Abstract
Objectives
We sought to determine if a durable bilayer implant composed of trabecular metal with autologous periosteum on top would be suitable to reconstitute large osteochondral defects. This design would allow for secure implant fixation, subsequent integration and remodeling.
Materials and Methods
Adult sheep were randomly assigned to one of three groups (n = 8/group): 1. trabecular metal/periosteal graft (TMPG), 2. trabecular metal (TM), 3. empty defect (ED). Cartilage and bone healing were assessed macroscopically, biochemically (type II collagen, sulfated glycosaminoglycan (sGAG) and double-stranded DNA (dsDNA) content) and histologically.
Results
At 16 weeks post-operatively, histological scores amongst treatment groups were not statistically different (TMPG: overall 12.7, cartilage 8.6, bone 4.1; TM: overall 14.2, cartilage 9.5, bone 4.9; ED: overall 13.6, cartilage 9.1, bone 4.5). Metal scaffolds were incorporated into the surrounding bone, both in TM and TMPG. The sGAG yield was lower in the neo-cartilage regions compared with the articular cartilage (AC) controls (TMPG 20.8/AC 39.5, TM 25.6/AC 33.3, ED 32.2/AC 40.2 µg sGAG/1 mg respectively), with statistical significance being achieved for the TMPG group (p < 0.05). Hypercellularity of the neo-cartilage was found in TM and ED, as the dsDNA content was significantly higher (p < 0.05) compared with contralateral AC controls (TM 126.7/AC 71.1, ED 99.3/AC 62.8 ng dsDNA/1 mg). The highest type II collagen content was found in neo-cartilage after TM compared with TMPG and ED (TM 60%/TMPG 40%/ED 39%). Inter-treatment differences were not significant.
Conclusions
TM is a highly suitable material for the reconstitution of osseous defects. TM enables excellent bony ingrowth and fast integration. However, combined with autologous periosteum, such a biocomposite failed to promote satisfactory neo-cartilage formation.
Cite this article: E. H. Mrosek, H-W. Chung, J. S. Fitzsimmons, S. W. O’Driscoll, G. G. Reinholz, J. C. Schagemann. Porous tantalum biocomposites for osteochondral defect repair: A follow-up study in a sheep model. Bone Joint J 2016;5:403–411. DOI: 10.1302/2046-3758.59.BJR-2016-0070.R1.
Article focus
-
In our previous studies, we have reported on the biocompatibility of periosteum and its known chondrogenic potential in the presence of tantalum both in vitro and in vivo.
-
The present study was a follow-up in a large animal model and was designed to test the hypothesis that a durable bilayer implant composed of trabecular metal with autologous periosteum on top will be suitable to reconstitute large osteochondral defects.
Key messages
-
Trabecular metal is a highly suitable material for the reconstitution of osseous defects.
-
Trabecular metal enables excellent bony ingrowth and fast integration.
-
However, combined with autologous periosteum, such a trabecular-metal/periosteum biocomposite failed to promote satisfactory neo-cartilage formation.
Strengths and limitations
-
Large animal study and use of critical size defects with a more realistic translation of the results into humans.
-
Proof-of-concept for trabecular metal as a bone substitute in osteochondral defect repair.
-
Limitation: n = 8 for each treatment group, thus further validation with higher numbers necessary.
Introduction
Joint lesions due to degenerative diseases such as osteoarthritis, osteochondritis dissecans or trauma are frequent and devastating.1-3 Left untreated, substandard scar tissue replaces lesions, which are thereafter predisposed to progressive joint destruction resulting in pain and impaired function.4 In particular, osteochondral defects in adults need clinical attention due to the high prevalence of early-onset osteoarthritis.5 This patient and health economic dilemma is due to the poor intrinsic capacity of cartilage for self-regeneration.6
To date, partial or total joint replacement is the benchmark for the elderly once the joint surface has significantly degenerated. However, even partial joint replacement is not an option for younger and more active patients. Consequently, there is considerable interest in the development of regenerative techniques in order to replace or restore the damaged or lost osteochondral tissue biologically, or to avoid or at least delay the need for partial or total joint replacement.
However, the challenge is to deliver a well integrated and structurally sound, regenerated tissue that has functional and metabolic properties resembling the osteochondral tissue it is replacing. The right combination of viable cells and scaffolds is the key to creating functional repair constructs.7,8 Not only the cartilage layer but also the subchondral bone and its interface have become increasingly important.9 Therefore, the interaction and homeostasis present in osteochondral tissue must be considered when developing cartilage repair strategies.10 Regardless of advancements that have recently been achieved, each with specific indications including lesion size,11 location,12 and activity demands of the patient,13 the ideal construct has not yet been found.
The treatment of extensive and/or uncontained osteochondral lesions remains especially troublesome. The underlying rationale is that the individual demands on cartilage and bone need to be addressed separately but in concert within the entire construct.14,15 Both cell transplantation and bone marrow stimulating techniques were hypothesised to be suitable to overcome this obstacle when combined with, for example, supportive cancellous bone grafting.16-18 When using scaffolds, uncertainties prevail with respect to chemical composition, biochemical and biomechanical properties, and architecture.19 Scaffolds used for the reconstitution of osteo-chondral tissue must be functional and conducive substitutes for three-dimensional cell arrangement, phenotype preservation, differentiated tissue formation and maturation while resisting mechanical forces until the growing regenerate is capable of taking over.7 Scaffolds can define the overall shape of the regenerated tissue thereby eliminating donor site scarcity and morbidity, as are inevitable for osteochondral allo- and autografting.20 Suitable biomaterials are either made of naturally derived or synthetic polymers, having specific benefits and disadvantages,21 or are of a hybrid nature. Polymeric implants are true biological substitutes due to their biodegradability, and biodegradable bilayer implants were shown to promote compartmented tissue repair.22-25 However, it appears to be infeasible to match the scaffold’s degradation kinetics with the evolving regenerative processes, particularly when facing large and/or uncontained osteochondral lesions.26 Degradation issues ultimately lead to incomplete filling of the defect with heterogeneous repair tissue. Moreover, it is highly demanding to simultaneously initiate chondrogenesis and osteogenesis within one single construct.
This led us to the hypothesis that a durable bilayer implant composed of trabecular metal (TM) with an autologous periosteum graft (PG) on top will be suitable to reconstitute large osteochondral defects. This novel design would allow for secure implant fixation, subsequent integration, and remodelling instead of degradation and replacement as postulated for polymeric scaffolds. TM has lately been used in revision arthroplasty and for various applications in reconstructive orthopaedic surgery.27,28 Elemental tantalum has been known since the 1940s for its biocompatibility, low elasticity, minor frictional characteristics, corrosion-resistance and excellent bone ingrowth properties.29 It can be manufactured as a highly interconnected porous scaffold with regular pore shapes and sizes and even in complex configurations. Instead of covering TM with an artificial construct for the regeneration of the cartilage layer, e.g. made of fibrin as described by Jamil et al,30 we chose a biological graft: autologous periosteum.31 Periosteum contains pluripotential stem cells with the potential to form either cartilage or bone. It can be transplanted as a whole tissue, it can serve as its own scaffold or a matrix onto which other cells and/or growth factors can adhere, and it produces bioactive factors that are known to be chondrogenic. In our previous studies, we have reported on the biocompatibility of periosteum and its known chondrogenic potential,32,33 in the presence of tantalum both in vitro and in vivo.24,34 The present study was a follow-up in a large animal model. In order to test our hypothesis, critical size osteochondral defects in skeletally mature sheep were treated either with a biocomposite made of TM and PG on top or TM alone. Repair tissue was analysed according to the recommendations of the International Cartilage Repair Society (ICRS).35
Materials and Methods
Study design
Skeletally mature, castrated male sheep were randomly assigned to one of the following treatment groups: trabecular metal/periosteal graft (TMPG), trabecular metal (TM), or empty defect (ED) (n = 8 each). Surgeries were conducted under general anaesthesia and sterile conditions. All procedures were approved by the Institutional Animal Care and Use Committee. Post-operative care was professionally managed by trained personnel and supervised by veterinarians.
Osteochondral defect preparation
After skin incision (approximately 6 cm in length) and subcutaneous preparation, the standard medial approach was used for the mini-arthrotomy (approximately 2 cm in length) of left knee joints. Eight-mm core cutters were then used to create an osteochondral defect (8 mm diameter, 13 mm deep) on the medial aspect of the medial femoral condyle in the main weight bearing area (Fig. 1). These lesions were left untreated in the ED control group.

Fig. 1
Surgical procedure of different treatments. Step 1, periosteal graft elevation from the medial aspect of the tibial head. Step 2, periosteal graft sutured to trabecular metal cylinder with cambium facing away from the metal implant. Large pictures show defects that were left untreated (ED) or after implantation of trabecular metal (TM) or trabecular metal in combination with a periosteal graft (TMPG).
Trabecular metal/periosteal graft osteochondral defect repair
In the TMPG treatment group, a 10 mm round periosteal flap was harvested using a core cutter and periosteal elevator from the medial proximal tibia. The grafts shrunk to approximately 8 mm in diameter once elevated due to their elastin content. The periosteal graft was then sutured (3/0 Vicryl) to a TM cylinder (8 mm diameter × 12 mm depth) with the cambium layer facing away from the TM (Fig. 1). The TM cylinders had a porosity of 75% to 80% by volume and a repeating arrangement of slender interconnecting struts which formed a regular array of dodecahedron-shaped pores (Zimmer Biomet Inc., Warsaw, Indiana). The graft composite was immediately placed in a culture medium containing 100 ng/ml TGF-β1 and kept there at room temperature until implantation (approximately five to ten minutes). Subsequently, an osteochondral defect was created as described above and the TMPG composite was press-fit implanted with the periosteal layer below adjacent cartilage level (Fig. 1). The joint was then repeatedly flexed and extended to ensure secure fit of the implant without loosening.
Trabecular metal osteochondral defect repair
The TM treatment group was processed as described above, yet without a periosteal flap on top of the TM (Fig. 1). Moreover, the TM cylinder was implanted level with the adjacent subchondral bone.
Post-operative procedures
Buprenorphine (0.01 m/kg, intramuscular (IM)) was administered every four to six hours and Ketoprofen (1 mg/kg, IM) daily for least 48 hours post-operatively. The sheep were housed in small kennels to restrict movement for the first three post-operative days and allowed unrestricted movement (pasture) thereafter. Animals were sacrificed approximately 16 weeks after treatment. Two animals of the ED control group and one animal of the TMPG group died or were sacrificed prior to study completion due to complications resulting from penile hypoplasia, which is common among castrated male sheep.
Macroscopic analysis
Both knees were opened for macroscopic analysis (contralateral side as healthy AC control). Documentation was performed using digital photography with a macro lens. Subsequently, a portion of the regenerated neo-cartilage was removed for biochemical analysis and the remaining samples were fixed and prepared for Exakt system histology (Exakt Technologies Inc., Oklahoma City, Oklahoma).
sGAG analysis
In order to quantitatively assess sulfated glycosaminoglycan (sGAG) content of the cartilage matrix, a dimethylmethylene blue assay (DMMB, Blyscan; Biocolor, Northern Ireland, United Kingdom) was applied to quantify sGAG content within the neo-cartilage regions. Samples were digested in 1 ml of 50 µg/ml proteinase K (Roche, Warsaw, Indiana) in 100 mM K2HPO4 (pH 8.0) at 60°C in a water bath. After 16 hours, 100 µl of sample digest were mixed in with 1 ml DMMB containing dye reagent, mechanically shaken for 30 minutes and micro-centrifuged at 10 000 g for ten minutes to precipitate sGAG dye complex out of solution. Unbound dye solution was removed and 1 ml dissociation reagent was added. Bound dye values were quantified at 656 nm using a SpectraMax Plus spectrophotometer (Molecular Devices, Sunnyvale, California) and compared with standard curve of chondroitin-4-sulphate.
dsDNA content
In order to quantify cellularity, a fluorescent PicoGreen double-stranded DNA (dsDNA) quantification assay (Molecular Probes, Eugene, Oregon) was used to analyse cell content within the neo-cartilage regions. Samples were digested according to the sGAG assay. A working reagent solution was prepared as a 200-fold dilution of the concentrated dimethyl sulfoxide (DMSO) solution in 1 x TE (20 mM Tris-HCl, 2 mM EDTA, pH 7.5). A total of 100 µl of sample digest were mixed in with 100 µl of the working solution and incubated for five minutes at room temperature, protected from light, and then excited at 480 nm. Fluorescence emission intensity was measured at 520 nm using a FLUOstar Galaxy plate reader (BMG LABTECH Inc., Cary, North Carolina) and compared against the DNA standard curve.
Collagen typing
Quantitative collagen typing was run in an automated fashion using the PhastSystem gel electrophoresis system (Pharmacia-LKB Biotechnology Group, Quebec, Canada) and microgram-sized samples. A 1 µl volume of sample, 8 µg/µl in sample buffer, was applied to and separated on 20% homogeneous SDS-PAGE Phast-Gels. The gels were scanned using an LKB laser densitometer and the absorbance curves were integrated with a computer software package (GelScan; Sebia, Camberly, United Kingdom). The total percentage of type II collagen was determined by calculating the ratio of the area under the α1(II)CB10 peak to that under the α1(I)CB7,8 and α1(II)CB11 peaks.
Histological analysis
The main osteochondral defect repair samples were processed for histology using the Exakt system, which uses plastic embedding (Technovit; Heraeus Kulzer Ltd., Hanau, Germany) to allow sectioning of metallic joint implants. Histological sections were stained with Safranin-O and counterstained with Fast Green. Morphologic details of both bone and neo-cartilage were evaluated using a 30-point modification of the O’Driscoll score34 by five blinded researchers (EHM, H-WC, JSF, GGR, JCS) in an independent manner for unbiased assessment.
Statistical analysis
Collagen typing, sGAG and dsDNA results were analysed statistically with 1- and 2-factor analysis of variance (ANOVA) with means-contrast comparison or Newman-Keuls post hoc testing being performed where appropriate. Histological scores were analysed as follows: statistical differences between each treatment group and corresponding healthy AC controls were evaluated using ANOVA and a Student’s t-test. Statistical differences between treatment groups were evaluated using the Least Squares Means Differences. All data are presented as mean and standard error (sem). A p-value < 0.05 was considered significant unless otherwise specified.
Results
Macroscopic appearance
Defect sites in the TMPG treatment group were almost completely covered with a repair tissue that had a predominantly cartilaginous and smooth appearance (Fig. 2). However, a fragmented surface structure could also be seen in a few cases. Moreover, the periosteal graft seemed only to be partly remodeled and integrated into the adjacent cartilage. Defect sites in the TM treatment group were also covered with a cartilaginous and smooth repair tissue, yet the regeneration of the cartilage layer appeared to be incomplete leaving the TM cylinder partly visible (Fig. 2). However, the cartilaginous repair tissue was well integrated into the bordering cartilage. Remarkably, defects that had been left untreated (ED) were also almost completely covered with a cartilaginous repair tissue, yet with an incomplete integration and a rather fragmented surface in common (Fig. 2). In general, regenerates after different treatments appeared to be approximately level with the adjacent cartilage. Joints were void of repair tissue overgrowth or bulging.
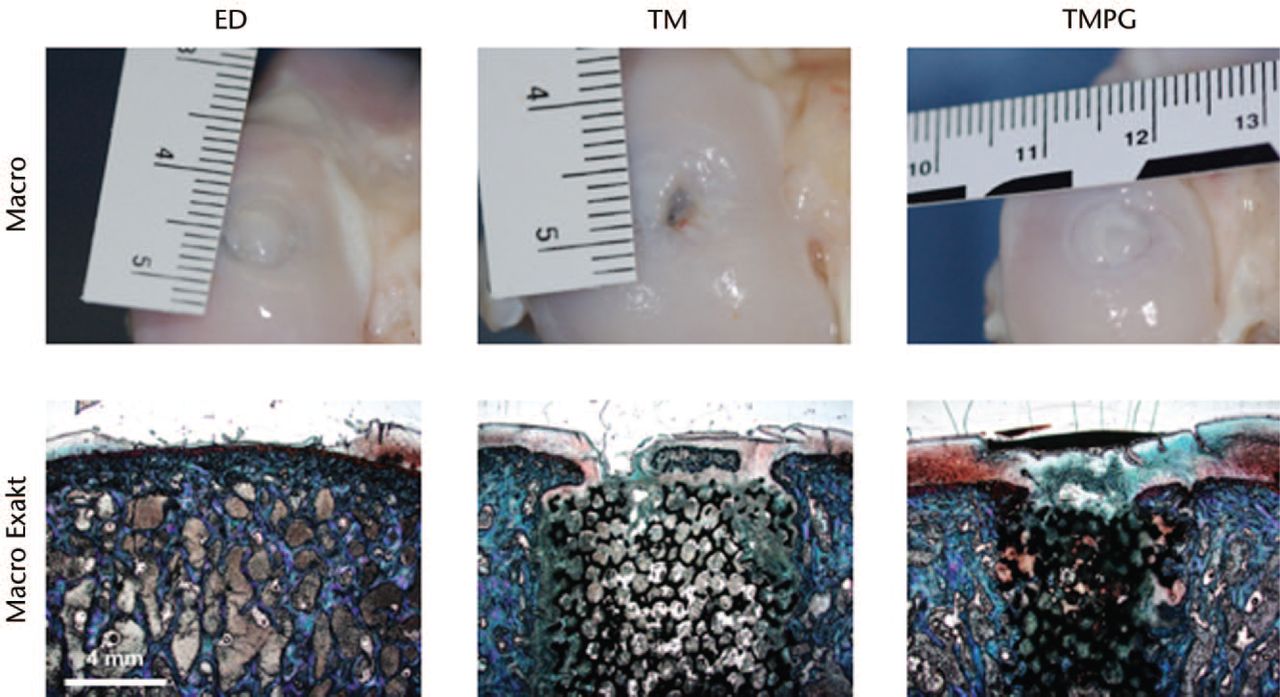
Fig. 2
Upper row shows the macroscopic appearance of the best regenerate found after different treatments. Notice the metal implant shining through the glistening white neo-cartilage both in the trabecular metal (TM) and trabecular metal in combination with a periosteal graft (TMPG) sections. Bottom row shows best histological results of the different treatments (Exakt system, Safranin-O/ Fast Green). Microscopic pictures do not necessarily correspond to macroscopic sections. ED untreated
Microscopic appearance and histological score
Gross histological appearance generally suggested a better regeneration in the TMPG group with a hyaline-like regenerate on top of the metal scaffold (Fig. 2). A rather fibrocartilaginous regenerate was primarily seen in the TM and ED group. However, neither the overall mean score values (maximum 30 points), nor the cartilage (maximum 22 points) or bone (maximum 8 points) breakdown revealed a statistical difference between the different treatment groups (Fig. 3; TMPG: overall 12.7, cartilage 8.6, bone 4.1; TM: overall 14.2, cartilage 9.5, bone 4.9; ED: overall 13.6, cartilage 9.1, bone 4.5). Nevertheless, the different treatment groups always scored lower than the matching healthy controls (p < 0.05). Moreover, the healthy controls (AC) were scored as significantly different (total and cartilage breakdown) and the maximum score yield was not reached, which indicates degenerative changes of AC. Nonetheless, in both the TM and TMPG treatment groups, metal scaffolds were nicely incorporated into the bordering subchondral bone. There were no signs of implant loosening or inflammatory response.

Fig. 3
Total histological score (maximum 30 points), and cartilage (maximum 22 points) and bone (maximum 8 points) breakdown of regenerates found after different treatments. There was no statistically significant difference between the treatment groups but the score yield of matching healthy controls was always higher, which was statistically significant (p <0.05). Moreover, the histological sub-scores for the healthy controls were different. This was significant for the total score and cartilage breakdown (ED, defects that were left untreated; TM, after implantation of trabecular metal; TMPG, trabecular metal in combination with a periosteal graft).*p<0.05
sGAG content
sGAG yield was consistently lower in the neo-cartilage regions compared with contralateral AC controls (Fig. 4 left; mean values after TMPG 20.8 / AC 39.5, TM 25.6 / AC 33.3, ED 32.2 / AC 40.2 µg sGAG / 1 mg tissue). This was statistically significant for the TMPG treatment group only (p < 0.05). Although the neo-cartilage of the ED group contained more sGAG than the neo-cartilage of the implant groups, neither significant inter-treatment nor inter-AC control differences were found.
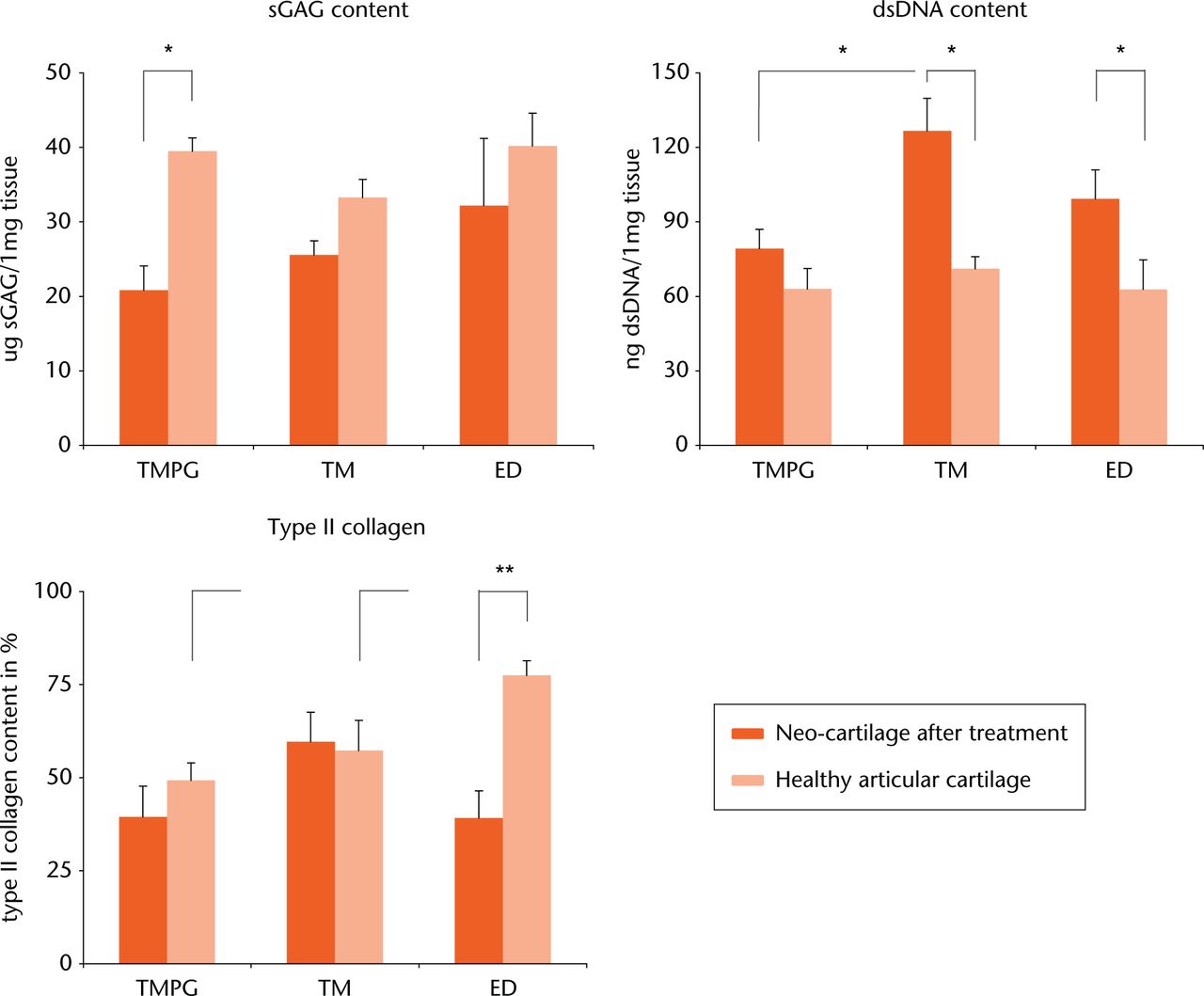
Fig. 4
Left: Sulfated glycosaminoglycan (sGAG) content of neo-cartilage found after different treatments. Neo-cartilage in the trabecular metal (TM) and defects that were left untreated (ED) group had a sGAG content similar to matching healthy controls, whereas the sGAG content was significantly (p < 0.05) lower in the TMPG group. Middle: Double-stranded DNA content of neo-cartilage found after different treatments. Hypercellularity was counted in the TM and ED groups, which was statistically significant (p < 0.05). Cell count after trabecular metal in combination with a periosteal graft (TMPG) treatment was similar to the matching healthy control. Right: type II collagen content of neo-cartilage found after different treatments. Neo-cartilage found in ED defects contained significantly (p < 0.005) less type II collagen than the matching healthy control. No differences were found for the TM and TMPG groups.
*p < 0.05, **p < 0.005
dsDNA content
A hypercellularity of the neo-cartilage was found in the TM and ED treatment groups, as the dsDNA content was significantly higher (p < 0.05) compared with relating AC controls (Fig. 4 middle; mean values after TM 126.7 / AC 71.1, ED 99.3 / AC 62.8 ng dsDNA / 1 mg tissue). dsDNA content in neo-cartilage after TMPG treatment was also higher compared with contralateral AC (mean values after TMPG 79.2 / AC 63.0 ng dsDNA / 1 mg tissue), yet this difference was not significant. Neo-cartilage after TM contained significantly more dsDNA than after TMPG (mean values after TM 126.7 / TMPG 79.2 ng dsDNA / 1 mg tissue). No significance was found comparing TM or TMPG with ED. Nor were inter-AC control differences statistically significant.
Type II collagen content
The highest type II collagen content was found in the neo-cartilage of the TM treatment group compared with the TMPG and ED groups (Fig. 4 right; mean values after TM 59.6 / TMPG 39.4 / ED 39.2 %). Inter-treatment differences were not significant. Neo-cartilage in the ED group contained significantly less (p < 0.005) type II collagen compared with the relating AC control (mean values after ED 39.2 / AC 77.5 %). Differences between the implant groups and contralateral AC were not statistically significant (mean values after TM 59.6 / AC 57.3, TMPG 39.4 / AC 49.3 %). As for histological scoring, healthy controls (AC) were different regarding type II collagen content. A significantly (p < 0.005) higher type II collagen content was found in AC controls in ED treated animals (mean value 77.5 %) compared with AC controls in the other two treatment groups (mean values 49.3% and 57.3 %, respectively).
Discussion
In this study, a biocomposite implant composed of trabecular metal with autologous periosteum failed to reconstitute the cartilage surface of osteochondral defects although the tantalum plugs did obtain secure implant fixation and subsequent integration in the bone.
Implants made of porous tantalum are well known for their excellent bone ingrowth and interface mechanics.36,37 However, little data were available about the use of porous tantalum for the restoration of osteochondral defects.27,38 Also, it is perfectly obvious that porous tantalum is inappropriate for the regeneration of the cartilage layer. Or in a broader sense, macroporous scaffolds in general may cause neo-cartilage surface irregularity, structural disintegration, and unfavourable tribology as seen in studies conducted by Shao et al.22,39 Therefore, a decision was made for an autologous periosteal graft on top of the TM cylinder. Periosteum is a biological graft that fulfils the major prerequisites for cartilage repair.31 It contains pluripotential stem cells that are capable of differentiating into bone and cartilage. Besides superior cellularity, periosteum serves as its own scaffold, providing attachment sites and growth factors. For the repair of major osteochondral defects, osteoperiosteal grafts have been used successfully in the past.32,33 However, the use of a cancellous bone graft may present additional complications to the procedure and produces graft site morbidity. We believed that substituting the autologous bone graft with an artificial scaffold (TM) for osteochondral defect repair could be a suitable alternative strategy.
Previously, we demonstrated, using rabbit experimental models, the biocompatibility and chondrogenic potential of periosteum32,33 in the presence of TM both in vitro and in vivo.24,34 In our in vitro experiment, when periosteum was cultured on porous tantalum under chondrogenic conditions, robust hyaline-like cartilage outgrowth was formed, and the periosteal graft became firmly attached to the scaffold by fibrous tissue ingrowth. In our in vivo rabbit experiment, porous TM scaffolds promoted excellent bone regeneration and integration of the construct into the adjacent tissue. Neo-cartilage formation from periosteum supported by the metal scaffold was promising. Wherever we found a healthy layer of neo-cartilage it was well bonded to the underlying subchondral bone. The overall mean histological score for TMPG was 13.4. This is in line with our present study with a mean histological score of 12.7 for the TMPG group. However, biochemical and histological analysis in the present study revealed that the TM and TMPG treatment groups resulted in a regenerate that was inferior compared with healthy controls (AC). This was somewhat expected but there were no gross differences between the TM and TMPG treatment groups. Although periosteum has established chondrogenic potential and is a compatible partner of TM,24,34,40,41 neo-cartilage formation after TMPG treatment performed less well than expected especially when being compared with the histological score yield after treatment with TM alone. Thus, the high quality neo-cartilage obtained by optimised chondrogenic culture conditions in vitro could not be reproduced in vivo in this sheep study.24
Moreover, the defects that were left untreated performed almost identically to the implant groups. Neo-cartilage in the ED group even contained the highest sGAG content. These observations do not correlate with our preliminary study on mature rabbits34 nor with our previous hydrogel study on mature sheep,42 demonstrating that critical-sized osteochondral defects do not heal spontaneously. Our defects (8 mm diameter, 13 mm depth) were even larger than those in our previous sheep model (6 mm diameter, 12 mm depth) and it is generally recognised that empty critical-sized defects such as those in our model do not heal properly.22,34,39 One reason for this divergence could be the accidentally inconsistent depth of the created defects. As for the untreated defects, the interpretation of the findings remains uncertain.
Nonetheless, it is clear that the novel design of a TMPG biocomposite allowed for secure implant fixation and fast integration within three months. This could potentially enable early weight-bearing and progressive rehabilitation protocols for treated patients in the future. Also, TMPG composites were kept in a culture medium containing transforming growth factor-β1 (TGF)-β1 prior to implantation in order to enhance cambium layer cellularity and chondrogenic potential of aged periosteum to levels comparable with younger individuals, thereby rejuvenating aged periosteum.43 However, this design failed to reconstitute large osteochondral defects in a large animal model. Possible reasons are the implantation method of the biocomposite that were press fitted using a hammer after having sutured the periosteal flap onto the TM cylinder. This may have caused damage to the cambium layer of the periosteum as discussed previously.32,33 In order to avoid this it might have been better if we had sutured the periosteal flap onto the TM cylinder after the metal was implanted. Moreover, the method of creating the defects - either using a drill34 or a core cutter42 - potentially caused bone necrosis.44 Other critical factors are unfavourable biomechanical properties of the implant including high stiffness and low elasticity that might prevent cartilage formation. Stimulation by continuous passive movement might have helped to improve chondrogenesis.32,33
In conclusion, TM is a highly suitable material for the reconstitution of osseous defects. TM enables excellent bony ingrowth and fast integration. However, in the form of a biocomposite by combining it with autologous periosteum, it failed to promote satisfactory neo-cartilage formation. We could not translate our promising in vitro results in our rabbit model to a large animal model.
Funding Statement
Funding was provided by the Mayo Foundation and Zimmer Inc. to undertake this study.
Provisional Patents regarding Periosteal Tissue Grafts and the use of trabecular metal for the repair of osteochondral defects have been filed.
S. W. O’Driscoll reports funding received from Tornier Inc. and Aircast Corp, neither of which is related to this article.
ICMJE conflict of interest
S. W. O’Driscoll and the Mayo Foundation receive royalties from Acumed, LLC; Tornier, Inc.; and Aircast, Inc. The Mayo Foundation receives royalties from Zimmer, Inc. All other authors have no conflicts of interest to report.
References
1 Smith GD , KnutsenG, RichardsonJB. A clinical review of cartilage repair techniques. J Bone Joint Surg [Br]2005;87-B:445-449.CrossrefPubMed Google Scholar
2 Curl WW , KromeJ, GordonES, et al.. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy1997;13:456-460.CrossrefPubMed Google Scholar
3 Hjelle K , SolheimE, StrandT, MuriR, BrittbergM. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy2002;18:730-734.CrossrefPubMed Google Scholar
4 Buckwalter JA , BrownTD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res2004;423:7-16.PubMed Google Scholar
5 Smith GD , RichardsonJB, BrittbergM, et al.. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. J Bone Joint Surg [Am]2003;85-A:2487-2488.CrossrefPubMed Google Scholar
6 Hunziker EB . Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage2002;10:432-463.CrossrefPubMed Google Scholar
7 Sittinger M , HutmacherDW, RisbudMV. Current strategies for cell delivery in cartilage and bone regeneration. Curr Opin Biotechnol2004;15:411-418.CrossrefPubMed Google Scholar
8 Risbud MV , SittingerM. Tissue engineering: advances in in vitro cartilage generation. Trends Biotechnol2002;20:351-356.CrossrefPubMed Google Scholar
9 Gomoll AH , MadryH, KnutsenG, et al.. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc2010;18:434-447.CrossrefPubMed Google Scholar
10 Hoemann CD , Lafantaisie-FavreauCH, Lascau-ComanV, ChenG, Guzmán-MoralesJ. The cartilage-bone interface. J Knee Surg2012;25:85-97.CrossrefPubMed Google Scholar
11 Salzmann GM , NiemeyerP, SteinwachsM, et al.. Cartilage repair approach and treatment characteristics across the knee joint: a European survey. Arch Orthop Trauma Surg2011;131:283-291.CrossrefPubMed Google Scholar
12 Kreuz PC , SteinwachsMR, ErggeletC, et al.. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage2006;14:1119-1125.CrossrefPubMed Google Scholar
13 Harris JD , BrophyRH, SistonRA, FlaniganDC. Treatment of chondral defects in the athlete’s knee. Arthroscopy2010;26:841-852. Google Scholar
14 Reinholz GG , LuL, SarisDB, YaszemskiMJ, O’DriscollSW. Animal models for cartilage reconstruction. Biomaterials2004;25:1511-1521.CrossrefPubMed Google Scholar
15 Klein TJ , MaldaJ, SahRL, HutmacherDW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev2009;15:143-157.CrossrefPubMed Google Scholar
16 Brittberg M , TallhedenT, Sjögren-JanssonB, LindahlA, PetersonL. Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res2001;391:S337-S348.CrossrefPubMed Google Scholar
17 Benthien JP , BehrensP. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc2011;19:1316-1319.CrossrefPubMed Google Scholar
18 Peterson L , VasiliadisHS, BrittbergM, LindahlA. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med2010;38:1117-1124.CrossrefPubMed Google Scholar
19 Seo SJ , MahapatraC, SinghRK, KnowlesJC, KimHW. Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng2014;5:2041731414541850.CrossrefPubMed Google Scholar
20 Hutmacher DW , GohJC, TeohSH. An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med Singapore2001;30:183-191.PubMed Google Scholar
21 Tuan RS , ChenAF, KlattBA. Cartilage regeneration. J Am Acad Orthop Surg2013;21:303-311.CrossrefPubMed Google Scholar
22 Shao X , GohJC, HutmacherDW, LeeEH, ZigangG. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng2006;12:1539-1551.CrossrefPubMed Google Scholar
23 Kandel RA , GrynpasM, PilliarR, et al.; CIHR-Bioengineering of Skeletal Tissues Team. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials2006;27:4120-4131.CrossrefPubMed Google Scholar
24 Mardones RM , ReinholzGG, FitzsimmonsJS, et al.. Development of a biologic prosthetic composite for cartilage repair. Tissue Eng2005;11:1368-1378.CrossrefPubMed Google Scholar
25 Frenkel SR , BradicaG, BrekkeJH, et al.. Regeneration of articular cartilage–evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthritis Cartilage2005;13:798-807. Google Scholar
26 Li WJ , JiangYJ, TuanRS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng2006;12:1775-1785.CrossrefPubMed Google Scholar
27 Gordon WJ , ConzemiusMG, BirdsallE, et al.. Chondroconductive potential of tantalum trabecular metal. J Biomed Mater Res B Appl Biomater2005;75:229-233.CrossrefPubMed Google Scholar
28 Borland WS , BhattacharyaR, HollandJP, BrewsterNT. Use of porous trabecular metal augments with impaction bone grafting in management of acetabular bone loss. Acta Orthop2012;83:347-352.CrossrefPubMed Google Scholar
29 Cohen R . A porous tantalum trabecular metal: basic science. Am J Orthop (Belle Mead NJ)2002;31:216-217.PubMed Google Scholar
30 Jamil K , ChuaKH, JoudiS, NgSL, YahayaNH. Development of a cartilage composite utilizing porous tantalum, fibrin, and rabbit chondrocytes for treatment of cartilage defect. J Orthop Surg Res2015;10:27.CrossrefPubMed Google Scholar
31 O’Driscoll SW , FitzsimmonsJS. The role of periosteum in cartilage repair. Clin Orthop Relat Res2001;391:S190-S207.CrossrefPubMed Google Scholar
32 O’Driscoll SW , KeeleyFW, SalterRB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg [Am]1986;68-A:1017-1035.PubMed Google Scholar
33 O’Driscoll SW , SalterRB. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res1986;208:131-140.PubMed Google Scholar
34 Mrosek EH , SchagemannJC, ChungHW, et al.. Porous tantalum and poly-epsilon-caprolactone biocomposites for osteochondral defect repair: preliminary studies in rabbits. J Orthop Res2010;28:141-148.CrossrefPubMed Google Scholar
35 Hoemann C , KandelR, RobertsS, et al.. International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials. Cartilage2011;2:153-172.CrossrefPubMed Google Scholar
36 Meneghini RM , LewallenDG, HanssenAD. Use of porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement. J Bone Joint Surg [Am]2008;90-A:78-84.CrossrefPubMed Google Scholar
37 Bobyn JD , TohKK, HackingSA, TanzerM, KrygierJJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty1999;14:347-354.CrossrefPubMed Google Scholar
38 Levine BR , SporerS, PoggieRA, Della ValleCJ, JacobsJJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials2006;27:4671-4681.CrossrefPubMed Google Scholar
39 Shao XX , HutmacherDW, HoST, GohJC, LeeEH. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials2006;27:1071-1080.CrossrefPubMed Google Scholar
40 O’Driscoll SW , ReckliesAD, PooleAR. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg [Am]1994;76-A:1042-1051.CrossrefPubMed Google Scholar
41 Lorentzon R , AlfredsonH, HildingssonC. Treatment of deep cartilage defects of the patella with periosteal transplantation. Knee Surg Sports Traumatol Arthrosc1998;6:202-208.CrossrefPubMed Google Scholar
42 Schagemann JC , ErggeletC, ChungHW, et al.. Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Eng Part A2009;15:75-82.CrossrefPubMed Google Scholar
43 Reinholz GG , FitzsimmonsJS, CasperME, et al.. Rejuvenation of periosteal chondrogenesis using local growth factor injection. Osteoarthritis Cartilage2009;17:723-734.CrossrefPubMed Google Scholar
44 Chen H , SunJ, HoemannCD, et al.. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res2009;27:1432-1438.CrossrefPubMed Google Scholar









