6); Female fracture patients after menopause (n = 13) | N/A | N/A | 32 to 97 yrs, mean = 75 yrs; Subgroup Day 0: Male patients: 32 to 97 yrs, mean: 69 yrs; Female patients: 57 to 87 yrs, mean: 78 yrs | Femur, tibia, humeri, radii, ulna | Long-bone fracture | N/A | Days 0, 14, 42 | Mdk |
| Ginaldi et al28 (2015) | C | Fractured women with osteoporosis (n = 15); unfractured women (n = 41) with osteoporosis; age-matched healthy post-menopausal women (n = 26) | N/A | N/A | 65 yrs old or above | Multiple | Fragility fracture | N/A | N/A | IL-31 |
| Lipovetzki et al69 (2017) | C | Postmenopausal women: Group 1 - Hospitalized from Jan 2008 to Dec 2012 due to diagnosis of non-traumatic hip fracture (n = 49); Group 2 - without history of hip fracture (n = 66) | N/A | Hip | Group 1: average age: 65.1 yrs old; Group 2: average age: 78.4 yrs old | Hip | Non-traumatic hip fracture | N/A | N/A | CRP |
| Meyer et al70 (2014) | C | Women (n = 18 (7 osteoporotic; 8 osteopenic by DXA; 2 no DXA data)) | N/A | N/A | 50 yrs old or above | Distal radii | Stable distal radii fracture | N/A | 1 to 2, 3 to 4, 6 to 8, and 12 wks post fracture | hsCRP |
| Pesic et al64 (2017) | C | Elderly patients with femoral neck fracture (n = 65) | N/A | N/A | Older than 65 yrs; Mean: 79.8 yrs | Femoral neck | Femoral neck fracture | N/A | 12 hrs after fracture, first, third, and seventh postoperative days | TNF-α, IL-6 |
| Tsangari et al66 (2004) | C | Patients undergoing hip arthroplasty surgery for a subcapital fractured neck of femur) – all characterized as having fragility fracture (n = 13); Control (from postmortem) (n = 13) | N/A | N/A | 70 yrs old or above | Intertrochanteric region of the proximal femur | Subcapital fractured neck of femur | N/A | Up to 6 days after fracture | IL-6 |
| Saribal et al65 (2019) | C | Osteoporotic patients with hip fracture (n = 40); Age-matched non-osteoporotic healthy controls (n = 40) | N/A | N/A | Patients (mean 74 yrs (SD 1)); Controls (mean 67 yrs (SD 7)) | Hip | Hip fracture due to osteoporosis | N/A | Before receiving surgery; On the first and second days of postoperative period | TNF-α, IL-6 |
-
C, clinical; CCL2, chemokine (C-C motif) ligand 2; CXCL1, chemokine (C-X-C motif) ligand 1; hsCRP, high-sensitive CRP; IL, interleukin; DXA, dual energy X-ray absorptiometry; MCP-1, monocyte chemoattractant protein 1; Mdk, midkine; MIP-1α, macrophage inflammatory protein 1 alpha; N/A, not applicable; OVX, ovariectomy; PBS, phosphate-buffered saline; PC, preclinical; rhTNF, recombinant human tumour necrosis factor; SD, Sprague-Dawley; SHAM, normal control; TNF, tumour necrosis factor
As for the clinical studies, all included subjects with fragility fracture, of which only two studies involved patients confirmed with osteoporosis by dual energy x-ray absorptiometry (DXA). Three included patients suffering from hip fracture and one involved patients having subcapital fracture at the intertrochanteric region of the proximal femur. One included distal radius fracture, one included long-bone fracture, and one involved femoral neck fracture. One study did not specify the fractured region of patients (Table I).
Inflammatory factors
Preclinical studies
Five out of seven preclinical studies (Table II) included TNF-α, a proinflammatory cytokine that enhances mesenchymal stromal cell (MSC) recruitment and osteogenic differentiation.36 Chan et al37 reported that in OVX mice, treatment with 1 ng recombinant human tumour necrosis factor (rhTNF) at the fracture site at days 0 and 1 resulted in an increased relative callus mineralization at day 14 post-fracture compared to SHAM mice. However, healing was found equivalent between the two groups at day 28. Chow et al38 revealed that TNF-α level at the fracture callus by immunohistochemistry (IHC) was significantly lower compared to SHAM rats at week 1 post-fracture, while the serum level of TNF-α by enzyme-linked immunosorbent assay (ELISA) test was significantly higher in OVX rats at weeks 1 and 2.38 Wang F et al39 showed that the serum level of TNF-α by ELISA test was significantly higher in OVX mice at day 5 compared to SHAM mice but no significant difference was found at day 3 post-fracture. The level of TNF-α in serum by ELISA test was also found to be significantly higher in OVX rats at week 5 and week 6 by Zhang et al40 and Wang J et al,41 respectively.
Table II.
Summary of inflammatory factors in the included preclinical studies. All factors were compared between ovariectomized and normal control fractured animals.
| Inflammatory factor | Type | Role in fracture healing | Included studies | Type | Experiment | Systemic/local (Location) | Finding |
|---|---|---|---|---|---|---|---|
| TNF-α | Proinflammatory cytokine | Enhance MSC recruitment and proliferation and osteoblast differentiation | Chan et al37 2015 | PC | Treatment with 1 ng rhTNF on days 0 and 1 (immediately and 24 hrs) | Local injection at the fracture site | Increased relative callus; mineralization observed in OVX* mice on day 14 compared to SHAM* mice; equivalent healing on day 28 between groups |
| Chow et al38 2019 | PC | IHC | Local (fracture callus) | Significantly lower in OVX group compared to SHAM group at week 1 | |||
| ELISA | Systemic (serum) | Significantly higher in OVX group compared to SHAM group at weeks 1 and 2 | |||||
| Wang F et al39 2018 | PC | ELISA | Systemic (serum) | Similar between SHAM† and OVX† mice on day 3; significantly higher in OVX mice on day 5 | |||
| Wang J et al41 2018 | PC | ELISA | Systemic (serum) | Significantly higher in OVX* rats at week 6 compared to SHAM* rats | |||
| Zhang et al40 2019 | PC | ELISA | Systemic (serum) | Significantly higher in OVX rats at week 5 compared to normal control rats | |||
| IL-6 | Proinflammatory cytokine | Stimulate angiogenesis and MSCs to differentiate towards the osteoblast lineage; modulate osteoblast and osteoclast activities | Chow et al38 2019 | PC | IHC | Local (fracture callus) | Significantly lower in OVX group compared to SHAM at week 1 |
| ELISA | Systemic (serum) | Significantly lower in OVX group compared to SHAM at week 8 | |||||
| Fischer et al34 2018 | PC | Multiplex cytokine assay | Systemic (plasma) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 | |||
| ELISA | Local (fracture callus) | Significantly higher in OVX mice on day 3 compared to SHAM mice | |||||
| Haffner-Luntzer et al35 2017 | PC | IHC | Local (fracture callus) | Increased expression in OVX mice on day 3 compared to SHAM mice | |||
| Wang J et al41 2018 | PC | ELISA | Systemic (serum) | Significantly higher in OVX* rats at week 6 compared to SHAM* rats | |||
| IL-1β | Proinflammatory cytokine | Stimulate osteoblast proliferation and production of mineralized bone matrix; inhibit proliferation and differentiation of chondrocytes | Fischer et al34 2018 | PC | ELISA | Local (fracture callus) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 |
| Wang F et al39 2018 | PC | ELISA | Systemic (serum) | Similar between SHAM† and OVX† mice on day 3; significantly higher in OVX mice on day 5 | |||
| Zhang et al40 2019 | PC | ELISA | Systemic (serum) | Significantly higher in OVX rats at week 5 compared to normal control rats | |||
| IL-10 | Anti-inflammatory cytokine | Suppress excessive or prolonged inflammation | Chow et al38 2019 | PC | IHC | Local (fracture callus) | Significantly higher in OVX group at week 1 and lower at week 4 compared to SHAM |
| ELISA | Systemic (serum) | Significantly lower in OVX group compared to SHAM at week 2 | |||||
| IL-13 | Anti-inflammatory cytokine | Involved in osteoblast recruitment | Fischer et al34 2018 | PC | Multiplex cytokine assay | Systemic (plasma) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 |
| IL-4 | Anti-inflammatory cytokine | Inhibit monocyte production of IL-1 and TNF-α; chemoattract osteoblasts; inhibit bone resorption | Fischer et al34 2018 | PC | ELISA | Local (fracture callus) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 |
| Neutrophil | Immune cell | Secrete proinflammatory and immunomodulatory cytokines and MCP-1 to attract monocytes; involved in fibrin thrombus | Haffner-Luntzer et al35 2017 | PC | FACS | Systemic (bone marrow) | Significantly decreased on day 1 in OVX mice |
| FACS | Local (fracture haematoma) | Similar between SHAM and OVX mice on day 1 | |||||
| IHC | Local (fracture callus) | Significantly increased on day 3 in OVX mice | |||||
| Macrophage | Immune cell | Undergo polarization and modulate the inflammatory phase; produce growth factors such as BMP-2; induce osteogenesis of mesenchymal progenitor cells | Haffner-Luntzer et al35 2017 | PC | FACS | Systemic (bone marrow) | Similar between SHAM and OVX mice on day 1 |
| FACS | Local (fracture haematoma) | Similar between SHAM and OVX mice on day 1 | |||||
| IHC | Local (fracture callus) | Similar between SHAM and OVX mice on day 3 | |||||
| Inflammatory monocyte | Immune cell | Differentiate into macrophages in tissues | Haffner-Luntzer et al35 2017 | PC | FACS | Systemic (bone marrow) | Similar between SHAM and OVX mice on day 1 |
| FACS | Local (fracture haematoma) | Similar between SHAM and OVX mice on day 1 | |||||
| B-lymphocyte | Immune cell | Suppress proinflammatory signals; produce OPG to regulate osteoclastic differentiation and activity | Haffner-Luntzer et al35 2017 | PC | FACS | Systemic (bone marrow) | Significantly higher on day 1 in OVX mice |
| FACS | Local (fracture haematoma) | Similar between SHAM and OVX mice on day 1 | |||||
| IHC | Local (fracture callus) | Similar between SHAM and OVX mice on day 3 | |||||
| T-lymphocyte | Immune cell | Produce RANKL to recruit, differentiate, and activate osteoclasts; secrete IL-17 to induce anti-inflammatory functions from MSCs and induce osteogenic differentiation and activity | Haffner-Luntzer et al35 2017 | PC | FACS | Systemic (bone marrow) | Significantly reduced in OVX mice on day 1 (cytotoxic T-cells significantly reduced, whereas T-helper cells significantly increased) |
| FACS | Local (fracture haematoma) | Similar between SHAM and OVX mice on day 1 | |||||
| IHC | Local (fracture callus) | No difference in cytotoxic T-cells on day 3 | |||||
| Mdk | Proinflammatory cytokine | Chemoattract neutrophils and macrophages | Fischer et al34 2018 | PC | Multiplex cytokine assay | Systemic (plasma) | Significantly higher in OVX mice on day 3 compared to SHAM mice |
| ELISA | Local (fracture callus) | Significantly higher in OVX mice on day 3 compared to SHAM mice | |||||
| Haffner-Luntzer et al35 2017 | PC | IHC | Local (fracture callus) | Increased expression in OVX mice on day 3 compared to SHAM mice | |||
| CCL2 | Chemokine | Regulate migration and infiltration of monocytes/macrophages | Haffner-Luntzer et al35 2017 | PC | IHC | Local (fracture callus) | Similar between SHAM and OVX mice on day 3 |
| MCP-1 | Fischer et al34 2018 | PC | Multiplex cytokine assay | Systemic (plasma) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 | ||
| ELISA | Local (fracture callus) | Significantly higher in OVX mice on day 3 compared to SHAM mice | |||||
| CXCL1 | Proinflammatory cytokine | Recruit neutrophils | Fischer et al34 2018 | PC | Multiplex cytokine assay | Systemic (plasma) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 |
| ELISA | Local (fracture callus) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 | |||||
| Haffner-Luntzer et al35 2017 | PC | IHC | Local (fracture callus) | Similar between SHAM and OVX mice on day 3 | |||
| MIP-1α | Chemokine | Recruit monocytes | Fischer et al34 2018 | PC | ELISA | Local (fracture callus) | Similar between SHAM and OVX mice at 6 hrs, day 1, 2, 3 |
-
BMP-2, bone morphogenetic protein 2; CCL2, chemokine (C-C motif) ligand 2; CXCL1, chemokine (C-X-C motif) ligand 1; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IHC, immunohistochemistry; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; Mdk, midkine; MIP-1α, macrophage inflammatory protein 1 α; MSC, mesenchymal stromal cell; OPG, osteoprotegerin; OVX, ovariectomy; PC, preclinical; RANKL, receptor activator of nuclear factor kappa-B ligand; rhTNF, recombinant human tumour necrosis factor; SHAM, normal control; TNF-α, tumour necrosis factor-α.
-
*
Treated with phosphate buffered saline (PBS), no other treatment added.
-
†
Treated with physiological saline.
Four out of seven preclinical studies included the proinflammatory cytokine IL-6, which stimulates angiogenesis42 and MSCs to differentiate towards the osteoblast lineage43 and modulates osteoblast and osteoclast activities.44 Chow et al38 demonstrated that IL-6 level at the fracture callus by IHC was significantly lower in OVX rats compared to SHAM rats at week 1 post-fracture. Significantly lower serum level of IL-6 was detected by ELISA test in OVX rats at week 8.38 Fischer et al34 showed that IL-6 level at the fracture callus by ELISA test was significantly higher compared to SHAM mice on day 3 post-fracture, while no significance was detected in plasma IL-6 level by multiplex cytokine assay at six hours and days 1, 2, and 3 after fracture. Haffner-Luntzer et al35 reported that IL-6 expression by immunostaining was increased in periosteal callus in OVX mice compared to SHAM mice on day 3 post-fracture. Wang J et al41 found that the serum level of IL-6 by ELISA test was significantly higher in OVX rats at week 6.
Three studies examined the proinflammatory cytokine IL-1β, which stimulates osteoblast proliferation and production of mineralized bone matrix as well as inhibiting proliferation and differentiation of chondrocytes.45 Fischer et al34 reported that its levels at the fracture callus by ELISA test were similar between SHAM and OVX mice at six hours and days 1, 2, and 3 post-fracture. Wang F et al39 showed that the serum level of IL-1β by ELISA test was significantly higher in OVX mice at day 5 compared to SHAM mice but no significant difference was detected at day 3 post-fracture. The level of IL-1β in serum by ELISA test was also found to be significantly higher in OVX rats at week 5 by Zhang et al.40
One study found that the anti-inflammatory cytokine IL-10, which suppresses excessive or prolonged inflammation.46 Chow et al38 reported that IL-10 level at the fracture callus by IHC was significantly higher at week 1 but lower at week 4 in OVX rats compared to SHAM rats. Significantly lower serum IL-10 level was detected by ELISA test in OVX rats at week 2.38
The anti-inflammatory cytokine IL-13 was investigated in another study, which is closely related to IL-447 and involved in osteoblast recruitment.48 Fischer et al34 reported that its levels in plasma by multiplex cytokine assay were similar between SHAM and OVX mice at six hours and days 1, 2, and 3 post-fracture.
The anti-inflammatory cytokine IL-4 was investigated in an additional study; this inhibits monocyte production of IL-1 and TNF-α,49 chemoattracts osteoblasts,48 and inhibits bone resorption.50 Fischer et al34 reported that its levels at the fracture callus by ELISA test were similar between SHAM and OVX mice at six hours and days 1, 2, and 3 post-fracture.34
One study investigated the immune cell neutrophils reported to secrete proinflammatory and immunomodulatory cytokines such as IL-1, IL-6, TNF-α, IL-10, and monocyte chemoattractant protein 1 (MCP-1) to attract monocytes and be involved in fibrin thrombus.51 Haffner-Luntzer et al35 showed that the number of neutrophils by fluorescent-activated cell sorting (FACS) analysis significantly decreased in the bone marrow of OVX mice at day 1 post-fracture, although no significant difference was found in the fracture haematoma. Besides, OVX mice displayed significantly greater numbers of neutrophils in the periosteal callus at day 3 post-fracture.35
Macrophages were analyzed in another study; these undergo polarization and modulate the inflammatory phase,52 produce growth factors such as bone morphogenetic protein 2 (BMP-2),53 and induce osteogenesis of mesenchymal progenitor cells.54 Haffner-Luntzer et al35 found that the number of macrophages in the fracture haematoma and bone marrow by FACS analysis at day 1 post-fracture, and that at the marrow cavity near the fracture gap by immunostaining at day 3 post-fracture, were similar between SHAM and OVX mice.
One study involved the analysis of inflammatory monocytes that differentiate into macrophages in tissues.52 Haffner-Luntzer et al35 found that the number of inflammatory monocytes in the bone marrow and fracture haematoma of OVX mice was not significantly different compared to SHAM mice at day 1 post-fracture.
Another study involved the analysis of B-lymphocytes, which suppress proinflammatory signals of TNF-α and IL-255 and produce osteoprotegerin (OPG) to regulate osteoclastic differentiation and activity.56 Haffner-Luntzer et al35 demonstrated that at day 1 post-fracture, the number of B-lymphocytes by FACS analysis was significantly higher in the bone marrow of OVX mice but similar between SHAM and OVX mice in the fracture haematoma. Also, the expression of B-lymphocytes by immunostaining was not found to be significantly altered at the periosteal callus at day 3 post-fracture.35
One study involved the analysis of T-lymphocytes, which produce receptor activator of nuclear factor kappa-B ligand (RANKL) to recruit, differentiate, and activate osteoclasts57 and secrete IL-17 to induce anti-inflammatory functions from MSCs58 and induce osteogenic differentiation and activity.59 Haffner-Luntzer et al35 found that the number of T-lymphocytes was significantly lower by FACS analysis in the bone marrow, but no significant difference was detected in the fracture haematoma in OVX mice at day 1 post-fracture. Within the T-lymphocyte population in the bone marrow, cytotoxic T-cells were significantly reduced whereas T-helper cells were significantly increased in OVX mice. However, the number of cytotoxic T-lymphocytes at the periosteal callus by immunostaining was similar between SHAM and OVX mice at day 3 post-fracture.35
Two studies examined the proinflammatory cytokine midkine (Mdk) reported to chemoattract neutrophils and macrophages.60 Fischer et al34 found that OVX mice showed significantly higher Mdk levels in plasma by multiplex cytokine assay and at the fracture site by ELISA test compared to SHAM mice at day 3 after fracture. Haffner-Luntzer et al35 showed that Mdk was expressed more in the fracture callus in OVX mice compared to SHAM-operated animals at day 3 post-fracture.
Two studies examined the proinflammatory cytokine chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP-1), which regulates migration and infiltration of monocytes/macrophages.61 Fischer et al34 found that its level at the fracture callus by ELISA test was significantly higher in OVX mice compared to SHAM mice on day 3 post-fracture, but no significance was detected in plasma MCP-1 level by multiplex cytokine assay at six hours and days 1, 2, and 3 after fracture.34 Haffner-Luntzer et al35 found that the protein expression of CCL2 at the fracture callus was similar between SHAM and OVX mice at day 3 post-fracture.
Two studies examined the proinflammatory cytokine chemokine (C-X-C motif) ligand 1 (CXCL1), which is found to recruit neutrophils.62 Fischer et al34 reported that its levels in plasma by multiplex cytokine assay and at the fracture site by ELISA test were similar between SHAM and OVX mice at six hours and days 1, 2, and 3 post-fracture. Haffner-Luntzer et al35 found that CXCL1 protein expression at the fracture callus was similar between SHAM and OVX mice at day 3 post-fracture.
One study involved the analysis of macrophage inflammatory protein 1 α (MIP-1α), which recruits monocytes.51 Fischer et al34 reported that MIP-1α level at the fracture callus by ELISA test was similar between SHAM and OVX mice at six hours and days 1, 2, and 3 post-fracture.34
Clinical studies
Among all clinical studies included in this review (Table III), there was no clinical study investigating the difference in inflammatory response between osteoporotic and non-osteoporotic patients. All clinical data presented could, however, provide reference data for the temporal changes of common inflammatory cytokines in osteoporotic fracture patients.
Table III.
Summary of inflammatory factors in the included clinical studies.
| Inflammatory factor | Type | Role in fracture healing | Included studies | Experiment | Systemic/local (Place) | Fracture site | Finding |
|---|---|---|---|---|---|---|---|
| TNF | Proinflammatory cytokine | Enhance MSC recruitment and osteogenic differentiation | Caetano-Lopes et al63 2011 | qPCR | Local (Bone specimen) | Hip | Similar gene expression between the first 3 days, days 4 to 7, and days 8 or more post-fracture |
| TNF-α | Pesic et al64 2017 | ELISA | Systemic (Plasma) | Femoral neck | Similar between 12 hours after fracture, and days 1, 3, and 7 after surgical intervention | ||
| Saribal et al65 2019 | Quantitative sandwich enzyme immunoassay | Systemic (Plasma) | Hip | Similar level in fracture patients compared to healthy controls before and after surgery | |||
| IL-6 | Proinflammatory cytokine | Stimulate angiogenesis and MSCs to differentiate towards the osteoblast lineage; modulate osteoblast and osteoclast activities | Caetano-Lopes et al63 2011 | qPCR | Local (Bone specimen) | Hip | Gene expression was highest for the first 3 post-fracture days compared to days 4 to 7 and days 8 or more after fracture |
| Fischer et al34 2018 | ELISA | Systemic (Serum) | Long bone | Significantly higher in fracture patients at days 0 and 14 compared to healthy controls; similar to controls at day 42 | |||
| Pesic et al64 2017 | ELISA | Systemic (Plasma) | Femoral neck | Significantly increased at day 1 after surgical intervention compared to 12 hours after fracture and days 3 and 7 after intervention | |||
| Saribal et al65 2019 | Quantitative sandwich enzyme immunoassay | Systemic (Plasma) | Hip | Similar level in fracture patients compared to healthy controls before surgery; significantly higher in fracture patients on the first and second days of postoperative period | |||
| Tsangari et al66 2004 | qPCR | Local (Bone specimen) | Neck of femur | Significantly higher in fracture group compared to no-fracture group; mRNA levels associated with RANKL and RANK mRNA levels | |||
| IL-1β | Proinflammatory cytokine | Stimulate osteoblast proliferation and production of mineralized bone matrix; inhibit proliferation and differentiation of chondrocytes | Caetano-Lopes et al63 2011 | qPCR | Local (Bone specimen) | Hip | Similar gene expression between the first 3 days, days 4 to 7, and days 8 or more post-fracture |
| IL-31 | Proinflammatory cytokine | Induce the release of the proinflammatory cytokines such as IL-1β and IL-6 and chemokines such as CCL2 and CXCL1 | Ginaldi et al28 2015 | ELISA | Systemic (Serum) | (Not specified) | Higher in fractured women with osteoporosis compared to age-matched controls, but no significant difference (p = 0.068, Mann-Whitney U test); similar between unfractured and fractured osteoporotic women (p = 0.310, Mann-Whitney U test) |
| Mdk | Proinflammatory cytokine | Chemoattract neutrophils and macrophages | Fischer et al34 2018 | ELISA | Systemic (Serum) | Long bone | Significantly higher in fracture patients on days 0, 14, and 42 compared to healthy controls; significantly higher on day 14 compared to day 0 in fracture patients; significantly higher in female fracture patients after menopause on day 0 after fracture compared to male fracture patients |
| CRP | Acute inflammatory protein | Act as chemoattractant for monocytes and induce tissue factor expression in macrophages such as IL-1α, IL-1β, TNF-α, and IL-6 | Lipovetzki et al69 2017 | ELISA | Systemic (Blood) | Hip | Significantly higher in postmenopausal women with fracture compared to those without |
| hsCRP | Meyer et al70 2014 | Plasma protein analysis | Systemic (Serum) | Distal radii | No significant change between 1 to 2 and 3 to 4 wks post-fracture |
-
CCL2, chemokine (C-C motif) ligand 2; CXCL1, chemokine (C-X-C motif) ligand 1; ELISA, enzyme-linked immunosorbent assay; hsCRP, high-sensitive CRP; IL, interleukin; Mdk, midkine; mRNA, messenger RNA; MSC, mesenchymal stromal cell; qPCR, quantitative polymerase chain reaction; RANK, receptor activator of nuclear factor kappa-B; RANKL, receptor activator of nuclear factor kappa-B ligand; TNF, tumour necrosis factor.
Three studies examined the proinflammatory cytokine TNF, which enhances MSC recruitment and osteogenic differentiation.36 Caetano-Lopes et al63 found that gene expression of TNF locally at the fracture site of low energy hip arthroplasty patients were similar among all timepoints (the first three days, days 4 to 7, and days 8 or more post-fracture) without any apparent variation in pattern. Pesic et al64 reported that the level of TNF-α in plasma detected by ELISA was similar among all timepoints (12 hours after fracture, and days 1, 3, and 7) after surgical intervention. Saribal et al65 demonstrated plasma TNF-α levels by quantitative sandwich enzyme immunoassay were similar between healthy controls and osteoporotic hip fracture patients before and after surgery.
Five clinical studies included proinflammatory cytokine IL-6, which stimulates angiogenesis42 and MSCs to differentiate towards the osteoblast lineage43 and modulates osteoblast and osteoclast activities.44 Caetano-Lopes et al63 showed that gene expression of IL-6 locally at the fracture site was the highest during the first three days after fracture compared to days 4 to 7 (p = 0.021, analysis of variance (ANOVA)) and days 8 or more after fracture. In Fischer et al,34 serum level of IL-6 was found to be significantly higher in fracture patients at days 0 and 14 post-fracture compared to healthy controls.34 Pesic et al64 demonstrated that IL-6 level in plasma was significantly higher at day 1 after surgical intervention when compared to 12 hours after fracture and days 3 and 7 after intervention. Saribal et al65 demonstrated that plasma IL-6 levels by quantitative sandwich enzyme immunoassay were similar between healthy controls and osteoporotic hip fracture patients before surgery. Significantly higher IL-6 levels were detected in osteoporotic hip fracture patients on the first (p = 0.005, Mann-Whitney U test) and second (p = 0.010, Mann-Whitney U test) days of postoperative periods compared to controls.65 Tsangari et al66 reported that IL-6 messenger RNA (mRNA) level of the trabecular bone cores from the intertrochanteric region obtained from patients suffering from fragility subcapital fracture of the neck of femur was significantly higher than that of aged-matched control without fracture. IL-6 mRNA levels were found to be associated with RANKL and receptor activator of nuclear factor kappa-B (RANK) mRNA levels (Pearson’s correlation of r = 0.77, p < 0.001; r = 0.95, p < 0.001, respectively) in the fracture group.66
One study investigated the proinflammatory cytokine IL-1β, which stimulates osteoblast proliferation and production of mineralized bone matrix as well as inhibiting proliferation and differentiation of chondrocytes.45 Caetano-Lopes et al63 revealed that gene expression of IL-1β at the fracture site was similar among all timepoints (the first three days, days 4 to 7, and days 8 or more post-fracture). The proinflammatory cytokine IL-31 was examined in another study; this induces the release of proinflammatory cytokines such as IL-1β and IL-6 and chemokines such as CCL2 and CXCL1.67 Ginaldi L et al28 found that the serum level of IL-31 by ELISA test was similar between fractured women with osteoporosis and age-matched controls. Unfractured and fractured osteoporotic women also showed similar IL-31 serum levels.
Another study involved the examination of the proinflammatory cytokine, Mdk, which is reported to chemoattract neutrophils and macrophages.60 In Fischer et al,34 Mdk level in serum was reported to be significantly higher in fracture patients at days 0, 14, and 42 after fracture compared to healthy controls. In fracture patients, Mdk level was significantly higher at day 14 compared to day 0 post-fracture. Also, female fracture patients after menopause showed significantly higher Mdk levels than male patients at day 0 after fracture.34
Two clinical studies included the acute inflammatory protein, CRP, which acts as a chemoattractant for monocytes and induces tissue factor expression in macrophages such as IL-1, TNF-α, and IL-6.68 Lipovetzki et al69 reported that serum level of CRP by ELISA test was significantly higher in postmenopausal women with non-traumatic hip fracture than that of postmenopausal osteoporotic women without hip fracture. Meyer et al70 found that serum level of high-sensitive CRP (hsCRP) by plasma protein analysis showed no significant difference between one to two weeks post-fracture and three to four weeks post-fracture.
Healing outcomes
Preclinical studies
All included preclinical studies (Table IV) reported radiograph micro-CT (μCT) assessments at the fracture site, except one using DXA to measure bone mineral content (BMC) and bone mineral density (BMD) of intact femora. Significantly lower bone volume ratio (BV/TV), trabecular number (Tb.N), and increased trabecular separation (Tb.Sp) in tibial callus of OVX mice were found in Wang F et al.39 Wang J et al41 demonstrated significantly lower bone volume (BV), total volume (TV), BV/TV, trabecular thickness (Tb.Th), Tb.N, and cortical thickness (Ct.Th) in fracture callus of OVX rats compared to SHAM rats.41 Chow et al38 also found significantly lower BV, BV/TV, and BMD at the fracture callus of OVX rats.38 Chan et al37 presented significantly increased relative callus mineralization (p = 0.0099, independent-samples t-test) in mice treated with 1 ng rhTNF compared to phosphate buffered saline (PBS) controls.37 Disturbed bony callus development and poorer cortical bridging at the fracture callus were observed in OVX mice compared to SHAM mice in Haffner-Luntzer M et al35 Zhang et al40 reported significantly lower BMD but similar BMC in OVX rats compared to normal control rats.
Table IV.
Summary of healing outcomes in the included preclinical studies.
| Included studies | Experiment | Parameter | Timepoint | Location | Finding |
|---|---|---|---|---|---|
| Chan et al37 2015 | μCT analysis | Relative callus mineralization | Day 14 | Callus (Tibia) | Treatment with 1 ng rhTNF significantly increased relative callus mineralization in OVX mice compared to SHAM mice |
| Day 28 | Healing was equivalent between groups | ||||
| Chow et al38 2019 | μCT analysis | TV, BV, BV/TV, BMD | Week 8 | Fracture callus | Significantly lower BV, BV/TV, and BMD in OVX rats |
| Radiography | Callus width and area | Weeks 1, 2, 3 | Fracture callus | Significantly lower callus width and area in OVX rats | |
| Haffner-Luntzer et al35 2017 | μCT analysis | Callus development and bridging | Day 23 | Fracture callus (Femur) | Disturbed bony callus development and poor cortical bridging at the fracture callus in OVX mice |
| Wang F et al39 2018 | Three-point bending test | Callus stiffness | Day 35 | Callus (Tibia) | Significantly lower callus stiffness in OVX mice |
| μCT analysis | BV/TV, Tb.N, Tb.Sp | Day 24 | Significantly decreased BV/TV and Tb.N and increased Tb.Sp in OVX mice | ||
| H&E staining | Bone morphometry | Day 5 | More fibrous callus and hematoncus (haematoma formed around the fracture site) in OVX mice | ||
| Day 11 | Chondrocyte proliferation and hypertrophic differentiation still took place in OVX mice | ||||
| Day 24 | Less new bone tissue generated in OVX mice | ||||
| Real-time PCR | mRNA expression of aggrecan, Col10a, VEGF, MMP13, Col1a, OCN | Day 11 | Callus tissue | In OVX mice, significantly higher levels of aggrecan and Col10a; significantly lower VEGF, Col1a, and OCN levels; no apparent change in MMP13 | |
| Day 24 | Significantly higher mRNA expression levels of aggrecan and Col10a; no apparent change in VEGF; significantly higher MMP13 level; significantly lower expression of Col1a and OCN | ||||
| ELISA | Aggrecan, MMP13, VEGF | Day 5 | Serum | Significantly higher VEGF level; no significant increase in aggrecan and MMP13 levels | |
| Day 11 | Significantly higher aggrecan, lower MMP13 and VEGF levels in OVX mice | ||||
| Wang J et al,41 2017 | μCT analysis | BV, TV, BV/TV, Tb.Th, Tb.N and Ct.Th | Week 6 | Fractured femur | Significantly lower BV, TV, BV/TV, Tb.Th, Tb.N, and Ct.Th in OVX rats compared to SHAM rats |
| Biomechanical measurement | Maximal fracture load | Femoral neck | Significantly lower maximal fracture load in OVX rats | ||
| H&E staining | Callus bony area, cartilage area, callus total area | Femur callus callous area | Significantly lower callus bony area, higher cartilage area, and lower callus total area in OVX rats | ||
| IHC | BMP-2 | Fractured femur section | Significantly lower BMP-2 expression in OVX rats | ||
| Real-time PCR | mRNA expression of BMP-2, RUNX2, ALP, OPG, RANKL | Fractured femur | Significantly lower BMP-2, RUNX2, and ALP expression in OVX mice; significantly lower OPG and higher RANKL expression in OVX rats | ||
| Western blot | BMP-2, RUNX2, ALP | Fractured femur | Significantly lower BMP-2, RUNX2, and ALP expression in OVX rats | ||
| TRAP staining | Number of osteoclasts | Fracture callus section | Significantly higher numbers of osteoclasts in OVX rats | ||
| Zhang et al40 2019 | DXA | BMC, BMD | Week 5 | Femur | No significance in BMC; significant reduction of BMD in OVX rats relative to normal control group |
| ELISA | TRAP, ALP, CTX, OCN | Serum | Significantly higher in OVX rats compared to normal control rats |
-
Aggrecan, chondrocyte proliferation marker; ALP, alkaline phosphatase; BMC, bone mineral content; BMD, bone mineral density; BMP-2, bone morphogenetic protein 2; BV, bone volume; BV/TV, bone volume ratio; Col1a, collagen type I (osteogenesis marker); Col10a, collagen type X (chondrocyte hypertrophic marker); CTX, C-terminal telopeptide of type 1 collagen; Ct.Th, cortical thickness; DXA, dual energy x-ray absorptiometry; ELISA, enzyme-linked immunosorbent assay; H&E, haematoxylin and eosin; IHC, immunohistochemistry; MMP13, matrix metallopeptidase 13 (cartilage matrix degradation marker); mRNA, messenger RNA; OCN, osteocalcin (osteogenesis marker); OPG, osteoprotegerin; OVX, ovariectomy; PCR, polymerase chain reaction; RANKL, receptor activator of nuclear factor kappa-B ligand; rhTNF, recombinant human tumour necrosis factor; RUNX2, runt-related transcription factor 2; SHAM, normal control; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TRAP, tartrate-resistant acid phosphatase; TV, tissue volume; VEGF, vascular endothelial growth factor (vascularization marker); μCT, x-ray micro-CT
One study measured callus width and area through weekly lateral radiography. Chow et al38 reported that OVX rats had significantly lower callus width and area than SHAM rats at weeks 1, 2, and 3 post-fracture.
Two studies included mechanical testing to evaluate the bone properties of the fracture bones, including Wang F et al39 and Wang J et al.41 Wang F et al39 reported significantly lower callus stiffness by three-point bending test in fractured tibia of OVX mice. Wang J et al41 presented significantly lower maximal fracture load of the femoral neck by loading vertical pressure on the femoral head in OVX rats.
Histomorphometric analysis was reported in two preclinical studies, including Wang F et al39 and Wang J et al.41 Wang F et al39 demonstrated slower bone regeneration as shown by less new bone tissue formed to replace cartilage callus around the fracture site of OVX mice compared to controls. Wang J et al41 also reported significantly higher osteoclast numbers in fracture callus of OVX rats compared to SHAM groups. In addition, significantly lower callus bony area (-10.7%), higher cartilage area, and lower callus total area were reported at the fracture callus of OVX rats in Wang J et al.41
Two studies evaluated the gene expression at the fracture site, including Wang F et al39 and Wang J et al.41 Wang F et al39 found that mRNA expression of aggrecan and collagen type X (Col10a), which are related to chondrogenesis, and matrix metallopeptidase 13 (MMP13) were found to be significantly higher, whereas expression of collagen type I (Col1a) and osteocalcin (OCN), which are related to osteogenesis, and vascular endothelial growth factor (VEGF) was significantly lower in the callus tissue of OVX mice.39 Wang J et al41 also reported significantly lower levels of osteogenic marker, alkaline phosphatase (ALP), bone morphogenetic protein 2 (BMP-2), and runt-related transcription factor 2 (RUNX2) expression in fractured femora of OVX rats. Significantly lower OPG and higher RANKL expression was also shown in fractured femora of OVX rats.41
Three studies involved the assessments of protein expression. Wang J et al41 performed western blot on the fractured femora, and Wang F et al39 and Zhang et al40 performed ELISA in serum. Wang J et al41 found that expression of RUNX2 and ALP was significantly lower in fractured femora of OVX rats compared to the SHAM group. Significantly lower BMP-2 expression was also reported at the fracture site of OVX rats.41 Systematically, Wang F et al39 demonstrated that the serum level of VEGF was significantly higher at day 5 post-fracture but lower at day 11 in OVX mice. Significantly higher aggrecan and lower MMP13 levels in serum were also found in OVX mice.39 Zhang et al40 presented significantly higher serum levels of TRAP, ALP, C-terminal telopeptide of type 1 collagen (CTX), and OCN in OVX rats.
Clinical studies
CT parameters were reported in a study by Meyer et al70 This study found that total bone mineral density (D.tot), cortical bone mineral density (D.cort), trabecular bone mineral density (D.trab), and Tb.Th were significantly increased at the fracture site at three to four weeks post-fracture when compared to one to two weeks post-fracture in osteoporotic/osteopenic women, but no difference was reported in compression stiffness (S.comp), torsion stiffness (S.tors), or bending stiffness (S.bend) (Table V).70
Table V.
Summary of healing outcomes in the included clinical studies.
| Publication | Experiment | Parameter | Source of sample | Finding |
|---|---|---|---|---|
| Caetano-Lopes et al63 2011 | Real-time PCR | BMP-2, BMP-4, TGF-β1, IGF-1, FGF-2, PDGF-β | Femoral epiphysis | BMP-2: highest until 3 days post-fracture and decreased thereafter; BMP-4: remained stable over time; TGF-β1: decreased constantly over time; IGF-1: remained stable over time; FGF-2, PDGF-β: decreased slightly until 8 days after fracture; decreased clearly after 8 days after fracture |
| OPG, RANK, RANKL, RANKL/OPG | OPG: Decreased over time; RANK: increased over time; RANKL, RANKL/OPG: highest at days 4 to 7 post-fracture and decreased thereafter | |||
| CBFA1/RUNX2, Osx, ALP | CBFA1/RUNX2: slightly higher at days 4 to 7 post-fracture and remained constant later; Osx: remained constant and increased after days 4 to 7 post-fracture; ALP: slightly lower at days 4 to 7 post-fracture and remained constant later | |||
| SOST | Decreased over time | |||
| TRAP, CTSK, ITGB3, ATP6V0D2 | TRAP: increased over time; CTSK: increased over time; ITGB3: remained constant; ATP6V0D2: increased over time | |||
| Lipovetzki et al69 2017 | ELISA | OPG | Serum | Significantly higher in postmenopausal women with fracture compared to those without |
| Meyer et al70 2014 | HR-pQCT | D.tot, D.cort, D.trab, Ct.Th, Tb.N, Tb.Th, Tb.Sp | Distal radii | Significantly increased D.tot, D.cort, D.trab, and Tb.Th at 3 to 4 wks compared to 1 to 2 wks post-fracture, similar between 1 to 2 and 3 to 4 wks post-fracture for other parameters |
| μFEA | S.comp, S.tors, S.bend | No significant change between 1 to 2 and 3 to 4 wks post-fracture | ||
| Radioimmunoassay | ICTP, PINP | Serum | No significant change between 1 to 2 and 3 to 4 wks post-fracture | |
| Tsangari et al66 2004 | Real-time PCR | RANKL, OPG, RANKL/OPG, RANK, CTR, OCN | Intertrochanteric trabecular bone | Significantly greater ratio of RANKL/OPG and RANK mRNA expression in fracture group compared to no-fracture group, whereas no significance for other parameters |
-
ALP, alkaline phosphatase; ATP6V0D2, ATPase H+ transporter; BMP-2, bone morphogenetic protein 2; BMP-4, bone morphogenetic protein 4; CBFA1/RUNX2, core-binding factor, alfa subunit 1/runt-related transcription factor 2; CTR, calcitonin receptor; CTSK, cathepsin K; Ct.Th, cortical thickness; D.cort, cortical bone mineral density; D.tot, total bone mineral density; D.trab, trabecular bone mineral density; ELISA, enzyme-linked immunosorbent assay; FGF-2, fibroblast growth factor 2; HR-pQCT, high-resolution peripheral quantitative CT; ICTP, carboxy-terminal telopeptide of type I collagen; IGF-1, insulin-like growth factor 1; ITGB3, β3 subunit of the avb3 integrin; mRNA, messenger RNA; OPG, osteoprotegerin; PCR, polymerase chain reaction; PDGF-β, platelet-derived growth factor subunit B; PINP, procollagen type-I N-terminal propeptide; OCN, osteocalcin; Osx, Osterix; RANK, receptor activator of nuclear factor kappa-B; RANKL, receptor activator of nuclear factor kappa-B ligand; SOST, sclerostin; S. bend, bending stiffness; S.comp, compression stiffness; S.tors, torsion stiffness; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TGF-β1, transforming growth factor beta 1; TRAP, tartrate-resistant acid phosphatase; μFEA, micro-finite element analysis
Gene expression at the fracture site was evaluated in two clinical studies. Tsangari et al66 demonstrated significantly greater ratio of RANKL/OPG and RANK mRNA expression in the fracture group compared to the no-fracture group.66 In Caetano-Lopes et al,63BMP-2 expression was found to be the highest at the first three post-fracture days compared to days 4 to 7 and days 8 or more after fracture. BMP-4 and insulin-like growth factor 1 (IGF-1) expression were similar among the timepoints (the first three days, days 4 to 7, and days 8 or more post-fracture). Transforming growth factor β 1 (TGF-β1) expression decreased constantly after fracture, while fibroblast growth factor 2 (FGF-2) and platelet-derived growth factor subunit B (PDGF-β) expression was found to decrease slightly from the first three days to days 4 to 7, and drop clearly at days 8 or more post-fracture. Moreover, OPG expression was reported to decrease over time after fracture, while RANK expression showed the opposite trend. Both RANKL expression and RANKL/OPG ratio were found to be highest at days 4 to 7 post-fracture and decrease thereafter. Osteoblast-related genes were also investigated. Core-binding factor, α subunit 1/runt-related transcription factor 2 (CBFA1/RUNX2) expression was shown to be slightly higher at days 4 to 7 post-fracture and remain constant thereafter. Osterix (Osx) expression remained constant and increased after days 4 to 7 post-fracture. On the other hand, ALP expression was slightly lower at days 4 to 7 post-fracture and remained constant later. For osteocyte-related genes, sclerostin (SOST) expression was found to decrease over time after fracture. For osteoclast-related genes, expression of tartrate-resistant acid phosphatase (TRAP), cathepsin K (CTSK), and ATPase H+ transporter (ATP6V0D2) increased over time, while β3 subunit of the avb3 integrin (ITGB3) expression remained constant.63 Protein expression in serum was investigated in three studies. Lipovetzki et al69 presented significantly higher OPG levels in postmenopausal women with fracture compared to those without. Meyer et al70 found no significance in carboxy-terminal telopeptide of type I collagen (ICTP) and procollagen type-I N-terminal propeptide (PINP) among all timepoints (one to two and three to four weeks post-fracture).
Discussion
We reviewed both the local and systemic inflammatory responses in ovariectomy-induced osteoporotic fractures or fragility fractures, and the potential effects of inflammatory response on differential healing outcomes.
In terms of systemic inflammatory response, six out of the seven preclinical studies in this review demonstrated that OVX animals had higher systemic inflammatory response. In terms of proinflammatory cytokines, OVX animals showed significantly higher levels of TNF-α, IL-1β, and Mdk at the early healing stage.34,38,39 Also, at later stages of fracture healing markedly increased serum levels of TNF-α, IL-1β, and IL-6 were detected in OVX animals.38,40,41 As for immune cells, Haffner-Luntzer et al35 reported significantly increased numbers of B-lymphocytes and T-helper cells in the bone marrow of OVX mice at day 1, with the numbers of other immune cells including macrophages and inflammatory monocytes being similar between SHAM and OVX mice. The findings in these six studies demonstrated that at both shorter and longer timepoints post-fracture, OVX groups showed systemically higher levels of proinflammatory cytokines, implying that systemic inflammation remains higher in OVX animals, which would interfere with the local inflammatory response and the subsequent regulation of the healing cascade.
We also reviewed literature that addressed the local inflammatory response following fracture injury. Fischer et al34 reported that significantly higher levels of IL-6, Mdk, and MCP-1 were detected at the fracture callus in OVX mice at day 3 after fracture. Haffner-Luntzer et al35 also showed that IL-6 and Mdk expression were higher in the fracture callus of OVX mice at day 3 post-fracture. In terms of immune cells at the fracture callus, only neutrophil population was found to be higher at day 3 in OVX mice, with other immune cell expression being similar between SHAM and OVX mice.35 These two studies together reported a higher local inflammatory response in OVX animals. On the other hand, Chow et al38 found that at the fracture site of OVX rats, the levels of TNF-α and IL-6 were lower while IL-10 level was higher at week 1 post-fracture. In the study by Chan et al,37 with the local injection of 1 ng rhTNF at the fracture site immediately or day 1 after fracture, OVX mice showed increased relative callus mineralization at day 14 compared to SHAM mice. This study demonstrated that the local inflammatory response during fracture healing was reduced in OVX animals and thus healing could be augmented with the enhancement of the inflammatory response via administration of proinflammatory cytokines at the fracture site. Taking these studies together, it is inconclusive whether osteoporotic fracture has higher or lower local inflammatory response compared to normal fracture. Despite being controversial, all of these preclinical studies showed that OVX animals demonstrated inferior healing outcomes, including poorer bone formation35,38–41 and biomechanical strength.39,41 Besides, lower levels of osteogenic markers were detected in OVX animals.39–41 One study also demonstrated that the number of osteoclasts at the fracture site was increased in OVX animals.41 Thus, it can be seen that healing outcomes in OVX animal fracture models are impaired at either elevated or reduced local inflammatory response, thus indicating that an optimal level of response was disrupted under oestrogen-deprived conditions.
Controlled release of cytokines is of critical importance for normal bone regeneration. Previous studies showed that disrupted inflammatory response would exert a negative effect on fracture healing outcomes. In general, summarizing the findings from all preclinical studies, the level of systemic proinflammatory cytokines was found to be higher in OVX-induced osteoporotic fracture compared to normal fracture at the early inflammation and later bone remodelling stages (Figure 2). Indeed, inflammatory cytokines secreted by different immune cells have been shown to have close interactions with bone cells, including osteoblasts, osteoclasts, and osteocytes.71,72 Inflammatory cytokines including TNF-α and IL-6 have been shown to influence osteoblastogenesis or osteoclastogenesis, which would have direct impacts on callus formation, maturation, and remodelling,36,42 suggesting that these inflammatory cytokines could be key regulators of bone formation. However, excessive expression of proinflammatory cytokines had negative impact on bone formation. In culture, continuous exposure of osteoblasts to IL-1β inhibits their functions, including inhibition of collagen and non-collagen protein synthesis, ALP expression, and cell replication.73 The administration of a high concentration of TNF-α (10 ng/ml) in C2C12 and primary mouse calvaria cells is shown to inhibit osteoblast differentiation through enhancing the transcription of Smurf1 in an activator protein-1 (AP-1) and Runx2-dependent manner.74
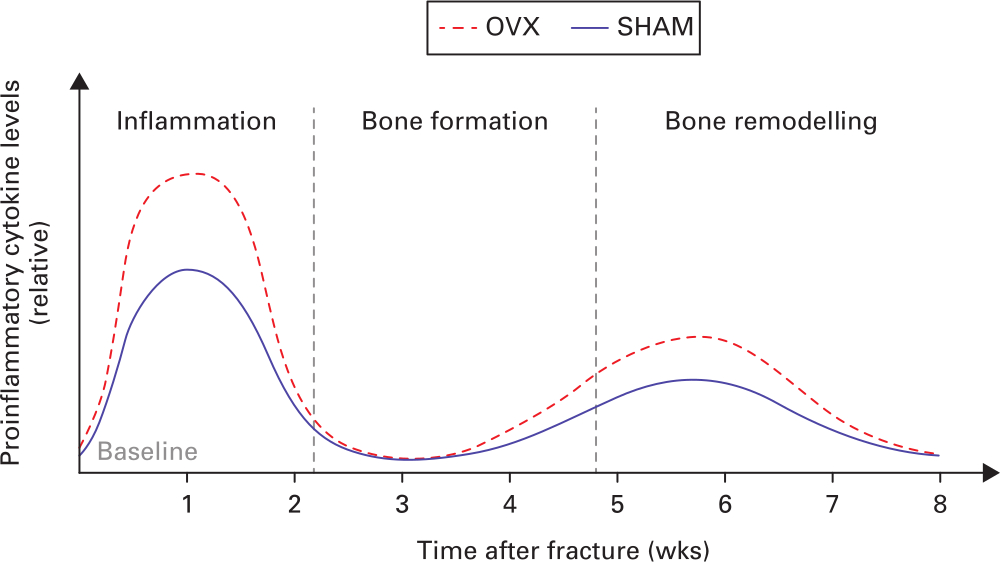
Fig. 2
Schematic diagram depicting the relative levels of systemic proinflammatory cytokines of normal and osteoporotic fracture healing. OVX, ovariectomy; SHAM, normal control.
Inflammatory cytokines have been reported to have distinct functions at different healing stages. TNF-α has been shown to stimulate MSC recruitment at the early healing stage and later enhance bone resorption activity of osteoclasts to encourage the bone remodelling process.75 Impaired callus strength and reduced osteoclast numbers were shown in the absence of IL-6 in the early healing stage, while enhanced callus stiffness was observed at the later remodelling stage.76 Both local and systemic inflammatory cytokines play key roles in different stages of fracture healing. However, the cell types that release these cytokines locally are under-investigated for their roles in coordinating the inflammatory cascade and subsequent healing phases. Therefore, further studies should be conducted to dissect the regulatory role of the inflammatory response in osteoporotic fracture healing. Different fracture regions such as the metaphyseal region could be examined as different fracture sites may show different inflammatory responses after injury.77,78 In addition, parabiosis mice models could be used to investigate factors that are responsible for age-dependent tissue regeneration, as previous studies identified that a youthful circulation can rejuvenate aged-bone healing.79,80
All the included clinical studies reported either the temporal changes of inflammatory markers after fragility fracture or comparison of inflammatory factor levels between fragility fracture patients and normal controls without fracture. So far, there is no clinical study investigating the inflammatory response between osteoporotic fracture and normal fracture, probably due to the difficulties in recruiting such types of patients. Therefore, the differences in the inflammatory response between elderly or osteoporotic patients and normal patients cannot be known at this stage until more studies in this respect are conducted.
It is suggested that in the study design of future clinical studies, fracture patients of younger age should be recruited to serve as the control group and be compared with elderly/osteoporotic patients with fragility fracture, similar to the design of preclinical studies in which SHAM and OVX animals are compared. This way we can really find out the differences of the inflammatory response between normal and fragility/osteoporotic fracture healing in human subjects and fill the existing knowledge gap.
All of the included clinical studies are prospective cohort studies in which human subjects with different characteristics such as age, fracture site, and fracture type were recruited. Although four studies looked at the temporal changes of inflammatory factors, the timepoints and outcome measures were different. The remaining four studies examined the differences of inflammatory factor expression between fragility fracture and controls without fracture, but their parameters were different as well. Also, the studies do not involve common treatment. Thus, it is not feasible to perform a meta-analysis due to the heterogeneity in terms of participants, interventions, and outcomes of the included clinical studies.
Taking all the studies into consideration, of all the inflammatory markers reported IL-6 and TNF-α were two major markers shown to have a close relationship with bone formation or healing-related outcomes. Currently, these two markers are being commonly studied in both animal and human studies on fracture healing. Thus, they are suggested to be included in future clinical studies by examining their levels in serum/plasma or gene expression locally at the fracture site.
In this review, three clinical studies reported the temporal changes of inflammatory cytokines in fragility fracture. All of them included the investigation of IL-6, and they all demonstrated that IL-6 increased considerably at earlier stages of fragility fracture healing and diminished over time.34,63,64 It has been postulated that IL-6 is responsible for stimulating the early stage of fracture healing, as reported in a previous study showing that IL-6 level in trauma patients decreased six months after injury.81 Another study also reported that IL-6 elevated to a great level in patients with head injury within hours of injury, as IL-6 and receptor complex may be capable of stimulating cells of the mesenchymal lineage and therefore may enhance the formation of osteoblastic bone.82 Besides, two included preclinical studies (Haffner-Luntzer M et al35 and Fischer V et al34) also found significantly higher IL-6 levels at the fracture callus of OVX mice compared to normal mice at day 3 post-fracture, further showing the significant increase of IL-6 at the early phase of fracture healing in osteoporotic subjects. Therefore, the expression of IL-6 or other major proinflammatory markers in osteoporotic fracture patients at the first three days as compared to non-osteoporotic fracture patients is a key knowledge gap that should be further investigated.
In addition, one preclinical study (Wang J et al41) found that OVX rats showed significantly higher serum IL-6 level and RANKL mRNA expression at week 6 post-fracture. One clinical study (Tsangari et al66) reported that the IL-6 mRNA level was shown to be positively correlated with the RANKL level. These two studies showed a positive association between levels of IL-6 and RANKL, which was shown to activate osteoclasts and promote bone resorption.83 However, there is no other study investigating the relationships between inflammatory cytokines and fracture healing outcomes. This proposes a pressing need for further studies to provide more evidence on the effects of changes of inflammatory response on different fracture healing outcomes.
As seen from the findings of all the included studies, the inflammatory response has a great impact on the healing process; any disruption of the inflammatory reaction will negatively affect different healing outcomes. Bone healing in aged or osteoporotic individuals has higher risk of failure, usually accompanied with delayed healing or nonunions. Novel therapies to improve age-associated impairment in fracture healing are needed. So far, there have been different studies targeting bone formation-related pathways such as the BMP signalling pathway.84,85 The tight interaction between the immune system and the skeletal system has been recognized in the emerging research field of osteoimmunology.86 Thus, the inflammatory response could also be one potential target for immune-modulatory approaches to enhance fracture healing, as a previous study demonstrated that modulation of inflammatory reaction could enhance osteogenesis.87 Further investigation into the roles and interaction of inflammatory factors and bone cells is necessary for a better understanding of fragility/osteoporotic fracture healing and development of treatment to enhance it.
There are several limitations in this review. Firstly, not all subjects in the clinical studies were confirmed as having osteoporosis. Secondly, meta-analysis was not feasible due to the heterogeneity of the included studies, which may weaken the conclusion. Thirdly, only studies written in English were included, which may not fully cover all the evidence available.
In conclusion, the systemic inflammatory response during osteoporotic fracture healing is generally higher compared to normal fractures as demonstrated by the expression of proinflammatory cytokines and elevation in immune cell counts in animals. As for the local inflammatory response, it is inconclusive whether OVX animals have higher or lower local inflammatory response. But the healing outcomes have been shown to be impaired. Regarding the clinical studies, there is no study investigating the difference in the inflammatory response between osteoporotic and non-osteoporotic patients. All clinical data presented could only serve as reference data for the temporal changes of common inflammatory cytokines in osteoporotic fracture patients. Taken together, a suboptimal inflammatory response could imply poorer fracture healing in OVX subjects or postmenopausal osteoporotic or aged individuals with fragility fracture, thus there is a need for further studies to better understand the role of the inflammatory response in the healing cascade for potential immunomodulation to enhance osteoporotic fracture healing.
References
1. Wei FY , Leung KS , Li G , et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing . PLoS One . 2014 ; 9 ( 9 ): e106722 . Crossref PubMed Google Scholar
2. Sanghani-Kerai A , Coathup M , Samazideh S , et al. Osteoporosis and ageing affects the migration of stem cells and this is ameliorated by transfection with CXCR4 . Bone Joint Res . 2017 ; 6 ( 6 ): 358 – 365 . Crossref PubMed Google Scholar
3. Cheung WH , Sun MH , Zheng YP , et al. Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model-evaluation of pulsed-wave doppler, 3-D power Doppler ultrasonography and micro-CT microangiography . Ultrasound Med Biol . 2012 ; 38 ( 12 ): 2120 – 2129 . Crossref PubMed Google Scholar
4. Kampschulte M , Krombach GA , Richards DC , et al. Neovascularization of osteoporotic metaphyseal bone defects: A morphometric micro-CT study . Microvasc Res . 2016 ; 105 : 7 – 14 . Crossref PubMed Google Scholar
5. Shi HF , Cheung WH , Qin L , Leung AH , Leung KS . Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone . Bone . 2010 ; 46 ( 5 ): 1299 – 1305 . Crossref PubMed Google Scholar
6. Chow DH , Leung KS , Qin L , et al. LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing . J Orthop Res . 2011 ; 29 ( 5 ): 746 – 752 . Google Scholar
7. Chow SK , Leung KS , Qin L , Wei F , Cheung WH . Callus formation is related to the expression ratios of estrogen receptors-alpha and -beta in ovariectomy-induced osteoporotic fracture healing . Arch Orthop Trauma Surg . 2014 ; 134 ( 10 ): 1405 – 1416 . Crossref PubMed Google Scholar
8. Claes L , Recknagel S , Ignatius A . Fracture healing under healthy and inflammatory conditions . Nat Rev Rheumatol . 2012 ; 8 ( 3 ): 133 – 143 . Crossref PubMed Google Scholar
9. Loi F , Córdova LA , Pajarinen J , et al. Fracture and bone repair . Bone . 2016 ; 86 : 119 – 130 . Google Scholar
10. Walsh MC , Kim N , Kadono Y , et al. Osteoimmunology: interplay between the immune system and bone metabolism . Annu Rev Immunol . 2006 ; 24 : 33 – 63 . Crossref PubMed Google Scholar
11. Schmidt-Bleek K , Schell H , Schulz N , et al. Inflammatory phase of bone healing initiates the regenerative healing cascade . Cell Tissue Res . 2012 ; 347 ( 3 ): 567 – 573 . Crossref PubMed Google Scholar
12. Schindeler A , McDonald MM , Bokko P , Little DG . Bone remodeling during fracture repair: the cellular picture . Semin Cell Dev Biol . 2008 ; 19 ( 5 ): 459 – 466 . Crossref PubMed Google Scholar
13. Cheung WH , Miclau T , Chow SK , Yang FF , Alt V . Fracture healing in osteoporotic bone . Injury . 2016 ; 47 ( suppl 2 ): S21 – S26 . Crossref PubMed Google Scholar
14. Thomas MV , Puleo DA . Infection, inflammation, and bone regeneration: a paradoxical relationship . J Dent Res . 2011 ; 90 ( 9 ): 1052 – 1061 . Crossref PubMed Google Scholar
15. Dishowitz MI , Mutyaba PL , Takacs JD , et al. Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing . PLoS One . 2013 ; 8 ( 7 ): e68726 . Crossref PubMed Google Scholar
16. Lim JC , Ko KI , Mattos M , et al. TNFα contributes to diabetes impaired angiogenesis in fracture healing . Bone . 2017 ; 99 : 26 – 38 . Crossref PubMed Google Scholar
17. Gruver AL , Hudson LL , Sempowski GD . Immunosenescence of ageing . J Pathol . 2007 ; 211 ( 2 ): 144 – 156 . Crossref PubMed Google Scholar
18. Franceschi C , Bonafè M , Valensin S , et al. Inflamm-aging. An evolutionary perspective on immunosenescence . Ann N Y Acad Sci . 2000 ; 908 : 244 – 254 . Crossref PubMed Google Scholar
19. Ferrucci L , Fabbri E . Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty . Nat Rev Cardiol . 2018 ; 15 ( 9 ): 505 – 522 . Google Scholar
20. Sebastián C , Herrero C , Serra M , et al. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation . J Immunol . 2009 ; 183 ( 4 ): 2356 – 2364 . Crossref PubMed Google Scholar
21. Ramanathan R , Kohli A , Ingaramo MC , et al. Serum chitotriosidase, a putative marker of chronically activated macrophages, increases with normal aging . J Gerontol A Biol Sci Med Sci . 2013 ; 68 ( 10 ): 1303 – 1309 . Crossref PubMed Google Scholar
22. Compston JE . Bone marrow and bone: a functional unit . J Endocrinol . 2002 ; 173 ( 3 ): 387 – 394 . Crossref PubMed Google Scholar
23. Duscher D , Rennert RC , Januszyk M , et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells . Sci Rep . 2014 ; 4 : 7144 . Crossref PubMed Google Scholar
24. Sethe S , Scutt A , Stolzing A . Aging of mesenchymal stem cells . Ageing Res Rev . 2006 ; 5 ( 1 ): 91 – 116 . Google Scholar
25. Gómez-Barrena E , Rosset P , Lozano D , et al. Bone fracture healing: cell therapy in delayed unions and nonunions . Bone . 2015 ; 70 : 93 – 101 . Crossref PubMed Google Scholar
26. Ginaldi L , Di Benedetto M , De Martinis M , Osteoporosis DMM . Inflammation and ageing . Immun Ageing . 2005 ; 2 ( 1 ): 14 . Google Scholar
27. Pfeilschifter J , Köditz R , Pfohl M , Schatz H . Changes in proinflammatory cytokine activity after menopause . Endocr Rev . 2002 ; 23 ( 1 ): 90 – 119 . Crossref PubMed Google Scholar
28. Ginaldi L , De Martinis M , Ciccarelli F , et al. Increased levels of interleukin 31 (IL-31) in osteoporosis . BMC Immunol . 2015 ; 16 : 60 . Crossref PubMed Google Scholar
29. Weitzmann MN , Pacifici R . Estrogen regulation of immune cell bone interactions . Ann N Y Acad Sci . 2006 ; 1068 : 256 – 274 . Crossref PubMed Google Scholar
30. Silverman SL , Siris E , Kendler DL , et al. Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study . Osteoporos Int . 2015 ; 26 ( 1 ): 361 – 372 . Google Scholar
31. Straub RH . The complex role of estrogens in inflammation . Endocr Rev . 2007 ; 28 ( 5 ): 521 – 574 . Crossref PubMed Google Scholar
32. Torres M , Palomer X , Montserrat JM , Vázquez-Carrera M , Farré R . Effect of ovariectomy on inflammation induced by intermittent hypoxia in a mouse model of sleep apnea . Respir Physiol Neurobiol . 2014 ; 202 : 71 – 74 . Crossref PubMed Google Scholar
33. Khan D , Ansar Ahmed S . The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases . Front Immunol . 2016 ; 6 : 635 . Crossref PubMed Google Scholar
34. Fischer V , Kalbitz M , Müller-Graf F , et al. Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing . Int J Mol Sci . 2018 ; 19 ( 7 ): E2070 . Crossref PubMed Google Scholar
35. Haffner-Luntzer M , Fischer V , Prystaz K , Liedert A , Ignatius A . The inflammatory phase of fracture healing is influenced by oestrogen status in mice . Eur J Med Res . 2017 ; 22 ( 1 ): 23 . Crossref PubMed Google Scholar
36. Glass GE , Chan JK , Freidin A , et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells . Proc Natl Acad Sci U S A . 2011 ; 108 ( 4 ): 1585 – 1590 . Crossref PubMed Google Scholar
37. Chan JK , Glass GE , Ersek A , et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response . EMBO Mol Med . 2015 ; 7 ( 5 ): 547 – 561 . Crossref PubMed Google Scholar
38. Chow SKH , Chim YN , Wang J , et al. Vibration treatment modulates macrophage polarisation and enhances early inflammatory response in oestrogen-deficient osteoporotic-fracture healing . Eur Cell Mater . 2019 ; 38 : 228 – 245 . Crossref PubMed Google Scholar
39. Wang F , Han L , Wang X , et al. Sialoglycoprotein isolated from eggs of Carassius auratus promotes fracture healing in osteoporotic mice . J Food Drug Anal . 2018 ; 26 ( 2 ): 716 – 724 . Crossref PubMed Google Scholar
40. Zhang JL , Zong LZ , Bai DY . Boeravinone B promotes fracture healing in ovariectomy-induced osteoporotic rats via the regulation of NF-kappa B p65/I kappa B-alpha/SIRT-1 signaling pathway . Trop J Pharm Res . 2019 ; 18 ( 5 ): 955 – 960 . Google Scholar
41. Wang J , He M , Wang G , Fu Q . Organic Gallium Treatment Improves Osteoporotic Fracture Healing Through Affecting the OPG/RANKL Ratio and Expression of Serum Inflammatory Cytokines in Ovariectomized Rats . Biol Trace Elem Res . 2018 ; 183 ( 2 ): 270 – 279 . Crossref PubMed Google Scholar
42. Yang X , Ricciardi BF , Hernandez-Soria A , et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice . Bone . 2007 ; 41 ( 6 ): 928 – 936 . Crossref PubMed Google Scholar
43. Heymann D , Rousselle AV . gp130 Cytokine family and bone cells . Cytokine . 2000 ; 12 ( 10 ): 1455 – 1468 . Crossref PubMed Google Scholar
44. Blanchard F , Duplomb L , Baud’huin M , Brounais B . The dual role of IL-6-type cytokines on bone remodeling and bone tumors . Cytokine Growth Factor Rev . 2009 ; 20 ( 1 ): 19 – 28 . Crossref PubMed Google Scholar
45. Lange J , Sapozhnikova A , Lu C , et al. Action of IL-1beta during fracture healing . J Orthop Res . 2010 ; 28 ( 6 ): 778 – 784 . Crossref PubMed Google Scholar
46. Ono T , Takayanagi H . Osteoimmunology in Bone Fracture Healing . Curr Osteoporos Rep . 2017 ; 15 ( 4 ): 367 – 375 . Crossref PubMed Google Scholar
47. Zurawski G , de Vries JE . Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells . Immunol Today . 1994 ; 15 ( 1 ): 19 – 26 . Google Scholar
48. Lind M , Deleuran B , Yssel H , Fink-Eriksen E , Thestrup-Pedersen K . IL-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts . Cytokine . 1995 ; 7 ( 1 ): 78 – 82 . Google Scholar
49. Baumann H , Gauldie J . The acute phase response . Immunol Today . 1994 ; 15 ( 2 ): 74 – 80 . Google Scholar
50. Watanabe K , Tanaka Y , Morimoto I , et al. Interleukin-4 as a potent inhibitor of bone resorption . Biochem Biophys Res Commun . 1990 ; 172 ( 3 ): 1035 – 1041 . Crossref PubMed Google Scholar
51. Baht GS , Vi L , Alman BA . The Role of the Immune Cells in Fracture Healing . Curr Osteoporos Rep . 2018 ; 16 ( 2 ): 138 – 145 . Crossref PubMed Google Scholar
52. Wu AC , Raggatt LJ , Alexander KA , Pettit AR . Unraveling macrophage contributions to bone repair . Bonekey Rep . 2013 ; 2 : 373 . Crossref PubMed Google Scholar
53. Champagne CM , Takebe J , Offenbacher S , Cooper LF . Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2 . Bone . 2002 ; 30 ( 1 ): 26 – 31 . Crossref PubMed Google Scholar
54. Chang MK , Raggatt LJ , Alexander KA , et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo . J Immunol . 2008 ; 181 ( 2 ): 1232 – 1244 . Crossref PubMed Google Scholar
55. Sun G , Wang Y , Ti Y , et al. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells . Clin Exp Pharmacol Physiol . 2017 ; 44 ( 4 ): 455 – 462 . Crossref PubMed Google Scholar
56. Manabe N , Kawaguchi H , Chikuda H , et al. Connection between B lymphocyte and osteoclast differentiation pathways . J Immunol . 2001 ; 167 ( 5 ): 2625 – 2631 . Crossref PubMed Google Scholar
57. Könnecke I , Serra A , El Khassawna T , et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion . Bone . 2014 ; 64 : 155 – 165 . Crossref PubMed Google Scholar
58. Han X , Yang Q , Lin L , et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells . Cell Death Differ . 2014 ; 21 ( 11 ): 1758 – 1768 . Crossref PubMed Google Scholar
59. Nam D , Mau E , Wang Y , et al. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair . PLoS One . 2012 ; 7 ( 6 ): e40044 . Crossref PubMed Google Scholar
60. Weckbach LT , Muramatsu T , Walzog B . Midkine in inflammation . ScientificWorldJournal . 2011 ; 11 : 2491 – 2505 . Crossref PubMed Google Scholar
61. Deshmane SL , Kremlev S , Amini S , Sawaya BE . Monocyte chemoattractant protein-1 (MCP-1): an overview . J Interferon Cytokine Res . 2009 ; 29 ( 6 ): 313 – 326 . Crossref PubMed Google Scholar
62. Sadik CD , Kim ND , Luster AD . Neutrophils cascading their way to inflammation . Trends Immunol . 2011 ; 32 ( 10 ): 452 – 460 . Crossref PubMed Google Scholar
63. Caetano-Lopes J , Lopes A , Rodrigues A , et al. Upregulation of inflammatory genes and downregulation of sclerostin gene expression are key elements in the early phase of fragility fracture healing . PLoS One . 2011 ; 6 ( 2 ): e16947 . Crossref PubMed Google Scholar
64. Pesic G , Jeremic J , Nikolic T , et al. Interleukin-6 as possible early marker of stress response after femoral fracture . Mol Cell Biochem . 2017 ; 430 ( 1-2 ): 191 – 199 . Crossref PubMed Google Scholar
65. Saribal D , Hocaoglu-Emre FS , Erdogan S , et al. Inflammatory cytokines IL-6 and TNF-α in patients with hip fracture . Osteoporos Int . 2019 ; 30 ( 5 ): 1025 – 1031 . Crossref PubMed Google Scholar
66. Tsangari H , Findlay DM , Kuliwaba JS , Atkins GJ , Fazzalari NL . Increased expression of IL-6 and RANK mRNA in human trabecular bone from fragility fracture of the femoral neck . Bone . 2004 ; 35 ( 1 ): 334 – 342 . Crossref PubMed Google Scholar
67. Zhang Q , Putheti P , Zhou Q , Liu Q , Gao W . Structures and biological functions of IL-31 and IL-31 receptors . Cytokine Growth Factor Rev . 2008 ; 19 ( 5-6 ): 347 – 356 . Crossref PubMed Google Scholar
68. Sproston NR , Ashworth JJ . Role of C-Reactive Protein at Sites of Inflammation and Infection . Front Immunol . 2018 ; 9 : 754 . Crossref PubMed Google Scholar
69. Lipovetzki Y , Zandman-Goddard G , Feldbrin Z , Shargorodsky M . Elevated ferritin and circulating osteoprotegerin levels as independent predictors of hip fracture in postmenopausal women admitted for fragility fracture: time for new screening strategies? Immunol Res . 2017 ; 65 ( 1 ): 423 – 427 . Crossref PubMed Google Scholar
70. Meyer U , de Jong JJ , Bours SG , et al. Early changes in bone density, microarchitecture, bone resorption, and inflammation predict the clinical outcome 12 weeks after conservatively treated distal radius fractures: an exploratory study . J Bone Miner Res . 2014 ; 29 ( 9 ): 2065 – 2073 . Crossref PubMed Google Scholar
71. Adamopoulos IE . Inflammation in bone physiology and pathology . Curr Opin Rheumatol . 2018 ; 30 ( 1 ): 59 – 64 . Crossref PubMed Google Scholar
72. Metzger CE , Narayanan SA . The Role of Osteocytes in Inflammatory Bone Loss . Front Endocrinol (Lausanne) . 2019 ; 10 : 285 . Crossref PubMed Google Scholar
73. Stashenko P , Dewhirst FE , Rooney ML , Desjardins LA , Heeley JD . Interleukin-1 beta is a potent inhibitor of bone formation in vitro . J Bone Miner Res . 1987 ; 2 ( 6 ): 559 – 565 . Crossref PubMed Google Scholar
74. Osta B , Benedetti G , Miossec P . Classical and Paradoxical Effects of TNF-α on Bone Homeostasis . Front Immunol . 2014 ; 5 : 48 . Crossref PubMed Google Scholar
75. Karnes JM , Daffner SD , Watkins CM . Multiple roles of tumor necrosis factor-alpha in fracture healing . Bone . 2015 ; 78 : 87 – 93 . Crossref PubMed Google Scholar
76. Wallace A , Cooney TE , Englund R , Lubahn JD . Effects of interleukin-6 ablation on fracture healing in mice . J Orthop Res . 2011 ; 29 ( 9 ): 1437 – 1442 . Crossref PubMed Google Scholar
77. Wong RMY , Choy MHV , Li MCM , et al. A systematic review of current osteoporotic metaphyseal fracture animal models . Bone Joint Res . 2018 ; 7 ( 1 ): 6 – 11 . Crossref PubMed Google Scholar
78. Wong RM , Thormann U , Choy MH , et al. A metaphyseal fracture rat model for mechanistic studies of osteoporotic bone healing . Eur Cell Mater . 2019 ; 37 : 420 – 430 . Crossref PubMed Google Scholar
79. Xiong C , Zhang Z , Baht GS , Terrando N . A Mouse Model of Orthopedic Surgery to Study Postoperative Cognitive Dysfunction and Tissue Regeneration . J Vis Exp . 2018 ; 132 : 56701 . Crossref PubMed Google Scholar
80. Baht GS , Silkstone D , Vi L , et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin . Nat Commun . 2015 ; 6 : 7131 . Crossref PubMed Google Scholar
81. Volpin G , Cohen M , Assaf M , et al. Cytokine levels (IL-4, IL-6, IL-8 and TGFβ) as potential biomarkers of systemic inflammatory response in trauma patients . Int Orthop . 2014 ; 38 ( 6 ): 1303 – 1309 . Crossref PubMed Google Scholar
82. Beeton CA , Chatfield D , Brooks RA , Rushton N . Circulating levels of interleukin-6 and its soluble receptor in patients with head injury and fracture . J Bone Joint Surg Br . 2004 ; 86-B ( 6 ): 912 – 917 . Crossref PubMed Google Scholar
83. He LH , Liu M , He Y , et al. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation . Sci Rep . 2017 ; 7 : 42385 . Crossref PubMed Google Scholar
84. Qayoom I , Raina DB , Širka A , et al. Anabolic and antiresorptive actions of locally delivered bisphosphonates for bone repair: A review . Bone Joint Res . 2018 ; 7 ( 10 ): 548 – 560 . Crossref PubMed Google Scholar
85. Wang C , Zheng GF , Xu XF . MicroRNA-186 improves fracture healing through activating the bone morphogenetic protein signalling pathway by inhibiting SMAD6 in a mouse model of femoral fracture: an animal study . Bone Joint Res . 2019 ; 8 ( 11 ): 550 – 562 . Crossref PubMed Google Scholar
86. Bucher CH , Lei H , Duda G , et al. The role of immune reactivity in bone regeneration. In: Zorzi AR, de Miranda JB, eds. Advanced Techniques in Bone Regeneration . London : IntechOpen , 2016 : 169 – 194 . Google Scholar
87. Nathan K , Lu LY , Lin T , et al. Precise immunomodulation of the M1 to M2 macrophage transition enhances mesenchymal stem cell osteogenesis and differs by sex . Bone Joint Res . 2019 ; 8 ( 10 ): 481 – 488 . Crossref PubMed Google Scholar
Author contributions
S. K-H. Chow: Conceptualized the study, Wrote the manuscript.
Y-N. Chim: Conducted the literature search and data synthesis, Wrote the manuscript.
J-Y. Wang: Conducted the literature search and data synthesis.
R. M-Y Wong: Conceptualized the study.
V. M-H. Choy: Conducted the literature search and data synthesis.
W-H. Cheung: Conceptualized the study.
Funding statement
This study is supported by the Osteosynthesis and Trauma Care Foundation (OTC) Research Funds (2015-SCNT), Direct Grant of Research, The Chinese University of Hong Kong (CUHK) (2017.047), and General Research Fund of the Research Grants Council of Hong Kong (14113018).
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
None declared.
Acknowledgements
S.K-H. Chow and Y-N. Chim are co-first authors.
Ethical review statement
This study did not require ethical approval.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









