Abstract
Bone is a dynamic tissue with a quarter of the trabecular and a fifth of the cortical bone being replaced continuously each year in a complex process that continues throughout an individual’s lifetime. Bone has an important role in homeostasis of minerals with non-stoichiometric hydroxyapatite bone mineral forming the inorganic phase of bone. Due to its crystal structure and chemistry, hydroxyapatite (HA) and related apatites have a remarkable ability to bind molecules. This review article describes the accretion of trace elements in bone mineral giving a historical perspective. Implanted HA particles of synthetic origin have proved to be an efficient recruiting moiety for systemically circulating drugs which can locally biomodulate the material and lead to a therapeutic effect. Bone mineral and apatite however also act as a waste dump for trace elements and drugs, which significantly affects the environment and human health.
Cite this article: Bone Joint Res 2020;9(10):709–718.
Article focus
-
This review article provides a historical background on the structure of the bone mineral hydroxyapatite (HA) and its capacity to bind different agents.
-
The article explores how different chemicals bind to HA and the concomitant effect on human health and the environment.
Key messages
-
Due to the chemical affinity of a broad spectrum of drugs (including antibiotics, bisphosphonates, and radioactive tracers) to HA, it acts as a recruiting platform for systemically administered agents resulting in local targeted drug delivery.
-
HA also acts as a waste dump for several agents in nature and the human body, leading to unwanted harmful side-effects.
-
Stringent measures should be taken to measure the accretion and safety of contaminants in bone mineral.
Strengths and limitations
-
An innovative approach for drug accretion to particulate HA is described.
-
This article is not a systematic review but gives a perspective on how hydroxyapatite binds different agents and acts as a recruiting moiety.
Introduction
Apatite is a generic name for a large group of phosphate-containing minerals, essential to providing structural support in living and dead material in nature. Apatite existed before life started and was a prerequisite for the evolution of single cell organisms into vertebrates. Hydroxy(l)apatite (HA – Ca5(PO4)3(OH)), often stated as Ca10(PO4)6(OH)2 due to its hexagonal crystallographic symmetry, is the stoichiometric form of the inorganic mineral phase in bone and tooth (Figure 1a). Stoichiometric HA is rare in nature; geologically fluorapatite (FAP, Ca5(PO4)3F), chlorapatite (CAP, Ca5(PO4)3Cl), and multiple substituted apatites are found in rocks and corals.1,2 More than 300 apatite-type minerals exist, with elements from the entire periodic table replacing Ca, P, and OH in the fundamental apatite crystal structure.3 In mammalian bone and teeth, further substitutions are found, including both A and B position carbonate substituted apatites (Ca10[(PO4)6-y(CO3)y][(OH)2-2x(CO3)x]).4,5
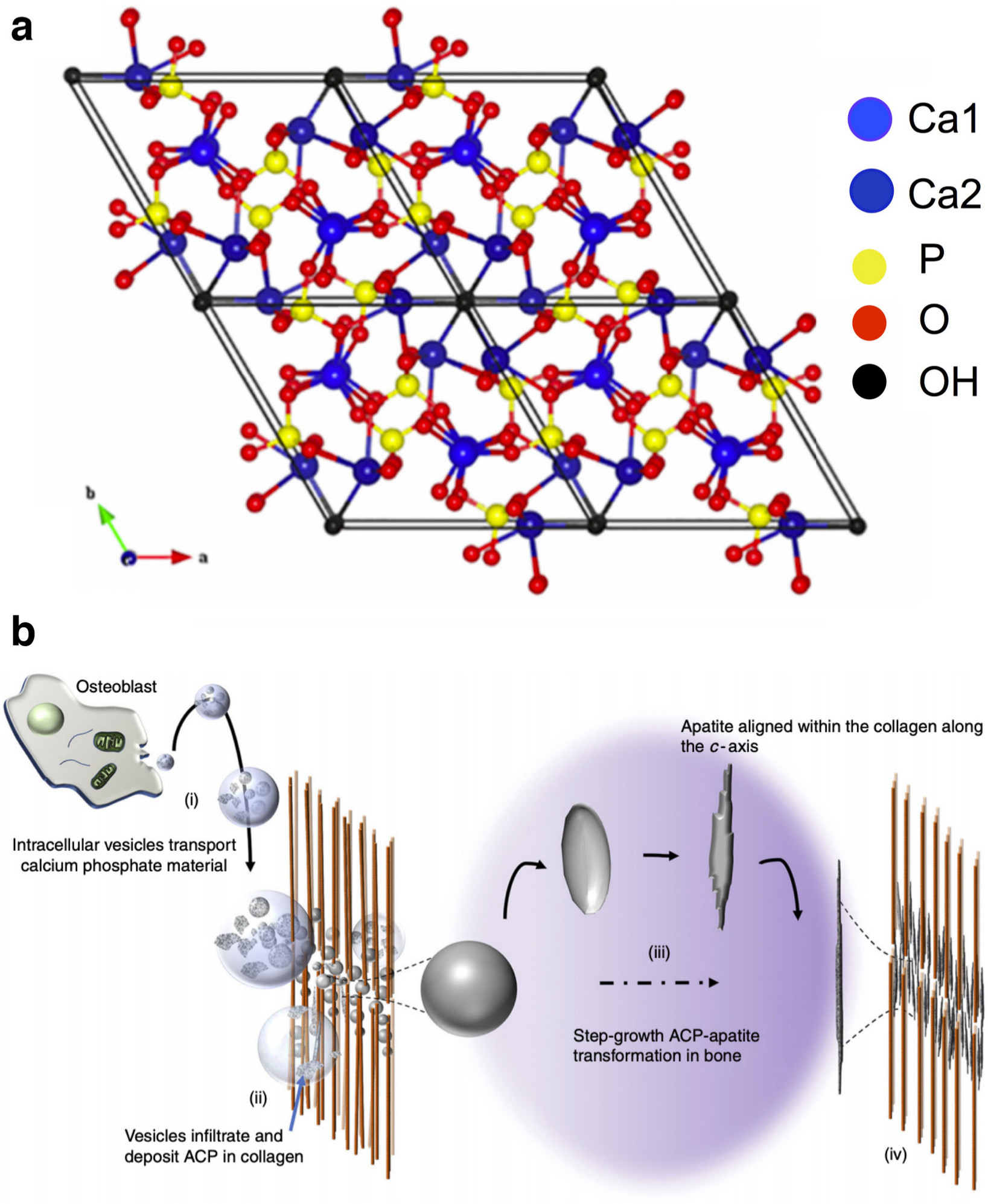
Fig. 1
Hydroxyapatite (HA) structure and speculated mechanism of apatite deposition in bone. a) Unit cell of HA in the (001) plane (reproduced with permission from the ICRP (Fihri A, Len C, Varma RS, Solhy A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coordination Chemistry Reviews. 2017;347:48-76).6 b) Possible mechanism of HA deposition by osteoblasts in the form of round vesicles (i) containing amorphous calcium phosphate nano-crystals. These vesicles infiltrate the collagen matrix and embed themselves (ii) where they undergo a step-growth (iii) from amorphous calcium phosphate (ACP) to crystalline HA. Finally, (iv) the apatite crystals are elongated and aligned with the long axis of the collagen fibers (reproduced from Lotsari A, Rajasekharan AK, Halvarsson M, Andersson M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat Commun. 2018;9(1):4170.7 Ca, calcium; P, phosphorus; O, oxygen; OH, hydroxyl.
Human bone consists of approximately 55% minerals, 35% collagen, and 10% water by weight.8 The mean annual bone turnover in a healthy adult has been approximated to be about 3.6% with clear differences between cortical and metaphyseal bone as reported by the International Commission on Radiological Protection in 1972.9 The process is continual and is started by osteoclast progenitors cleaning up old or damaged bone by a series of acidic and enzymatic treatments of old bone/bone mineral followed by infiltration of osteoprogenitor cells differentiating into HA-producing osteoblasts. HA is formed within the collagen fibrils down at the atomic scale.7 Cells send out vesicles containing calcium and phosphate ions, deposited within the collagen network. A series of biochemical processes lead to the formation of an ordered, crystalline, but non-stoichiometric HA structure (Figure 1b). The nano-apatite crystals located in the collagen fibrils however make detailed analysis of trace elements in bone mineral difficult. The mineral and collagen content are commonly not extracted directly from the bone, but from ash, which also contains trace elements from the collagen.
The most highly mineralized bone recorded, with a mineral content of 96%, is the rostrum (upper jaw bone) of the whale Mesoplodon densirostris, which can be used for understanding the chemistry of bone mineral.8 Recently an extensive bone mineral content analysis was applied using both bulk analysis (radiograph fluorescence, thermogravimetry, and carbon content) and elemental mapping (electron microprobe). These analyses showed that the mineral in the rostrum on average had a composition of (Ca8.40Mg0.20Na0.54)[(PO4)4.87(CO3)1.13](OH)0.87, different from geological HA (Ca10(PO4)6(OH)2) as the calcium is also replaced by magnesium and sodium while phosphate and hydroxyl groups are substituted by carbonate moieties as well.10
Tooth enamel is typically 97% mineral, 1% enamelin, a glycoprotein, and 3% water by weight.11,12 Again, the mineral phase is rarely stoichiometric with the most common substitution being fluorine-forming fluorapatite, which better resists the development of caries by providing extra protection to the teeth in acidic conditions.13 Dentine, the hard-inner structure of teeth, like bone, contains typically 40 vol% tooth mineral, which corresponds to 60 wt% mineral.14
Hydroxyapatite and its chemical interactions: a historical perspective
Besides being essential in high load-bearing structures like bones and teeth, HA has a remarkable ability to bind molecules, owing to its crystal structure and chemistry. The first report of a substance binding to bone was by Leminus in 1567, describing that the root of the madder plant made bone red (Figure 2).15
Fig. 2
Rendering of the Rubia tinctorum (the rose madder) by Franz Eugen Köhler (Original source: Köhlers Medizinal Pflanzen, 1897).
Almost 200 years later, Belchier in 1736 noticed at an evening dinner that the pork he was served had red bones and was later informed by the host that the pigs had been fed mashed madder root.15 This was the first report of a systemically administered agent traced in bone. John Hunter, in the late 1700s, studied bone growth by intermittent feeding of pigs with madder root.16 Hunter observed white and red bands in the growth zones of the pig mandible. More recently, Hoyte in the 1950s used Alizarin (1,2-dihydroxyanthraquinone), an ingredient of the madder root, for vital staining of bone.17 Based on its interaction with bone, Alizarin is still used today for dynamic histomorphometric studies of mineral apposition.17,18
Apart from Alizarin, several other substances have affinity for apatite. In 1935, while working with Niels Bohr (Nobel Prize winner in Physics, 1932) in Copenhagen, Georg de Hevesy, a Hungarian chemist, published the first study on radioactive bone mineral tracers in rats with 32P sodium phosphate.19 The results showed dynamic bone turnover and continuous uptake of the phosphorus atoms. He is regarded as the founder of radioanalytical chemistry, radiograph fluorescence analysis, father of nuclear medicine, and received the 1942 Nobel Prize in Chemistry. In 1950 in Lund, Sweden, influenced by Niels Bohr and his son Hans Bohr, Göran Bauer, together with Arvid Carlsson, the future 2002 Nobel Prize winner in Medicine, systemically administered a bone mineral-seeking radioactive tracer, 45Ca, to study bone accretion and metabolism.20,21 Even before the availability of MRI and CT imaging, they and others used bone-seeking radioactive isotopes such as 85Sr for identifying bone turnover. This eventually paved the way for 99Tc scintigraphy, now commonly used for detection of osseous infections, tumours, or other metabolic disorders. Further, with the advancement in chemistry/radiosynthesis techniques and modern imaging equipment, the same tracer 99Tc has been coupled with hydroxy diphosphonate (99Tc-HDP) or methylene diphosphonate (99Tc-MDP) to study mineralization of human stem cells in vitro22 and bone turnover in vivo in humans.23
Hydroxyapatite as a drug-seeking moiety
The most well-known bone-seeking drugs are the bisphosphonates, and their therapeutic use was found by Herbert Fleisch in the 1960s.24 The bisphosphonates are synthetic, non-hydrolyzable analogues of inorganic pyrophosphates having carbon as a bridge between two phosphate groups, resulting in high mineral-binding capacity. Of the antiresorptive bisphosphonates available today, those containing nitrogen in the heterocyclic ring, for example zoledronic acid, are 10,000 times more potent than non-nitrogen-containing bisphosphonates.25,26 Bisphosphonates are today widely used for retarding osteoporosis by acting on osteoclasts and slowing bone resorption.25,27
In our own studies, we have recently implanted stoichiometric synthetic particulate HA in an ectopic muscle pouch model in rats.28 Two weeks later, the animals were systemically administered zoledronic acid (ZA), which sought the HA particles in the abdominal muscle pouch (Figure 3a).28 The HA particles could even be reloaded and the local ZA accretion in the implanted HA pellet increased with reloading.
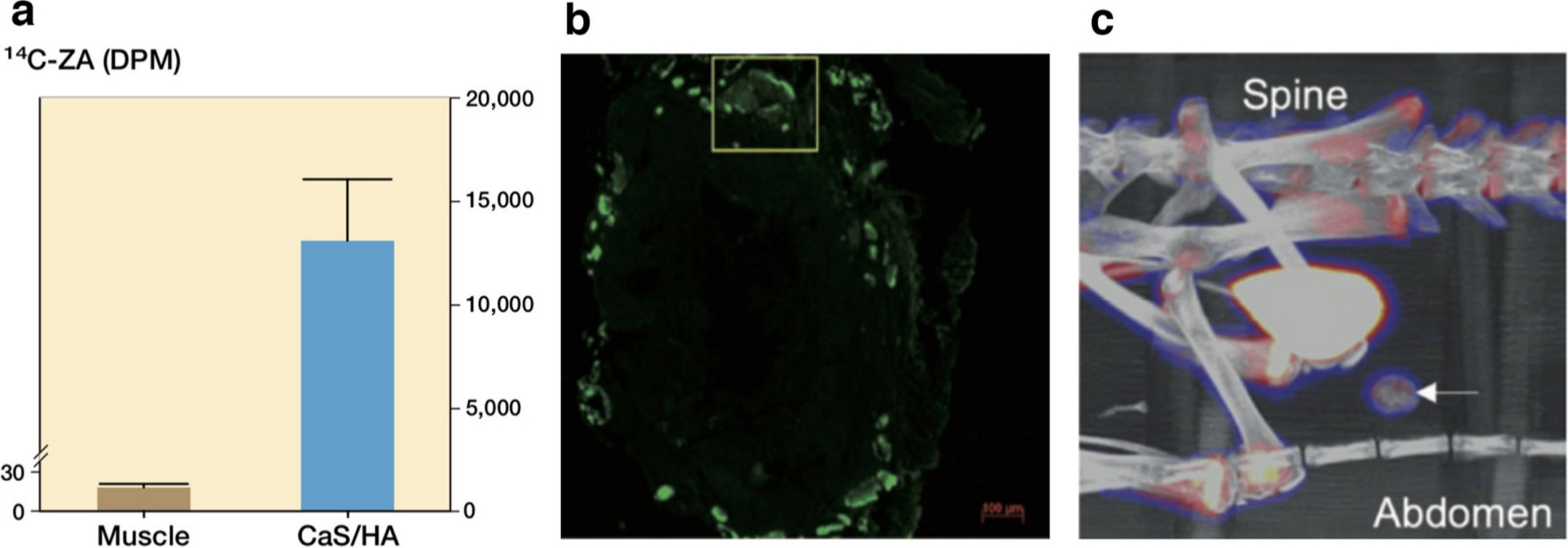
Fig. 3
Hydroxyapatite (HA) as a recruiting platform for systemically circulating drugs in rats. a) Uptake of 14C-zoledronic acid (ZA) in a pellet of calcium sulphate (CaS)/HA implanted in the abdominal muscle pouch. ZA was injected subcutaneously, two weeks post-implantation of CaS/HA biomaterial and animals were euthanized 24 hours later. b) and c) show the uptake of antibiotic tetracycline (injected subcutaneously) and radioactive tracer 18F (intravenous injection) in a pellet of CaS/HA biomaterial placed in the abdominal muscle pouch. Tetracycline uptake was measured using fluorescence microscopy while 18F uptake was accessed using positron emission tomography-CT. Figure has been modified from Raina et al.28
In another long-term in vivo study, radioactive ZA was added to apatite particles implanted in osteoporotic femoral necks in rats. Radioactive active ZA was measured in the synthetic HA particles as well as in the contralateral femur when the animals were euthanized at six months.29 It appears that bisphosphonates not only bind to the mineral, but remain bound in the bone for an extended period, potentially for years. The drug is eventually released in small amounts by osteoclast activity, only to be recirculated and rebound into vesicles that precipitate into new calcium phosphate mineral.
In several osteoporotic animal studies, we have administered ZA both systemically and locally in multiple anatomical locations, but have never been able to show a positive effect on either bone formation or biomechanical properties of ZA on cortical bone30 unlike cancellous bone.31 In patients treated long-term with bisphosphonates, a rare complication is the atypical femoral fracture, a low-energy diaphyseal fracture in a weight-bearing limb (Figure 4).32 The probable cause is decelerated osteoclastic activity and delayed or even aborted bone remodelling which, without the natural bone repair system, allows a 'critical length' crack to develop in the cortical bone.
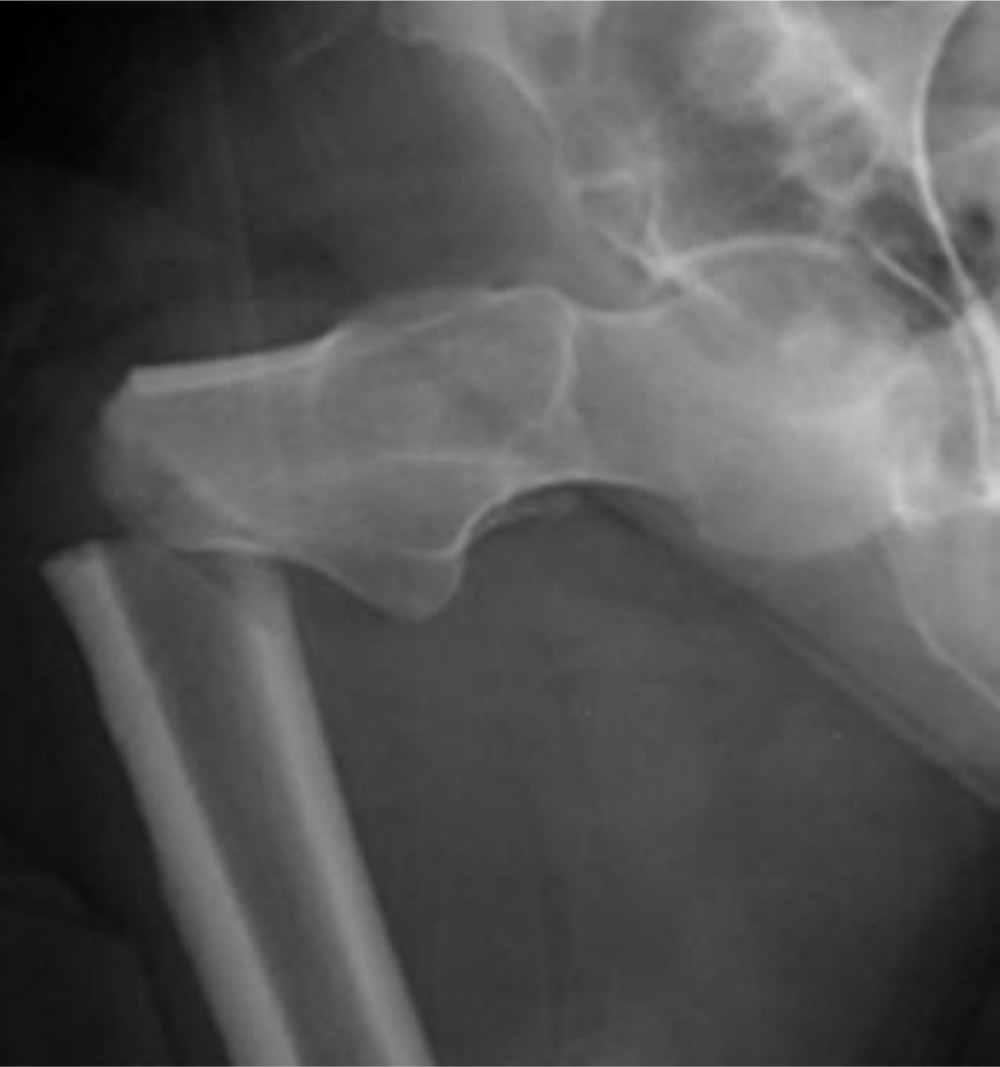
Fig. 4
Anteroposterior radiograph of an atypical femoral fracture caused by the prolonged use of bisphosphonates. Figure is reproduced from Lloyd et al.32
Antibiotics in bone mineral and infection
Tetracycline was the first antibiotic that was traced in bone after systemic administration as reported by Andre33 in 1956. Fluorescence studies have later verified that bone, enamel, and dentine incorporate tetracycline, leading to discoloured teeth and bone in foetuses, children, and adolescents.34,35 Perrin36 confirmed in 1967 that tetracycline strongly binds to HA, including mineral in dead bone, and proposed a chemical interaction between the mineral phase and tetracycline. HA can also absorb tetracycline in solution.37 We have recently shown that systemically administered tetracycline is capable of seeking and binding to a microparticulate apatite moiety implanted in a muscle pouch (Figure 3b).28 HA-binding substances can bind the substrate either by sequestering the calcium/phosphate ions or by forming hydrogen bonds.38 Hydrogen bonding can be efficient if the binding agent contains hydrophobic moieties. The affinity of an antibiotic to apatite particles would foremost depend on its chemical structure, i.e. binding capacity to the calcium, phosphate, and hydroxyl groups of HA, and the number and size of the particles.
During the last 50 years, aseptic and antiseptic measures have focused on prevention and hindering bacteria from colonizing and forming biofilms on implanted devices.39 If a deep bone or joint infection (DBJI) occurs, the implant is commonly removed and temporarily exchanged with an acrylate spacer containing very high levels of antibiotics such as gentamycin and vancomycin. Frequently, bacteria can still be found in the surrounding soft tissue and bone when the spacer is removed during a second stage, at prosthetic reimplantation.40 The costs associated with reoperations in the case of a revision total hip arthroplasty (THA) can be as high as £50,000 GBP, as recently reported by Ahmed et al41 from the UK.
A novel treatment in DBJI is to implant a local microparticulate biphasic calcium sulphate-apatite carrier containing antibiotics, i.e. gentamycin or vancomycin, released in a targeted location in supraphysiological concentrations 100 to 1,000 times above the minimal inhibitory concentration (MIC) levels, but avoiding systemic side effects.42,43 The carrier is injectable and sets in situ in the defect created by radical surgical debridement. A success rate of up to 95% healing has been reported without infection recurrence in chronic longstanding osteomyelitis.44 Gentamycin and vancomycin, in contrast to tetracycline, do not bind to HA, and consequently are completely released within a month, following the resorption of the soluble calcium sulphate (CaS) phase in the CaS/HA carrier.42,43 Even without the use of a carrier, prolonged use of antibiotics such as gentamycin or vancomycin have been reported to have no impact on bone turnover markers such as carboxy-terminal collagen crosslinks (CTX) or osteocalcin in humans.45 We have not been able to find any studies reporting a negative effect of long-term antibiotic usage or accumulation in bone with the exception of tetracycline. Recurrence of infection is reduced both in chronic osteomyelitis as well as in PJI, both with non-HA-binding antibiotics such as penicillin46 or with HA-binding antibiotics such as rifampicin,47 even with prolonged treatment.
So far, no one has provided a full rationale as to why certain systemic antibiotic combinations have superior effect when it comes to biofilm-associated bone infections; we suggest that the reason could be the difference in antibiotic accretion in bone mineral.48 As a possible coincidence, the recommended and second level systemic antibiotics in DBJI, such as rifampicin and daptomycin, all have chemical structures that allow them to bind to apatite.
Qayoom et al49 recently reported in an in vitro study that rifampicin used for DBJI and tuberculosis, added to a biphasic calcium sulphate/HA carrier, binds to the HA phase. They found that 50% of the antibiotic remained in the material at three months, contrary to the non-binding anti-tuberculosis antibiotic isoniazid, which was released within the first month. This may explain the clinical rationale behind the proven efficacy of systemic antibiotic treatment in DBJI, using a combination of vancomycin/gentamycin with rifampicin.50
It is well known that mycobacteria in bone tuberculosis can be dormant in their 'bone sarcophagus' for decades and then relapse. The senior author (LL) has in his clinical practice seen a tibial diaphyseal osteomyelitis recurring at the original location with Staphylococcus aureus after 50 years of dormancy. In the literature, an osteomyelitis case was published with a genetically identical S. aureus strain recurring after 75 years of quiescence.51 The bone marrow, surrounding soft tissue, and the glycocalyx on the surface of implanted hardware are all well-known reservoirs for bacteria. Bacteria can also be internalized in bone cells such as osteoblasts or osteoclasts but probably only during the acute or subacute phases of an infection and generally do not persist.52 However, in long-standing chronic osteomyelitis, bacteria have recently been shown to reside sessile in the osteocyte-lacuna canalicular network (OLCN) in the cortical bone (Figure 5).53 This is a plausible long term 'hide away' and could explain why bacteria could survive in bone lifelong. This cortical bone OLCN sarcophagus needs to be opened and 'the sleeping beauty' i.e. dormant sessile bacteria attacked using new treatment methods.
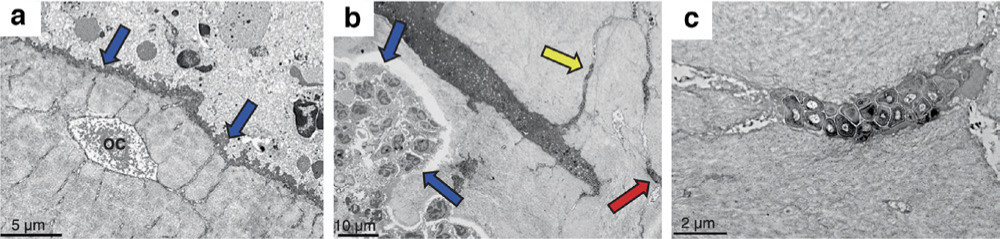
Fig. 5
Bacterial attack on bone via the osteocyte lacunocanalicular network. a) and b) show transmission electron microscopy (TEM) images of the cortical bone from a mouse model of implant infection created using Staphyloccocus aureus. S. aureus develops an attack front to eradicate the bone lining cells (blue arrows) and embedding the osteocyte (OC). After compromising the cortical bone, the bacteria then colonize the osteocyte lacunocanalicular network (yellow arrow) and migrate within the canaliculi by expanding the structure to reach other osteocytes (red arrow in Fig. 5b). They simultaneously demineralize the bone matrix. c) An osteocyte lacuna inhabited by infiltrating S. aureus bacteria. Marker bars are: a) 5 μm (6,000× magnification); b) 10 μm (1,800× magnification); and c) 2 μm (12,000× magnification). Image is reproduced from Masters et al.53
A possible future development could be to use a local HA or other calcium phosphate carriers preloaded or mixed with an HA-binding antibiotic, and combine it with a non-binding antibiotic added to a material locally. An initial high, effective, and sustained local release of, for instance, gentamycin or vancomycin will be achieved.42,43 By osteoclast resorption, a HA-bound antibiotic, such as rifampicin or a tetracycline, will be continuously released in the OLCN and thereby poison the sessile bacteria in the cortical bone sarcophagus in the long term. Even more intriguing is the recent finding that a local moiety containing tens of millions of HA particles can be loaded and reloaded after implantation by systemic drug administration.28,54
Bone: a waste dump that affects health
Fluorosis an environmental bone dump disease
Fluorine binds rapidly to carbonated HA in bone and is found in small amounts in nature, in ground water leaking out from fluorapatite (FAP) in rocks. HA precursors are octa-calcium phosphate (OCP) and in the case of long-term high F– exposure, the F– ion will be accumulated into bone and teeth by the formation of fluorapatite (FAP) (Ca5(PO4)3F) instead of HA.55,56
Fluorine can in certain endemic areas reach toxic levels in drinking water resulting in severe growth deformities in children (Figure 6a and 6b). In adults, fluorine causes 'marble bone disease' with severe secondary osteoarthritis (Figure 6c and 6d).57,58 The consumption of drinking water with a fluoride concentration higher than the World Health Organization's (WHO) recommended limit of 1.5 mg/l59 is a health concern for more than 250 million people today worldwide and an endemic causing serious disability in, for example, India.60 In many industrial countries, although controversial,61 fluorine is added to drinking water. It is also an ingredient in toothpaste to reduce dental caries and recommended by dentists as mouthwash, especially for children. FAP is more resistant to the low pH produced by dental caries than HA.
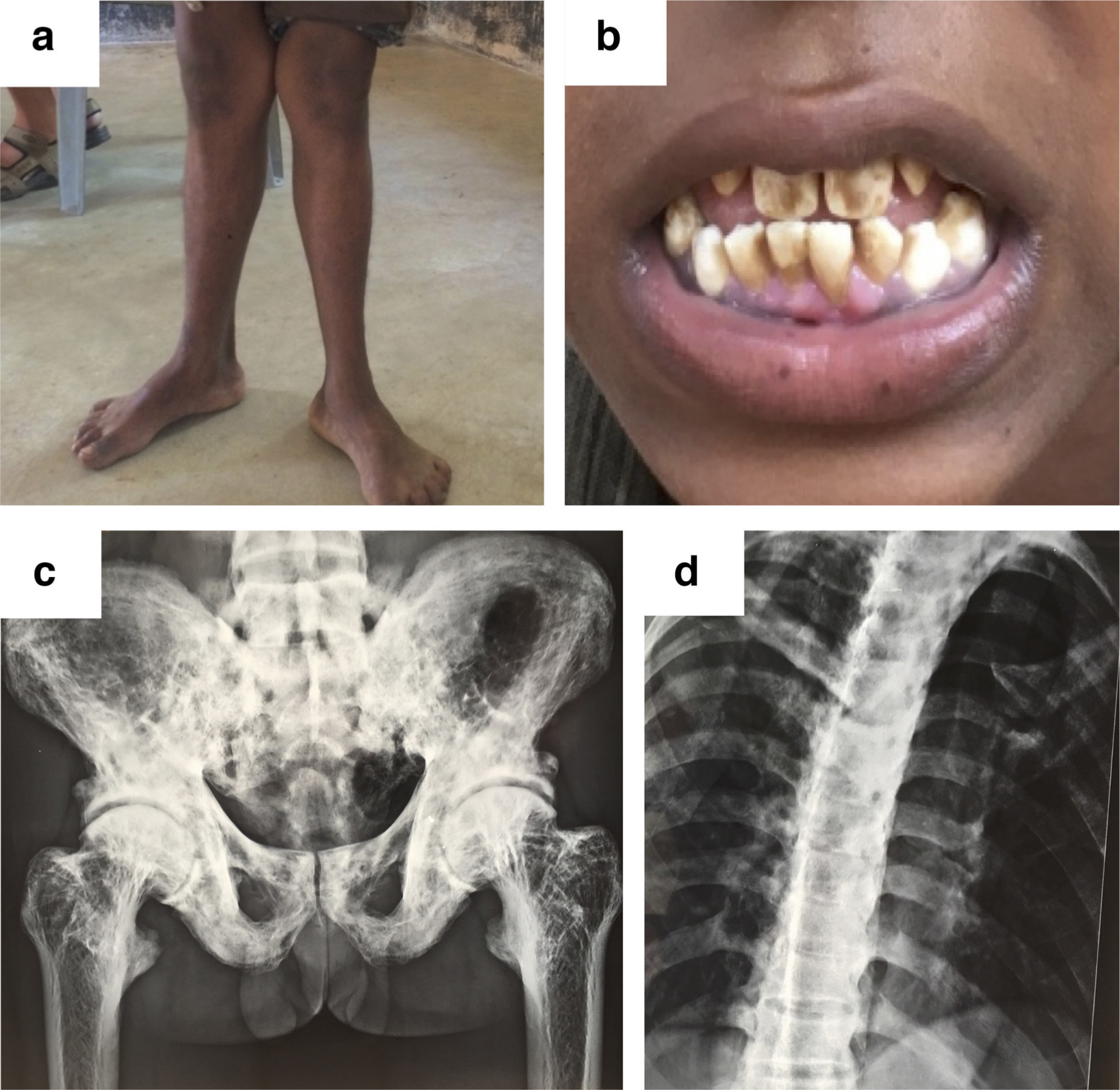
Fig. 6
Anteroposterior radiographs showing the skeletal effects of fluoride poisoning through drinking water in humans. a) Knee deformity in a 12-year-old boy and b) fluoride-induced discolouration of teeth. c) and d) show radiographs of patients exposed to high fluoride levels showing dense sclerotic pelvic bone and spine. Unpublished images used with permission from Prof. Urban Rydholm, Lund University Hospital, Lund, Sweden.
Triclosan and apatite: an environmental and health concern?
Triclosan (TC) is a phenyl ether with a broad antibacterial spectrum widely used in soap, surgical sutures, toothpaste, deodorants, clothes, sun protectors, etc. The impact on health is the subject of debate - over-the-counter soap containing TC has been banned in the USA and Canada - as is the antibacterial effect of TC. There is no conclusive evidence that sutures containing TC have any effect on deep surgical site infection (SSI), and very limited evidence for preventing superficial infection. In 2019 after a large randomized controlled trial, TC was not found to prevent superficial SSI and it was concluded that the use of TC-coated sutures in hip and knee arthroplasty does not lead to a reduction in the rate of SSI.62
In several studies, TC has been found in water,63 and in animal and human tissue.64 HA has been shown to interact with TC via hydrogen bonding and electrostatic interactions. Previously, Gilbert and Watson65 showed that TC binds to saliva-coated enamel. Coral reefs, like bone, are made up of calcium derivates, mainly calcium carbonate, and it has been reported that sunscreen ingredients, including TC, accelerate coral bleaching (Figure 7).66 In 2020 Adamson and Shinkai67 reported that substances in common sun lotions are being absorbed through human skin. The reported blood levels exceeded Food and Drug Administration (FDA) safety levels for several days.67,68 It was concluded that this needs to be investigated further to exclude any harmful effects. Countries with tourist beaches and coral reefs are therefore increasingly banning TC-containing sunscreens, to save the coral reefs.

Fig. 7
Image of a bleached coral reef in the Great Barrier Reef, courtesy of Jayne Jenkins, reproduced with permission.
In a large recent study, women with high TC levels in their urine, compared with those with low levels, suffered from osteoporosis as indicated by a low bone mineral density (BMD) at several anatomical locations.69 The study does not prove a causal relation between TC in urine and osteoporosis, or the risk of sustaining a fragility fracture, and long-term prospective studies with multiple timepoints are needed, but an explanation could be that women with lower BMD may be in a catabolic phase due to increased osteoclast resorption releasing TC bound to apatite. TC chemically mimics oestrogen, thus affecting bone turnover. TC binding to HA needs to be explored both in vitro as well as in vivo, and degradation and release measured. The level of TC in animal, human apatite, and coral apatite should be studied in exposed areas and compared to areas with non-contaminated water. In addition, the regulation of TC should be tightened, at least in consumer goods.
Husbandry contaminating the environment and human bone mineral with antibiotics
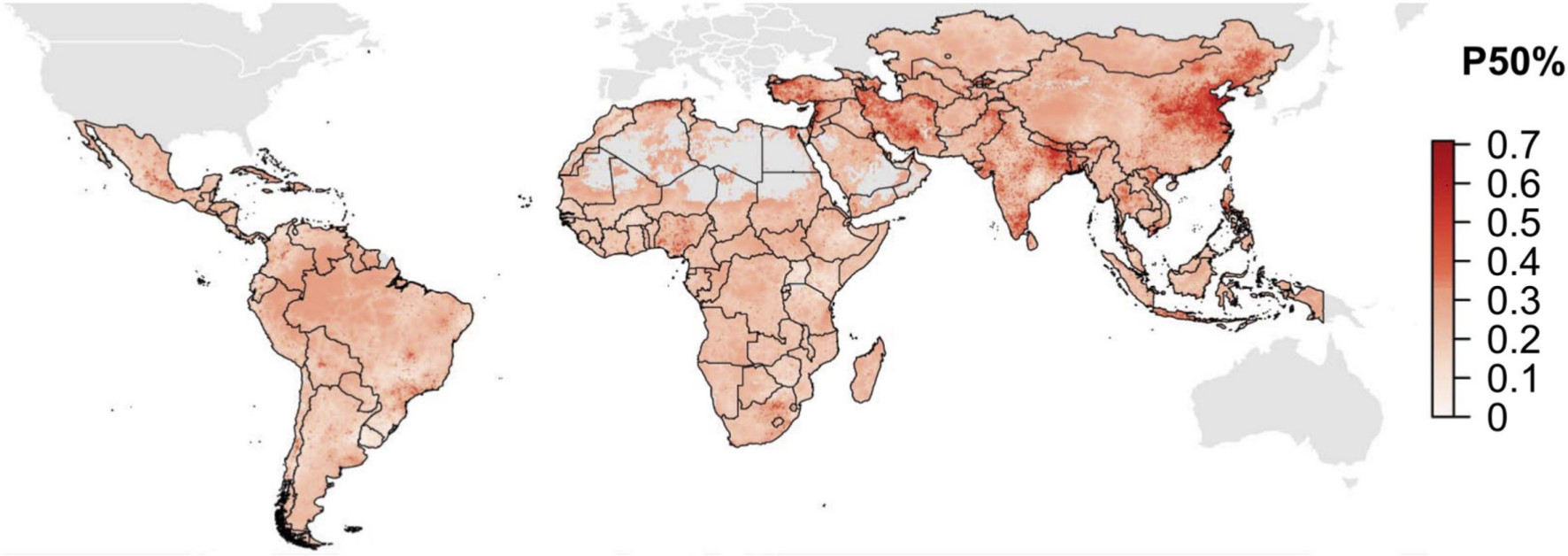
Husbandry accounts for three-quarters of all antibiotics consumed. The rationale is mainly economical and aims to increase the weight and improve the lives of cattle, pigs, and poultry.70 In recent years, farmed fish have been given antibiotics to reduce the infection burden during their short life cycle. Resistant bacteria, crossing over between husbandry and humans, have been documented: a recent analysis revealed that from 2000 to 2018, the proportion of antimicrobials showing more than 50% resistance increased from 0.15 to 0.41 in chickens, from 0.13 to 0.34 in pigs, and plateaued between 0.12 and 0.23 in cattle.71 A global map based on data for the antibiotic use and development of resistance in husbandry is available and shows middle-income countries to be the worst affected (Figure 8).71 Resistant bacteria travel light and can cross borders without passports. Regulation has improved and in the USA, use of tetracycline, a common antibiotic used in cattle that is given at any time during the animal’s life cycle, has been reduced from 80% to 40%. In Germany, probably the most regulated country in Europe, farming administers antibiotics seven times more often than in Sweden, although still on a low level. In some countries tetracyclines are still routinely added to daily feeds. Tigecycline, a commonly used tetracycline, is also used in humans. The concern is that tetracyclines bind to HA and remain in the bone for years.72 Thus, even if no antibiotic is given several weeks before slaughtering, the bone mineral remains loaded with antibiotics, which is highly relevant if the bone is industrially processed.
Bone settlers as waste dumpers
There is a large industrial sector with production based on the mineral and collagen components of husbandry bone post mortem.73 Collagen is extracted and used in several industrial processes, including food production, as gelatine. For bone, heating to 200°C and/or acid softening is common, followed by grinding into a bone powder. There are large differences in thermostability between antibiotics used in husbandry: for instance, doxycycline, another tetracycline, remains stable even above 150°C.74 The bone meal is a valuable additive used in pet food and as fertilizer for vegetables.73 In some countries it is added as a meat expander/substitute in hamburgers.
There is a lack of studies on bone meal as a source of secondary antibiotic contamination, and a lack of data regarding degradation, release, and resistance pattern in humans, the soil, and water. There is also a dearth of research on trace elements bound to HA in husbandry bone both in living animals and post mortem in the meat industry. We suggest that control of antibiotic food contamination extends to hard tissue, as well as the regulation of the 'bone settlers' production technology.
Antibiotic water contamination - a harmful circle
The rise in antibiotic-resistant bacteria is a global health emergency that may kill ten million people by 2050, according to a UN report.75 In Europe 33,000,76 and globally 700,000,75 people were recently reported to die annually due to an antibiotic-resistant bacterial infection. Researchers recently tested 711 rivers and lakes in 72 countries and found antibiotics in 65% of them.77,78 In 111 of the sites, the concentrations of antibiotics exceeded safe levels, possibly contributing to the development of bacterial resistance. Lower-income countries generally had higher antibiotic concentrations in rivers and were often lacking the technology to remove the drugs.
Samples taken from rivers in Europe, such as the Thames in the UK and the Danube in Austria, also showed severe antibiotic contamination for seven antibiotics at nearly four-times the 'safe' level. At worst, it was 300 times over the safe limit in some rivers.78 Some concentrations are even so high that drinking three glasses daily from the Danube could potentially be sufficient as oral antibiotic treatment for an upper respiratory infection. Some of the antibiotics, such as tetracyclines, may again result in animals drinking the water with their bone reactive mineral phase acting as a waste dump. An antibiotic-induced minor change in the microbiome may increase the risk for an infection and postoperative DBJI.79
In conclusion, we have described novel binding mechanisms for drug accretion to micro- and nano-apatite particles biologically activating the material. By systemic administration, pharmaceutical agents can seek and biomodulate a local moiety with apatite particles acting as a Trojan rechargeable drug reservoir. The chemical interactions between specific drugs and apatite, including non-stoichiometric HA found in bone, are however also responsible for a broad range of potential harmful effects. Antibiotics administered in husbandry pose considerable crossover risks of accrued drugs from animal bone to humans affecting the microbiome.
References
1. Broughm SG , Hanchar JM , Tornos F , Westhues A , Attersley S . Mineral chemistry of magnetite from magnetite-apatite mineralization and their host rocks: examples from Kiruna, Sweden, and El Laco, Chile . Mineralium Deposita . 2017 ; 52 ( 8 ): 1223 – 1244 . Google Scholar
2. Potts NJ , Barnes JJ , Tartèse R , Franchi IA , Anand M . Chlorine isotopic compositions of apatite in Apollo 14 rocks: Evidence for widespread vapor-phase metasomatism on the lunar nearside ∼4 billion years ago . Geochim Cosmochim Acta . 2018 ; 230 : 46 – 59 . Google Scholar
3. Ptáček P . Introduction to Apatites . Apatites and their synthetic Analogues - Synthesis, structure, properties and applications : IntechOpen , 2016 . Google Scholar
4. Zapanta LeGeros R . Apatites in biological systems . Prog Cryst Growth Charact . 1981 ; 4 ( 1-2 ): 1 – 45 . Google Scholar
5. LeGeros RZ . Properties of osteoconductive biomaterials: calcium phosphates . Clin Orthop Relat Res . 2002 ; 395 : 81 – 98 . Crossref PubMed Google Scholar
6. Fihri A , Len C , Varma RS , Solhy A . Hydroxyapatite: a review of syntheses, structure and applications in heterogeneous catalysis . Coord Chem Rev . 2017 ; 347 : 48 – 76 . Google Scholar
7. Lotsari A , Rajasekharan AK , Halvarsson M , Andersson M . Transformation of amorphous calcium phosphate to bone-like apatite . Nat Commun . 2018 ; 9 ( 1 ): 4170 . Crossref PubMed Google Scholar
8. Rogers KD , Zioupos P . The bone tissue of the rostrum of a Mesoplodon densirostris whale: a mammalian biomineral demonstrating extreme texture . J Mater Sci Lett . 1999 ; 18 ( 8 ): 651 – 654 . Google Scholar
9. International Commission on Radiological Protection . Alkaline earth metabolism in adult man . 1973 . Pergamon Press . Oxford . https://journals.sagepub.com/doi/pdf/10.1016/S0074-27407380001-7i . 20 . (date last accessed 1 October 2020 ). PubMed Google Scholar
10. Li Z , Pasteris JD . Chemistry of bone mineral, based on the hypermineralized rostrum of the beaked whale Mesoplodon densirostris . Am Mineral . 2014 ; 99 ( 4 ): 645 – 653 . Crossref PubMed Google Scholar
11. Beevers CA , McIntyre DB . The atomic structure of fluor-apatite and its relation to that of tooth and bone material. (with plates XVI-XVIII.) . Mineralogical Magazine and Journal of the Mineralogical Society . 1946 ; 27 ( 194 ): 254 – 257 . Google Scholar
12. Posner AS , Duyckaerts G . Infrared study of the carbonate in bone, teeth and francolite . Experientia . 1954 ; 10 ( 10 ): 424 – 425 . Crossref PubMed Google Scholar
13. Brudevold F , McCann HG , Nilsson R , Richardson B , Coklica V . The chemistry of caries inhibition problems and challenges in topical treatments . J Dent Res . 1967 ; 46 ( 1 ): 37 – 45 . Crossref PubMed Google Scholar
14. Goldberg M , Kulkarni AB , Young M , Boskey A . Dentin structure composition and mineralization . Front Biosci . 2011 ; E3 ( 2 ): 711 – 735 . Google Scholar
15. Premkumar S . Textbook of craniofacial growth . First edition : JP Medical Ltd , 2011 . Google Scholar
16. Hunter J . Experiments and observations on the growth of bones . Bone . 1798 ; 7 ( 315330 ): 3 . Google Scholar
17. Hoyte D . A histological study of bone growth using alizarin red AS . New York, New York, USA : Journal of Anatomy, Cambridge University Press , 1956 : 585 – 585 . Google Scholar
18. Hing KA , Best SM , Tanner KE , Revell PA , Bonfield W . Histomorphological and biomechanical characterization of calcium phosphates in the osseous environment . Proc Inst Mech Eng H . 1998 ; 212 ( 6 ): 437 – 451 . Crossref PubMed Google Scholar
19. Chiewitz O , Hevesy G . Radioactive indicators in the study of phosphorus metabolism in rats . Nature . 1935 ; 136 ( 3445 ): 754 – 755 . Google Scholar
20. Bauer GC . Tracer techniques for the study of bone metabolism in man . 10 : Academic Press , 1965 . Google Scholar
21. Bauer GC , Carlsson A , Lindquist B . Metabolism and homeostatic function of bone . Mineral metabolism . 1961 ; 1 ( Part B ): 609 – 676 . Google Scholar
22. Grossner TL , Haberkorn U , Gotterbarm T . (99m)Tc-Hydroxydiphosphonate quantification of extracellular matrix mineralization in 3D human mesenchymal stem cell cultures . Bone Joint Res . 2019 ; 8 ( 7 ): 333 – 341 . Google Scholar
23. Lenora J , Norrgren K , Thorsson O , et al. Bone turnover markers are correlated with total skeletal uptake of 99mTc-methylene diphosphonate (99mTc-MDP) . BMC Med Phys . 2009 ; 9 : 3 – 3 . Crossref PubMed Google Scholar
24. Fleisch H , Russell RG , Francis MD . Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo . Science . 1969 ; 165 ( 3899 ): 1262 – 1264 . Crossref PubMed Google Scholar
25. Drake MT , Clarke BL , Khosla S . Bisphosphonates: mechanism of action and role in clinical practice . Mayo Clin Proc . 2008 ; 83 ( 9 ): 1032 – 1045 . Crossref PubMed Google Scholar
26. Qayoom I , Raina DB , Širka A , et al. Anabolic and antiresorptive actions of locally delivered bisphosphonates for bone repair: a review . Bone Joint Res . 2018 ; 7 ( 10 ): 548 – 560 . Crossref PubMed Google Scholar
27. Favus MJ . Bisphosphonates for osteoporosis . N Engl J Med . 2010 ; 363 ( 21 ): 2027 – 2035 . Crossref PubMed Google Scholar
28. Raina DB , Liu Y , Isaksson H , Tägil M , Lidgren L . Synthetic hydroxyapatite: a recruiting platform for biologically active molecules . Acta Orthop . 2020 ; 91 ( 2 ): 126 – 132 . Crossref PubMed Google Scholar
29. Raina DB , Sirka A , Qayoom I , et al. Long term response to a bioactive biphasic biomaterial in the femoral neck of osteoporotic rats . Tissue Eng Part A . 2020 . Google Scholar
30. Raina DB , Qayoom I , Larsson D , et al. Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery . Biomaterials . 2019 ; 188 : 38 – 49 . Crossref PubMed Google Scholar
31. Širka A , Raina DB , Isaksson H , et al. Calcium Sulphate/Hydroxyapatite carrier for bone formation in the femoral neck of osteoporotic rats . Tissue Eng Part A . 2018 ; 24 ( 23-24 ): 1753 – 1764 . Crossref PubMed Google Scholar
32. Lloyd AA , Gludovatz B , Riedel C , et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance . Proc Natl Acad Sci U S A . 2017 ; 114 ( 33 ): 8722 – 8727 . Crossref PubMed Google Scholar
33. Andre T . Studies on the distribution of tritium-labelled dihydrostreptomycin and tetracycline in the body . Acta Radiol Suppl . 1956 ; 142 : 1 – 89 . PubMed Google Scholar
34. Milch RA , Rall DP , Tobie JE . Bone localization of the tetracyclines . J Natl Cancer Inst . 1957 ; 19 ( 1 ): 87 – 93 . PubMed Google Scholar
35. Milch RA , Rall DP , Tobie JE . Fluorescence of tetracycline antibiotics in bone . J Bone Joint Surg Am . 1958 ; 40-A ( 4 ): 897 – 910 . PubMed Google Scholar
36. Perrin DD . Binding of tetracyclines to bone . Nature . 1965 ; 208 ( 5012 ): 787 – 788 . Crossref PubMed Google Scholar
37. Harja M , Ciobanu G . Studies on adsorption of oxytetracycline from aqueous solutions onto hydroxyapatite . Sci Total Environ . 2018 ; 628-629 : 36 – 43 . Crossref PubMed Google Scholar
38. Misra DN . Adsorption on hydroxyapatite: role of hydrogen bonding and interphase coupling . Langmuir . 1988 ; 4 ( 4 ): 953 – 958 . Google Scholar
39. Gristina AG , Costerton JW . Bacterial adherence and the glycocalyx and their role in musculoskeletal infection . Orthop Clin North Am . 1984 ; 15 ( 3 ): 517 – 535 . PubMed Google Scholar
40. Carli AV , Bhimani S , Yang X , et al. Vancomycin-loaded polymethylmethacrylate spacers fail to eradicate periprosthetic joint infection in a clinically representative mouse model . J Bone Joint Surg Am . 2018 ; 100-A ( 11 ): e76 . Crossref PubMed Google Scholar
41. Ahmed SS , Haddad FS . Prosthetic joint infection . Bone Joint Res . 2019 ; 8 ( 11 ): 570 – 572 . Crossref PubMed Google Scholar
42. Stravinskas M , Horstmann P , Ferguson J , et al. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: in vitro and clinical release studies . Bone Joint Res . 2016 ; 5 ( 9 ): 427 – 435 . Crossref PubMed Google Scholar
43. Stravinskas M , Nilsson M , Vitkauskiene A , Tarasevicius S , Lidgren L . Vancomycin elution from a biphasic ceramic bone substitute . Bone Joint Res . 2019 ; 8 ( 2 ): 49 – 54 . Crossref PubMed Google Scholar
44. McNally MA , Ferguson JY , Lau ACK , et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases . Bone Joint J . 2016 ; 98-B ( 9 ): 1289 – 1296 . Crossref PubMed Google Scholar
45. Mikkelsen KH , Vilsbøll T , Holst JJ , Hartmann B , Knop FK , Frost M . No changes in levels of bone formation and resorption markers following a broad-spectrum antibiotic course . BMC Endocr Disord . 2018 ; 18 ( 1 ): 60 . Crossref PubMed Google Scholar
46. Tevell S , Christensson B . Oral flucloxacillin for staphylococcal osteomyelitis: obsolete or underused? J Bone Jt Infect . 2020 ; 5 ( 1 ): 25 – 27 . Google Scholar
47. Becker A , Kreitmann L , Triffaut-Fillit C , et al. Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France . J Bone Jt Infect . 2020 ; 5 ( 1 ): 28 – 34 . Crossref PubMed Google Scholar
48. Dall GF , Tsang S-TJ , Gwynne PJ , et al. Unexpected synergistic and antagonistic antibiotic activity against Staphylococcus biofilms . J Antimicrob Chemother . 2018 ; 73 ( 7 ): 1830 – 1840 . Crossref PubMed Google Scholar
49. Qayoom I , Verma R , Murugan PA , et al. A biphasic Nanohydroxyapatite/ calcium sulphate carrier containing rifampicin and isoniazid for local delivery gives sustained and effective antibiotic release and prevents biofilm formation . Sci Rep . 2019 ; 10 ( 1 ). Google Scholar
50. Zimmerli W , Sendi P . Role of Rifampin against Staphylococcal Biofilm Infections In Vitro, in Animal Models, and in Orthopedic-Device-Related Infections . Antimicrob Agents Chemother . 2019 ; 63 ( 2 ): e01746 – 18 . Crossref PubMed Google Scholar
51. Gallie WE . First recurrence of osteomyelitis eighty years after infection . J Bone Joint Surg Br . 1951 ; 33-B ( 1 ): 110 – 111 . Crossref PubMed Google Scholar
52. Hudson MC , Ramp WK , Nicholson NC , Williams AS , Nousiainen MT . Internalization of Staphylococcus aureus by cultured osteoblasts . Microb Pathog . 1995 ; 19 ( 6 ): 409 – 419 . Crossref PubMed Google Scholar
53. Masters EA , Trombetta RP , de Mesy Bentley KL , et al. Evolving concepts in bone infection: redefining "biofilm", "acute vs. chronic osteomyelitis", "the immune proteome" and "local antibiotic therapy" . Bone Res . 2019 ; 7 ( 1 ): 20 . Google Scholar
54. Ding M , Hvid I . Where do you want your drugs delivered? Acta Orthop . 2020 ; 91 ( 2 ): 121 – 122 . Crossref PubMed Google Scholar
55. Freeman JJ , Wopenka B , Silva MJ , Pasteris JD . Raman spectroscopic detection of changes in bioapatite in mouse femora as a function of age and in vitro fluoride treatment . Calcif Tissue Int . 2001 ; 68 ( 3 ): 156 – 162 . Crossref PubMed Google Scholar
56. Everett ET . Fluoride's effects on the formation of teeth and bones, and the influence of genetics . J Dent Res . 2011 ; 90 ( 5 ): 552 – 560 . Crossref PubMed Google Scholar
57. Krishnamachari KA . Skeletal fluorosis in humans: a review of recent progress in the understanding of the disease . Prog Food Nutr Sci . 1986 ; 10 ( 3-4 ): 279 – 314 . PubMed Google Scholar
58. Savas S , Çetin M , Akdoğan M , Heybeli N . Endemic fluorosis in Turkish patients: relationship with knee osteoarthritis . Rheumatol Int . 2001 ; 21 ( 1 ): 30 – 35 . Crossref PubMed Google Scholar
59. 2004 . Fluoride in drinking-water. Background document for developement of WHO guidelines for drinking-water quality . https://www.who.int/water_sanitation_health/dwq/chemicals/fluoride.pdf (date last accessed 29 September 2020 ). Google Scholar
60. Muralidharan D , Rangarajan R , Shankar G . Vicious cycle of fluoride in semi-arid India–a health concern . Current Science . 2011 ; 100 ( 5 ): 638 – 640 . Google Scholar
61. Peckham S , Awofeso N . Water fluoridation: a critical review of the physiological effects of ingested fluoride as a public health intervention . ScientificWorldJournal . 2014 ; 2014 : 1 – 10 . Crossref PubMed Google Scholar
62. Sukeik M , George D , Gabr A , Kallala R , Wilson P , Haddad FS . Randomised controlled trial of triclosan coated vs uncoated sutures in primary hip and knee arthroplasty . World J Orthop . 2019 ; 10 ( 7 ): 268 – 277 . Crossref PubMed Google Scholar
63. Kolpin DW , Furlong ET , Meyer MT , et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance . Environ Sci Technol . 2002 ; 36 ( 6 ): 1202 – 1211 . Crossref PubMed Google Scholar
64. Adolfsson-Erici M , Pettersson M , Parkkonen J , Sturve J . Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden . Chemosphere . 2002 ; 46 ( 9-10 ): 1485 – 1489 . Crossref PubMed Google Scholar
65. Gilbert R , Watson G . The tooth surface as a reservoir of antimicrobial activity . J Dent Res . 1986 ; 65 : 787 . Google Scholar
66. Danovaro R , Bongiorni L , Corinaldesi C , et al. Sunscreens cause coral bleaching by promoting viral infections . Environ Health Perspect . 2008 ; 116 ( 4 ): 441 – 447 . Crossref PubMed Google Scholar
67. Adamson AS , Shinkai K . Systemic absorption of sunscreen: balancing benefits with unknown harms . JAMA . 2020 ; 323 ( 3 ): 223 – 224 . Crossref PubMed Google Scholar
68. Matta MK , Zusterzeel R , Pilli NR , et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial . JAMA . 2019 ; 321 ( 21 ): 2082 – 2091 . Crossref PubMed Google Scholar
69. Cai S , Zhu J , Sun L , et al. Association between urinary triclosan with bone mass density and osteoporosis in US adult women, 2005–2010 . J Clin Endocrinol Metab . 2019 ; 104 ( 10 ): 4531 – 4538 . Google Scholar
70. Gaskins HR , Collier CT , Anderson DB . Antibiotics as growth promotants: mode of action . Anim Biotechnol . 2002 ; 13 ( 1 ): 29 – 42 . Crossref PubMed Google Scholar
71. Van Boeckel TP , Pires J , Silvester R , et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries . Science . 2019 ; 365 ( 6459 ): eaaw1944 . Crossref PubMed Google Scholar
72. Muralidharan G , Micalizzi M , Speth J , Raible D , Troy S . Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects . Antimicrob Agents Chemother . 2005 ; 49 ( 1 ): 220 – 229 . Crossref PubMed Google Scholar
73. Jayathilakan K , Sultana K , Radhakrishna K , Bawa AS . Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review . J Food Sci Technol . 2012 ; 49 ( 3 ): 278 – 293 . Crossref PubMed Google Scholar
74. Hassani M , Lázaro R , Pérez C , Condón S , Pagán R . Thermostability of oxytetracycline, tetracycline, and doxycycline at ultrahigh temperatures . J Agric Food Chem . 2008 ; 56 ( 8 ): 2676 – 2680 . Crossref PubMed Google Scholar
75. World Health Organization . 2019 . No time to wait: securing the future from drug-resistant infections. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/ (date last accessed 24 September 2020). Google Scholar
76. Cassini A , Högberg LD , Plachouras D , et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis . Lancet Infect Dis . 2019 ; 19 ( 1 ): 56 – 66 . Crossref PubMed Google Scholar
77. Science Daily . Antibiotics found in some of the worlds rivers exceed safe levels, global study finds . The University of York . www.sciencedaily.com/releases/2019/05/190527094120.htm (date last accessed 24 September 2020 ). Google Scholar
78. Wilkinsson J , Boxall A , Society of Environmental Toxicology and Chemistry Europe (SETAC Europe) . The first global study of pharmaceutical contamination in riverine environments . 2019 . https://helsinki.setac.org/wp-content/uploads/2019/05/SETAC-Helsinki-Abstract-Book-2019.pdf (date last accessed 29 September 2020 ). Google Scholar
79. Hernandez CJ , Yang X , Ji G , et al. Disruption of the gut microbiome increases the risk of periprosthetic joint infection in mice . Clin Orthop Relat Res . 2019 ; 477 ( 11 ): 2588 – 2598 . Crossref PubMed Google Scholar
Author contributions
D. B Raina: Drafted and edited the manuscript.
Y Liu: Drafted and edited the manuscript.
O. L. P Jacobson: Edited the manuscript.
K. E. Tanner: Edited the manuscript.
M. Tägil: Drafted and edited the manuscript.
L. Lidgren: Drafted and edited the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article
ICMJE COI statement
L. Lidgren reports board membership on Bone Support AB, Sweden and Orthocell Australia. L. Lidgren and M. Tägil report an institutional grant from the Swedish Research Council, unrelated to this study.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.