Abstract
Objectives
To explore the therapeutic potential of combining bone marrow-derived mesenchymal stem cells (BM-MSCs) and hydroxyapatite (HA) granules to treat nonunion of the long bone.
Methods
Ten patients with an atrophic nonunion of a long bone fracture were selectively divided into two groups. Five subjects in the treatment group were treated with the combination of 15 million autologous BM-MSCs, 5g/cm3 (HA) granules and internal fixation. Control subjects were treated with iliac crest autograft, 5g/cm3 HA granules and internal fixation. The outcomes measured were post-operative pain (visual analogue scale), level of functionality (LEFS and DASH), and radiograph assessment.
Results
Post-operative pain evaluation showed no significant differences between the two groups. The treatment group demonstrated faster initial radiographic and functional improvements. Statistically significant differences in functional scores were present during the first (p = 0.002), second (p = 0.005) and third (p = 0.01) month. Both groups achieved similar outcomes by the end of one-year follow-up. No immunologic or neoplastic side effects were reported.
Conclusions
All cases of nonunion of a long bone presented in this study were successfully treated using autologous BM-MSCs. The combination of autologous BM-MSCs and HA granules is a safe method for treating nonunion. Patients treated with BM-MSCs had faster initial radiographic and functional improvements. By the end of 12 months, both groups had similar outcomes.
Cite this article: H.D. Ismail, P. Phedy, E. Kholinne, Y. P. Djaja, Y. Kusnadi, M. Merlina, N. D. Yulisa. Mesenchymal stem cell implantation in atrophic nonunion of the long bones: A translational study. Bone Joint Res 2016;5:287–293. DOI: 10.1302/2046-3758.57.2000587.
Article focus
-
Explore the therapeutic potential of the mesenchymal stem cells for treating neglected nonunion of long bone fracture by evaluation of post-operative pain, level of functionality and plain radiographic assessments.
Key messages
-
Our findings showed that neglected atrophic nonunion cases that were treated using a combination of bone marrow-derived mesenchymal stem cells and hydroxyapatite granules demonstrated faster initial radiographic and functional improvements.
-
No immunological or neoplastic side effects were reported in the cases treated using mesenchymal stem cells.
Strengths and limitations
-
This is a quasi-experimental study with matched cases of severe atrophic nonunion of long bone fractures. More severe cases were allocated in the treatment group but blinding was performed during the evaluation.
-
The limitations of our study are the fact that the baseline patient characteristics are not similar and no randomisation was performed to strengthen the result. Longer follow-up would better illustrate the continuous progression and end result of both treatment arms.
Introduction
Nonunion is a serious orthopaedic complication. It signifies permanent failure of bone healing. Treatment of nonunion remains a great challenge for orthopaedic surgeons. Most cases will require a series of complex surgeries and a lengthy rehabilitation period. The condition is precipitated by history of neglected fracture, open fracture, compartment syndrome, peripheral nerve palsy, cigarette smoking and the presense of low insulin-like growth factor 1 (IGF-1).1-4 Aside from causing significant morbidity, the economic burden caused by nonunions are substantial. In the United Kingdom, Dahabreh, Dimitriou and Giannoudis et al5 estimated that £13 844.68 was needed to treat one case of nonunion, while the cost in Canada was as high as $C18 712 for each case.6
Approximately 5% to 10% of all fractures will result in delayed union or nonunion.7 The rate of nonunion of the tibial diaphysis is significantly higher than that of other sites: 21.7% in grade IIIA and 100% in grade IIIB open fracture.8 Due to the combination of traditional health paradigms and limited access to advanced orthopaedic centres in our country (there are only nine such centres in Indonesia), significant portions of the population still prefer an alternative approach for treating fractures (such as bone setting).9
Osseous defects further complicate the treatment of nonunions. They are classically managed by using bone graft, whether autogeneic, allogeneic or synthetically made.10 Each type of graft has its own limitations.11 Considered to be the benchmark, availability of autograft is limited and the harvesting procedure is associated with significant donor site morbidity. On the other hand, allograft carries the risk of transmission of infectious disease, post-operative infection and refracture. The strength of synthetic scaffolds is unpredictable in different individuals as various anatomical locations and clinical conditions may affect the material degradation differently.12 A different treatment approach that lacks such drawbacks is needed.
The multipotency of mesenchymal stem cells (MSCs) has gained worldwide attention for their immense potential to be used in the field of orthopaedics.13-15 However, osteogenicity alone is not sufficient to promote fracture healing. As described by Giannoudis, Einhorn and Marsh16 osteogenic cells must work in conjunction with osteoconduction, osteoinduction and a stable mechanical environment. Hence, we used a combination of osteogenic MSCs, osteoconductive hydroxyapatite (HA) granules and a stable mechanical environment provided by internal fixation.
The aim of this study was to explore the therapeutic potential of mesenchymal stem cells and HA granules for treating a nonunion of the long bones. Limited studies have documented the usage of stem cells in treating atrophic nonunions. Our hypothesis was that the combination treatment would be effective in atrophic nonunions of long bone fractures.
Materials and Methods
This quasi-experiment study enrolled ten subjects diagnosed with neglected atrophic nonunion of the long bones. Diagnosis was confirmed by clinical and radiographic assessment conducted by an orthopaedic surgeon (IHD). Ethical clearance was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (reference number: 158/PT02.FK/ETIK/2010). This study is registered at ClinicalTrials.gov (NCT 01626625). Written informed consent was obtained from all of the subjects before the start of the trial. Patients with atrophic nonunion of a long bone and who were willing to participate in this study were included. The exclusion criteria were immunodeficiency, history of pathological fracture, ongoing hormonal therapy and active osteomyelitis or related soft-tissue infection.
Subjects were selectively divided into two groups. A total of five subjects who were considered to suffer from a relatively more severe nonunion were assigned to the treatment group by an orthopaedic surgeon (IHD). The decisions were based on the length of fracture neglect and the presence of morbidity. The other five subjects were considered as a control, and were treated with a combination of autologous iliac crest bone graft and HA granules. All patients were treated at the same hospital by one surgeon (IHD). Patient and BM-MSC characteristics are summarised in Table I.
Table I.
Patient characteristics
| Patient number | Group | Age | Gender | Duration of fracture neglect (mths) | Fracture site | Morbidity | BM-MSC culture period (days) | Implanted BM-MSCs | Cell viability (%) |
|---|---|---|---|---|---|---|---|---|---|
| 01 | Treatment | 23 | Male | 72 | Right femur | Heavy smoker | 22 | 15 549 750 | 84.72 |
| 02 | Treatment | 37 | Male | 12 | Right humerus | Heavy smoker Low IGF-1 level | 27 | 14 965 000 | 86.32 |
| 03 | Treatment | 33 | Male | 48 | Right tibia | History of open fracture | 28 | 14 627 000 | 91.25 |
| 04 | Treatment | 18 | Male | 36 | Right femur | – | 29 (22) | 12 322 500 (17 212 000) | 94.48 |
| 05 | Treatment | 26 | Male | 18 | Right femur | Low IGF-1 level | 21 | 18 702 000 | 92.19 |
| 06 | Control | 43 | Female | 7 | Left humerus | – | – | – | – |
| 07 | Control | 32 | Male | 24 | Right femur | – | – | – | – |
| 08 | Control | 70 | Female | 7 | Left femur | – | – | – | – |
| 09 | Control | 57 | Male | 9 | Right tibia | History of open fracture | – | – | – |
| 10 | Control | 37 | Male | 48 | Left femur | – | – | – | – |
-
BM-MSC, bone marrow-derived mesenchymal stem cells
-
Control subjects received iliac crest autograft in place of BM-MSC
Bone marrow was harvested from patients in the treatment group in an outpatient clinic. The MSCs culture protocol used in this study has been previously described by Lubis et al.17 After a local anaesthetic injection using lidocaine, as much as 40 mL of bone marrow was aspirated (using bone marrow aspiration needle) from several locations within the posterior iliac crest. The aspirate then transferred into a container prefilled with 5000 U/mL of heparin.
Aspirate was diluted with phosphate-buffered saline at a ratio of 1:1 and centrifuged at room temperature at 3000 rpm for 30 minutes. The collected buffy coat was washed and transferred into a culture flask containing Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific Grand Island, New York) supplemented with 10% foetal bovine serum. Cells were incubated at 37°C at 5% CO2 with a routine culture medium change every two to three days. Subculture was performed between days 7 and 10.
Attached cells were cultured until reaching at least 15 million cells (by the fourth week). Cellular characterisation was then performed on plastic adherent confluent cells by flow cytometry (FACSCalibur, Franklin Lakes, New Jersey). Cultured cells were checked for typical MSC markers (CD73, CD105) and haematopoietic markers (HLA-DR, CD14, CD19, CD34 and CD45). To ensure safety, sterility of the BM-MSCs was checked three times throughout the culture process. All BM-MSC culture procedures were performed in a GMP-certified facility (ReGeniC Laboratory – Bifarma Adiluhung, Jakarta, Indonesia).
Cell viability was determined with trypan blue staining to differentiate between living and dead cells. The colour of the dead cells was blue due to dye infiltration into the cell cytoplasm, while living cells were transparent. Cell viability was calculated by dividing the number of living cells by the total number of living and dead cells.
Nonunion fractures were conventionally fixated using a plate and screws. The visible bone defect was filled with 5g/cm3 defect of HA granules (HA; Bongros-HA, Bioalpha, Seongnam, Korea) followed by prompt soft-tissue closure. Just before implantation, the granules were mixed in a container with approximately 14 million to 18 million BM-MSCs which were contained in 10 mL of plasma solution.
Four patients were injected with passage 1 cells and one patient with passage 2 cells. A similar procedure was carried out in five control subjects with transplantation of bone autograft from the iliac crest, instead of autologous BM-MSCs. The bone autograft was mixed with HA granules at a ratio of 1:1. Partial weight-bearing was allowed as soon as tolerated and full weight-bearing after eight weeks. All surgical procedures were performed by the same surgeon at the same institution (IHD).
Post-operative monthly follow-ups were conducted for 12 months in all subjects. The measured outcomes were post-operative pain level based on the visual analogue scale (VAS; scaled 0 no pain to 10 worst pain) and functionality of the involved extremity scored by either the Lower Extremity Functional Scale (LEFS; scaled 0 worst to 80 best)18 or the Disabilities of the Arms, Shoulder and Hand score (DASH; scaled 0 best to 100 worst).19 The LEFS and DASH were combined to create a functional score by using a percentage calculation. Clinical evaluations were conducted by two blinded orthopaedic residents (EK and YPD).
Radiological assessments for union were conducted by a blinded radiologist (NDY) using two radiological scoring systems: the Lane-Sandhu20 and Tiedeman radiological scores.21 Scoring descriptions for each system are listed in Tables II and III.
Table II.
Description of Lane-Sandhu20 radiographic scoring
| Lane-Sandhu | |
|---|---|
| Score | Description |
| 0 | No callus |
| 1 | Minimal callus formation |
| 2 | Callus evident and beginning osseous formation |
| 3 | Callus evident and fracture line almost obliterated |
| 4 | Complete union with complete remodelling |
Table III.
Description of Tiedeman21 radiographic scoring
| Tiedeman* |
|||
|---|---|---|---|
| Score | Bone formation | Union state | Remodelling state |
| 0 | No bone formation | Fracture line intact | No remodelling |
| 1 | Occurred in 25% of defect | – | Remodelling on one cortex |
| 2 | Occurred in 50% of defect | Fracture line partial | – |
| 3 | Occurred in 75% of defect | – | – |
| 4 | Occurred in 100% of defect | Fracture line disappeared | Remodelling on two cortices |
-
*
Tiedeman radiological scoring is based on the sum of three aspects of fracture healing: bone formation, union state and remodeling state
Statistical analysis
Outcome values were compared between the treatment and control groups using Student’s t-test. Data were presented as mean and standard deviation (sd). A p-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS v19 (SPSS Inc., Chicago, Illinois).
Results
A total of eight out of ten patients were male and both of the remaining females were allocated to the control group. The majority of nonunion cases involved the lower extremity with only two cases of nonunion of the humerus. The mean age of the patients was 27.4 years (18 to 37) in the treatment group (patient numbers 1 to 5) and 47.8 years (32 to 70) in the control group (patient numbers 6 to 10). The mean duration of fracture neglect in the treatment and control groups was 37.2 months and ten months, respectively (p = 0.211). Various comorbidities that might interfere with post-operative healing such as smoking habits, history of open fracture and lower IGF-1 levels were present in four patients in the treatment group. The duration of the BM-MSC culture ranged from 21 to 28 days, yielding 15 233 250 cells on average, with 89.79 sd 1.84 % cell viability. The cell viability was significantly lower in patients who were regular smokers.
Post-operative pain decreased significantly after the first four weeks in all subjects but it did so more quickly in the control group than in the treatment group. Post-operative pain subsided completely for all subjects after eight months but there were no clinically or statistically significant differences in the VAS between the two groups (Fig. 1).
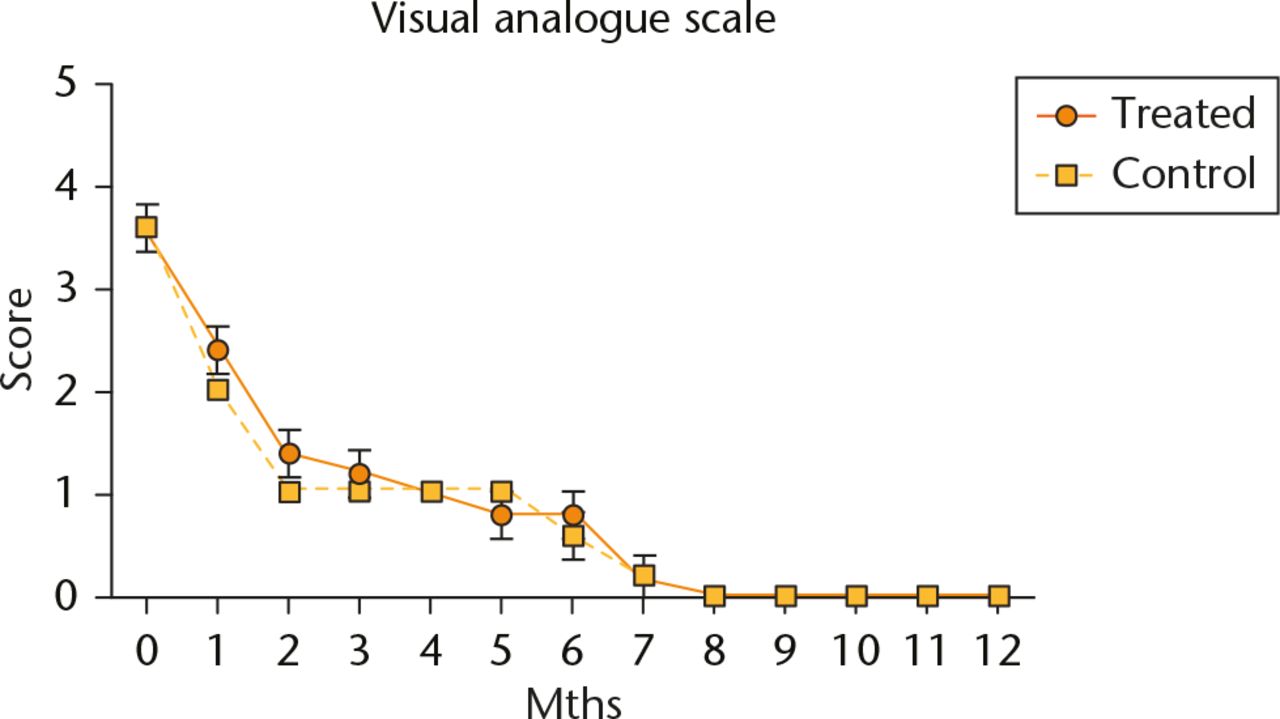
Fig. 1
Evaluation of post-operative pain using the visual analogue scale. Data are shown as mean and sd, n = 5 subjects/group, p > 0.05
The treatment group showed a general trend of greater functional improvements throughout the early post-operative period. Statistically significant differences were present during the first (p = 0.002), second (p = 0.005) and third (p = 0.01) month (Mann Whitney U test). By the end of the third month, the functional outcome percentage of the treatment group reached 43% and only 27% in the control group. The functional scores between the two groups converged after the seventh month (Fig. 2).

Fig. 2
Evaluation of functional scores. Data are shown as mean and sd, n = 5 subjects/group, *p < 0.05
All patients had progressive radiographic improvements. Treated patients demonstrated quicker radiographic improvements (bone growth) based on the Lane-Sandhu scoring system. On average, they achieved radiographic consolidation (Lane-Sandhu score ⩾ 3) by the eighth month, three months earlier than the control group (Fig. 3). Similar results were also evident when assessed using the Tiedeman scoring system (Fig. 4). Although the treatment group demonstrated faster radiological improvements, by the end of the one-year follow-up, both groups achieved similar radiographic results. By the end of our series, all cases from both groups had achieved union. No infection, nor immunological, neoplastic, or other, side effect was reported throughout the post-operative period.

Fig. 3
Evaluation of Lane-Sandhu Radiologic Score. Data are shown as mean and sd, n = 5 subjects/group, p > 0.05.

Fig. 4
Evaluation of Tiedema Radiologic Score. Data are shown as mean and sd, n = 5 subjects/group, p > 0.05.
Discussion
Bone graft possesses the basic components of bone healing: innate osteoprogenitor cells, pre-existing bone architecture and essential proteins for healing.22 Autologous bone graft was used as the control in this study because it is currently considered to be most effective for treating nonunions. Hence, direct comparison of BM-MSCs with autologous bone graft will provide the most clinically relevant results that may change the mainstay treatment of choice for nonunions. Atrophic nonunions cannot heal without augmentation of osteogenic factors such as bone graft.8 Thus, to ensure fracture union in every subject, we did not include control subjects that only received skeletal fixation.
In concordance with the regulations advocated by the ethics committee to limit the number of treated subjects, random assignment of treatment and control group was not performed. To increase the strength of this study, five subjects who suffered from more severe nonunion were assigned to the treatment group. The remaining subjects that were allocated to the control group were five subjects whose nonunion sites were matched with those of the treatment group in order to reduce the bias due to difference of healing potential of each fracture site. Clinical judgment was based on the length of fracture neglect, presence of comorbidities that affect bone healing such as cigarette smoking, low IGF-1 level and history of infection or open fracture. On average, the nonunions of treatment subjects had been neglected for more than three years, compared with ten months in the control group.
Functional score represents an imperative outcome for patients since it is closely related to their quality of life. It is a direct representation of the healing state of the soft tissue.23 On average, subjects treated with BM-MSCs were capable of performing their daily activity independently quicker than those in the control group. Post-operative pain was reduced to a negligible level at two months for both groups. As functional outcome is related to the status of bone healing and pain improvement, these findings indirectly suggest that the intervention group has achieved clinical union at a faster rate. This might be due to the accelerated bone healing effect by BM-MSCs. Even though there are previous reports regarding the effect of MSCs on the healing of nonunions, to our knowledge there are none that reported the functional outcome in nonunion patients treated with MSCs.
Diagnostic radiographic criteria for the diagnosis of nonunion includes the absence of bridging trabeculae, sclerotic fracture edges, persistent fracture lines and lack of evidence of progressive change towards union on serial radiographs.24 Despite published criteria, the radiographic diagnosis of nonunions is still affected by the subjectivity of the reader.25,26 Therefore, we implemented two radiographic scoring systems to improve the evaluation of bone union. Based on the Lane-Sandhu scoring system, the average treated subjects achieved radiological union by the eighth month, which was approximately three months earlier than the control group. Time needed for union after a revision surgery in nonunion cases ranged from 6.2 months (sd 4.1) in patients aged less than 65 and around 7.6 months (sd 6.6) in the older group.27
The Tiedeman scoring system evaluates three different aspects of bone healing. However, there is no consensus regarding score that confirms radiological union. Given the definition of radiological union of evident callus formation and obliteration of the fracture line, we deduced that a Tiedeman score of ⩾ 5 was the cut off point for radiographic union. On average, the treatment group achieved radiographic union one month earlier than the control group at the fifth month. Although the treatment group showed an accelerated healing, the radiographic scores at the end of the 12-month follow-up were similar between the two groups. This is explainable by the physiological process of cortical bone healing. The initial stage of fracture healing is marked by the rapid formation of primary woven bone. This process is followed by the hardening of newly formed callus through endochondral ossification. The BM-MSCs promoted quicker initial callus formation of the treated subjects, thus enabling them to achieve quicker functional return and higher radiologic scores.
The use of bone marrow-derived stromal cells in fracture nonunions has been reported by several authors, with positive results. There is also some variation in the method of introducing the cells into the defect area; some use percutaneous injection using a specified vehicle, others directly introduce the cells with a scaffold into the fracture site. Raulo et al28 have reported a case of percutaneous injection of bone marrow-derived stem cells for treating atropic nonunion of the tibia. Using a similar harvesting and culture technique, the cultured stem cells (3 × 107 cells) were injected in several places around the nonunion site. Callus formation and obliteration of fracture gap were identified at six months.
Despite having the advantage of minimising the soft-tissue injury caused by surgical procedures, percutaneous injection of the stem cells may risk losing a substantial amount of cells by apoptosis due to lack of cellular attachment. In order to overcome this, Kim et al29 used fibrin as a vehicle to facilitate the safe attachment of autologous cultured cells to the defect area. As this study evaluated the effect of stem cells on the healing of long bone fractures, the efficacy of this technique in fracture nonunions is still unknown. A good biological environment provided by preserved soft tissue is beneficial for the bone healing process, but in most long-standing cases of nonunion, such as those in the current study, decortication of the fracture site provided by the surgical procedures is essential.
The diamond concept of fracture healing, first introduced by Giannoudis, Einhorn and Marsh,16 is the landmark use of tissue engineering to provide a solution to impaired fracture healing. The diamond concept was further enhanced by adding the optimal biological chamber. An ideal biological environment (nonunion bed) will facilitate an early and successful osteogenesis leading to bone continuity and functional restoration. Surgical procedures such as decortication, while preserving the soft tissue as much as possible, should provide a biological chamber which is active enough to support the necessary physiological process.30
The concept of surgical decortication followed by implantation of scaffold loaded with autologous culture-expanded MSCs is further supported by the positive outcome demonstrated by several reported cases. Bajada et al31 presented a case of a nine-year hypertrophic tibial nonunion resistant to six previous surgical interventions which was then successfully treated by surgical decortication along with implantation of expanded bone marrow stromal cells carried by calcium sulphate pellets. Clinical union was identified at eight weeks after surgery. However, it is not known whether the combination of decortication, MSCs and CaSO4, or one of these factors alone, was responsible for the healing.31 Quarto et al32 reported 15 to 27 months’ follow-up of three patients with a bone defect of a critical size filled with a hydroxyapatite scaffold loaded with culture-expanded autologous MSCs. Graft integration was found within two months after surgery and all three patients regained the function of their limbs with no adverse effects. A similar study using bioceramic-HA scaffold loaded with MSCs was reported by Marcacci et al.33 Complete fusion was achieved five to seven months after surgery.
Both autograft and BM-MSCs have osteogenetic properties that are integral in bone healing. The number of osteoprogenitor cells administered plays an important role in fracture healing.32 Osteogenic precursor cells such as MSCs also exist in autograft but not in as highly concentrated a form as in the ex-vivo cultured MSCs.33 Thus, the osteogenetic properties of autologous bone graft are presumed not to be as extensive as that of MSCs. Moreover, interindividual variability of autologous bone graft may exist. Genetic factors, graft preparation techniques, and osteonecrosis may compromise its osteogenetic properties.34
Hydroxyapatite is a biomaterial with osteoconductive properties and it is known to be well accepted by bone tissue with no inflammatory reaction caused by hydroxyapatite granules. Although BM-MSCs have excellent osteogenetic properties, they need a scaffold with good osteoconductive capabilities in order to achieve union. Loading the serum-diluted BM-MSCs onto the scaffold before implantation allows immediate functional cellular attachment to the osteoconductive carrier.31
The last and the longest step of the bone healing process is the gradual replacement of callus with mature lamellar bone, followed by the remodeling phase. Radiographic changes at this point of the healing process are less discernible. The deceleration of the healing process during this phase is observable in the last months of follow-up. Thus, any difference in radiographic scores between the groups was less evident. This process is expected to continue into the second post-operative year. Overall, the BM-MSCs allowed faster functional return by one month and improved radiologic score by approximately one to three months. All subjects in the control group achieved union despite the slower healing rate. Bone healing in the control group may not be solely initiated by the iliac crest autograft. Pre-existing “dormant” MSCs in the site of atrophic nonunion may be mobilised by the presence of relevant growth factors from the transplanted autograft.35
One limitation of this study is the lack of randomisation. The mean age of the control group was 20 years older than that of the treatment group (47.8 years, 32 to 70 versus 27.4 years, 18 to 37). Arguably, the uneven age distribution might have contributed to the slower functional improvement and radiographic healing of the control group. Two patients in the control group were considerably older (patient 7 and patient 9). However, one large retrospective analysis of 288 patients conducted by Taormina et al27 did not find a negative correlation between older age and the healing rate of nonunions. Furthermore, their duration of fracture neglect was limited to ⩽ 9 months, compared with a mean of 3.1 years (1.5 to 6) of neglect in the treatment group. Assigning the more complicated cases to the treatment group introduces a selection bias and the possibility of a less accurate comparison, but on the other hand it might also further confirm the effectiveness of MSCs.
The cases of neglected atrophic nonunion of long bone fractures treated with a combination of autologous BM-MSCs and bone graft achieved functional and radiographic restoration more rapidly than the control group. Future studies should aim for similar patient baseline characteristics through randomisation to strengthen the result. Another limitation of this study was the short duration of follow-up. Longer follow-up would better illustrate the continuous progression and end result of both treatment arms.
All cases of nonunion fracture of the long bone presented in this study were successfully treated using both autologous BM-MSCs and bone graft. We found that patients treated with BM-MSCs achieved functional and radiographic restoration more rapidly than the autograft group. By the end of the one-year follow-up, both treatment and control groups showed similar functional and radiographic outcomes. The combination of autologous BM-MSCs and HA granules is safe and remains a good candidate for the definitive treatment of fracture nonunions.
Supplementary Material
Radiographs showing the treatment and control groups pre- and post-operatively can be found alongside this paper at http://www.bjr.boneandjoint.org.uk/
Funding Statement
The authors Ismail H.D., P. Phedy, E. Kholinne, Y. P. Djaja and N. D. Yulisa declare that funding was received from the Faculty of Medicine, Universitas Indonesia as a research grant for this study.
We would like to thank all laboratory technicians from the Laboratory of Regenerative and Cellular Therapy (ReGeniC) for performing the BM-MSCs in vitro cultivation.
ICMJE conflict of interest
None declared.
References
1 Al-Hadithy N , SewellMD, BhavikattiM, GikasPD. The effect of smoking on fracture healing and on various orthopaedic procedures. Acta Orthop Belg2012;78:285-290.PubMed Google Scholar
2 Gustilo RB , MendozaRM, WilliamsDN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma1984;24:742-746.CrossrefPubMed Google Scholar
3 Guntur AR , RosenCJ. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep2013;2:437.CrossrefPubMed Google Scholar
4 Reverte MM , DimitriouR, KanakarisNK, GiannoudisPV. What is the effect of compartment syndrome and fasciotomies on fracture healing in tibial fractures?Injury2011;42:1402-1407.CrossrefPubMed Google Scholar
5 Dahabreh Z , DimitriouR, GiannoudisPV. Health economics: a cost analysis of treatment of persistent fracture non-unions using bone morphogenetic protein-7. Injury2007;38:371-377.CrossrefPubMed Google Scholar
6 Busse JW , BhandariM, SpragueS, Johnson-MasottiAP, GafniA. An economic analysis of management strategies for closed and open grade I tibial shaft fractures. Acta Orthop2005;76:705-712.CrossrefPubMed Google Scholar
7 Gómez-Barrena E , RossetP, LozanoD, et al.. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone2015;70:93-101.CrossrefPubMed Google Scholar
8 Megas P . Classification of non-union. Injury2005;36:S30-S37.CrossrefPubMed Google Scholar
9 Darmawan J , ValkenburgHA, MuirdenKD, WigleyRD. Epidemiology of rheumatic diseases in rural and urban populations in Indonesia: a World Health Organisation International League Against Rheumatism COPCORD study, stage I, phase 2. Ann Rheum Dis1992;51:525-528.CrossrefPubMed Google Scholar
10 Dickson K , KatzmanS, DelgadoE, ContrerasD. Delayed unions and nonunions of open tibial fractures. Correlation with arteriography results. Clin Orthop Relat Res1994;302:189-193.PubMed Google Scholar
11 Betz RR . Limitations of autograft and allograft: new synthetic solutions. Orthopedics2002;25:s561-s570.CrossrefPubMed Google Scholar
12 Moore WR , GravesSE, BainGI. Synthetic bone graft substitutes. ANZ J Surg2001;71:354-361.PubMed Google Scholar
13 Bahney CS , MiclauT. Therapeutic potential of stem cells in orthopedics. Indian J Orthop2012;46:4-9.CrossrefPubMed Google Scholar
14 Hernigou P , PoignardA, BeaujeanF, RouardH. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg [Am]2005;87-A:1430-1437.CrossrefPubMed Google Scholar
15 Phedy P , DilogoIH, JusufAA, KholinneE, EfendiZ. Iliac crest and femoral bone marrow as the source of plastic-adherent cells. Med J Indones2011;20:100-104. Google Scholar
16 Giannoudis PV , EinhornTA, MarshD. Fracture healing: the diamond concept. Injury2007;38:S3-S6.CrossrefPubMed Google Scholar
17 Lubis AM , SandhowL, LubisVK, et al.. Isolation and cultivation of mesenchymal stem cells from iliac crest bone marrow for further cartilage defect management. Acta Med Indones2011;43:178-184.PubMed Google Scholar
18 Binkley JM , StratfordPW, LottSA, RiddleDL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther1999;79:371-383.PubMed Google Scholar
19 Hudak PL , AmadioPC, BombardierC. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med1996;29:602-608.CrossrefPubMed Google Scholar
20 Lane JM , SandhuHS. Current approaches to experimental bone grafting. Orthop Clin North Am1987;18:213-225.PubMed Google Scholar
21 Tiedeman JJ , LippielloL, ConnollyJF, StratesBS. Quantitative roentgenographic densitometry for assessing fracture healing. Clin Orthop Relat Res1990;253:279-286.PubMed Google Scholar
22 Finkemeier CG . Bone-grafting and bone-graft substitutes. J Bone Joint Surg [Am]2002;84-A:454-464.CrossrefPubMed Google Scholar
23 Shahid M , HussainA, BridgemanP, BoseD. Clinical outcomes of the Ilizarov method after an infected tibial non union. Arch Trauma Res2013;2:71-75.CrossrefPubMed Google Scholar
24 Bhattacharyya T , BouchardKA, PhadkeA, et al.. The accuracy of computed tomography for the diagnosis of tibial nonunion. J Bone Joint Surg [Am]2006;88-A:692-697.CrossrefPubMed Google Scholar
25 Hammer RR , HammerbyS, LindholmB. Accuracy of radiologic assessment of tibial shaft fracture union in humans. Clin Orthop Relat Res1985;199:233-238.PubMed Google Scholar
26 Marsh D . Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res1998;355:S22-S30.CrossrefPubMed Google Scholar
27 Taormina DP , ShulmanBS, KariaR, et al.. Older age does not affect healing time and functional outcomes after fracture nonunion surgery. Geriatr Orthop Surg Rehabil2014;5:116-121. Google Scholar
28 Raulo BC , DashC, RathS, et al.. Use of bone marrow derived stem cells in a fracture non-union. J Act Dis2012;156-158. Google Scholar
29 Kim SJ , ShinYW, YangKH, et al.. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast (Ossron) injection to treat fractures. BMC Musculoskelet Disord2009;10:20. Google Scholar
30 Calori GM , GiannoudisPV. Enhancement of fracture healing with the diamond concept: the role of the biological chamber. Injury2011;42:1191-1193.CrossrefPubMed Google Scholar
31 Bajada S , HarrisonPE, AshtonBA, et al.. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg [Br]2007;89-B:1382-1386.CrossrefPubMed Google Scholar
32 Quarto R , MastrogiacomoM, CanceddaR, et al.. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med2001;344:385-386.CrossrefPubMed Google Scholar
33 Marcacci M , KonE, MoukhachevV, et al.. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng2007;13:947-955.CrossrefPubMed Google Scholar
34 Pape HC , EvansA, KobbeP. Autologous Bone Graft: properties and Techniques. J Orthop Trauma2010;24:S36-S40.CrossrefPubMed Google Scholar
35 Ismail HD , PhedyP, KholinneE, et al.. Existence of mesenchymal stem cellsin sites of atrophic nonunion. Bone Joint Res2013;2:112-115.CrossrefPubMed Google Scholar









