Abstract
Objectives
During open orthopaedic surgery, joints may be exposed to air, potentially leading to cartilage drying and chondrocyte death, however, the long-term effects of joint drying in vivo are poorly understood. We used an animal model to investigate the subsequent effects of joint drying on cartilage and chondrocytes.
Methods
The patellar groove of anaesthetised rats was exposed (sham-operated), or exposed and then subjected to laminar airflow (0.25m/s; 60 minutes) before wounds were sutured and animals recovered. Animals were monitored for up to eight weeks and then sacrificed. Cartilage and chondrocyte properties were studied by histology and confocal microscopy, respectively.
Results
Joint drying caused extensive chondrocyte death within the superficial regions of cartilage. Histology of dried cartilage demonstrated a loss of surface integrity at four weeks, fibrillations at eight weeks, and an increased modified Mankin score (p < 0.001). Cartilage thickness increased (p < 0.001), whereas chondrocyte density decreased at four weeks (p < 0.001), but then increased towards sham-operated levels (p < 0.01) at eight weeks. By week eight, chondrocyte pairing/clustering and cell volume increased (p < 0.05; p < 0.001, respectively).
Conclusions
These in vivo results demonstrated for the first time that as a result of laminar airflow, cartilage degeneration occurred which has characteristics similar to those seen in early osteoarthritis. Maintenance of adequate cartilage hydration during open orthopaedic surgery is therefore of paramount importance.
Cite this article: Dr A. Hall. Drying of open animal joints in vivo subsequently causes cartilage degeneration. Bone Joint Res 2016;5:137–144. DOI: 10.1302/2046-3758.54.2000594.
Article focus
-
The drying of open living animal joints by laminar airflow subsequently causes deleterious changes to cartilage and in situ chondrocytes.
-
This leads to cartilage degeneration which has the characteristics of osteoarthritis (OA).
Key messages
-
Properties of cartilage were studied by histology and modified Mankin score, and in situ fluorescently-labelled chondrocytes by confocal laser scanning microscopy (CLSM).
-
Some of the changes observed resembled the degeneration that occurs during early osteoarthritis.
-
The results strongly suggest that limiting cartilage drying by maintaining effective cartilage hydration is crucial so as to minimise the damaging effect of laminar airflow on joint health.
Strengths and limitations
-
This is the first study demonstrating the effect of open joint drying by laminar airflow on the properties of cartilage and chondrocytes in an in vivo animal model.
-
The model cannot precisely mimic the changes occurring during open human orthopaedic surgery.
-
Nevertheless, it is highly likely that the deleterious changes to cartilage and chondrocytes reported here may occur in human cartilage unless adequate cartilage hydration is maintained under laminar airflow.
Introduction
The effects of drying on articular cartilage are of relevance during commonly performed orthopaedic procedures such as the treatment of intra-articular fractures or unicompartmental joint arthroplasties. The majority of fractures, especially those in larger joints such as the shoulder, elbow, hip, and knee, require open surgery for the restoration of articular surface congruity. These procedures can be complex and lengthy, and the native articular cartilage is exposed to allow visual inspection and reconstruction. In many orthopaedic theatres, the tissue is typically exposed to a constant laminar airflow of approximately 0.4 m/s.1,2 Articular cartilage is therefore at risk of drying unless regular irrigation is performed, however, there does not appear to be a defined protocol for cartilage irrigation and the tissue may be simply kept moist with saline-saturated gauze or irrigated at the surgeon’s discretion. There is a well-defined association between intra-articular fractures and secondary development of degenerative disorders within synovial joints.3 This raises the possibility that deleterious effects of articular cartilage drying during prolonged, complex surgery may decrease chondrocyte viability and potentially predispose the development of secondary osteoarthritis (OA).
Knee arthroplasty surgery for OA is now exclusively performed under laminar airflow and it is common practice to leave parts of the knee joint intact during surgery. For instance, during total knee arthroplasty (TKA) while the tibiotalar compartment is replaced, the patellar cartilage in the patellofemoral joint is often not resurfaced. Similarly, during partial knee arthroplasty the medial compartment of the knee joint is replaced but the lateral compartment and patellofemoral compartments are left intact. TKA surgery can last one to two hours and thus the native cartilage that is not replaced may be exposed to prolonged airflow. Inferior outcomes following TKA have been attributed to patellofemoral pain,4 and following partial knee arthroplasty to OA progression in the unresurfaced lateral compartment.5 Post-operative problems associated with these procedures could be due to a variety of factors, including the possibility that cartilage drying from the exposure of these unresurfaced parts of the knee joint may have resulted in loss of chondrocyte viability and initiation, and progression of OA.
Articular cartilage has a high water content (approximately 70% by weight) which increases in OA.6 In addition, the drying of cartilage in vitro changes the cartilage matrix and causes chondrocyte death. For example, Mitchell and Shepard7 reported changes to matrix constituents and chondrocyte3 H-proline labelling of rabbit cartilage exposed to static air. After 60 minutes of drying, cartilage was ‘necrotic’ but the changes were prevented by Ringer’s lactate irrigating solution. Chondrocyte necrosis and histological changes to cartilage have also been described after exposure of rabbit knees to static air causing drying.8 Interestingly, Speer et al9 noted that deleterious histochemical changes to rabbit cartilage following short-term drying were reversible if cartilage was not exposed to mechanical load. Pun et al10 reported that chondrocyte death within human osteochondral explants correlated with length of exposure to (static) air and cartilage depth, and noted the particular sensitivity of superficial zone (SZ) chondrocytes. Von Keudell et al11 demonstrated that periodic re-wetting of cartilage in vitro with Ringer’s lactate solution could limit SZ chondrocyte death. Recently, Paterson et al12 reported that chondrocytes at the cut edge of bovine or human cartilage were more sensitive to drying by laminar airflow than those distant from the edge. In addition, whereas rehydration could restore the normal appearance of cartilage, the extent of chondrocyte death was unaltered.12
Chondrocyte death arising from cartilage drying is a particular concern as cell division is absent in healthy articular cartilage.13 The reduced cell number will place an increasing burden on matrix maintenance and turnover by the remaining chondrocytes and there will be acellular areas of cartilage which are effectively dead. Articular cartilage has a very limited capacity for repair and the fibro-cartilaginous matrix produced is mechanically incompetent.14,15 Loss of SZ integrity is an early event in OA14 and thus maintenance of chondrocyte viability is crucial to prevent the OA cascade.16 Previous work on cartilage explants exposed to air emphasised the sensitivity of chondrocytes to cartilage drying.12 However, the long-term behaviour of cartilage to laminar airflow in vivo has not, to our knowledge, been reported. The aim of this investigation was therefore to test the hypothesis that the drying of exposed living animal joints would subsequently lead to degenerative changes in articular cartilage.
Materials and Methods
Surgical procedures and in vivo cartilage drying - animals
A total of 24 male Sprague Dawley rats (eight weeks old) were anaesthetised (3% isoflurane) and the patella dislocated laterally after medial parapatellar arthrotomy to expose the patellar groove.17 In preliminary experiments we observed that the amount of synovial fluid in opened joints between animals was variable and this appeared to influence the rate of cartilage drying and thus chondrocyte death. Accordingly, to standardise the amount of synovial fluid, excess synovial fluid was absorbed by gently swabbing with Melgisorb (Tendra 250600, Gothenburg, Sweden).18 The open joint was then exposed to laminar airflow (0.25 m/s; 60 minutes) before the patellar dislocation was reduced and the wound sutured in layers with coated Vicryl 6-0 (polyglactin 910; Ethicon Inc., Somerville, New Jersey). Control sham operations were performed in which the patella was displaced laterally, excess synovial fluid removed and the patella relocated without cartilage drying. Rats were given buprenorphine (0.01 mg/kg) subcutaneously and then allowed unrestricted activities in standard cages. Rats were randomly assigned to the experimental groups (four in each group) and were killed either immediately after surgery (week 0) or at four to eight weeks after surgery, and knee joints dissected. Rats were maintained under standard conditions of housing and husbandry. Procedures were approved by the Local Ethics Committee and performed under a United Kingdom Home Office licence in accordance with the Animals (Scientific Procedures) Act, 1986 (United Kingdom).
Imaging of in situ chondrocytes by confocal laser scanning microscopy (CLSM)
Knee joints were cleaned of loose tissue and incubated with 5-chloromethylfluorescein diacetate (CMFDA) and propidium iodide (PI) (one hour; both 10 µM; Invitrogen, Paisley, United Kingdom) to label living or dead cells, respectively.19,20 Fluorescently-labelled chondrocytes were imaged using a Zeiss Axioskop LSM510 (Carl Zeiss, Cambridge, United Kingdom) at low magnification (×10 dry; NA = 0.3) for chondrocyte density and viability, or with a high magnification dipping-water lens (DW; ×40 objective lens; NA = 1.2) for chondrocyte morphology and volume.19,21
Cartilage histology
Joints were decalcified and paraffin-embedded, sectioned at 5 µm intervals and stained with haematoxylin and eosin (H&E) and toluidine blue using standard protocols.22 For each joint, cartilage thickness was assessed in three non-consecutive full-depth histological sections, obtained 200 µm apart using Image J software (National Institutes of Health, Bethesda, Maryland), and the measurements averaged. Cartilage degeneration was assessed using the modified Mankin score in which cartilage structure, cellular appearance and intensity of matrix staining were assessed and scored.23,24 The changes to the joint-dried samples were very clear and could be observed with only low magnification of cartilage samples. Thus we felt that it would be obvious for even a ‘blinded’ assessor to differentiate between sham and dried cartilage by naked eye (macroscopic) or by just checking the slides under the microscope even without doing any further analysis/scoring. We therefore thought it would be more appropriate for the sham and dried cartilage to be scored by separate assessors. Accordingly, one assessor (NE) scored the sham-operated cartilage samples, while a different researcher (SP) independently scored the cartilage-dried samples using the same criteria, and the results were then collated.
Chondrocyte properties - density, viability and volume
For measurements of chondrocyte viability and density, a region of interest (ROI) (921 × 921 × 50 µm) was created using Volocity 3D (version 5.4.1; Perkin Elmer, Llantrisant, United Kingdom). A semi-automated protocol was then used to identify CMFDA- (green) and PI- (red) labelled cells and nuclei based on size and relative intensity.19 Live and dead cells were identified by voxel intensity and the percentage of cell death (PCD = 100 × number of PI-labelled cells/number of PI-labelled cells dead + number of CMFDA-labelled cells, respectively) assessed within the ROI. Chondrocyte density was determined as the total number of chondrocytes (i.e. CMFDA-labelled and PI-labelled) within the ROI to give chondrocyte number/mm3. In situ chondrocyte volume was determined from the CLSM images from a ×40 DW objective using a measurement protocol on Imaris (BitplaneAG, Zurich, Switzerland) as previously described.21 Cell arrangement was assessed visually using ×40 images and Imaris software to identify cells organised as single cells, pairs, or clusters (⩾ 3 cells in a group).
Data analysis and presentation
Data were compared between groups and time points using one- or two-way ANOVAs, or Student’s unpaired t-tests, as appropriate. Statistical tests and graphs were prepared using GraphPad Prism (GraphPad Software, La Jolla, California). Data are shown as means and 95% CI for N(n), where the number of rats is given as (N) and the number of samples from each animal is given as (n).
Results
Response of animals to joint drying
Animal behaviour was monitored routinely throughout the experimental periods as required for Home Office regulations. Pain behavioural signs such as curling toes, eversion of the foot, non-weight bearing, guarding and avoiding contact with the limb were observed following surgical procedures.25 These signs disappeared within 24 hours of surgery in both sham-operated animals and animals exposed to joint drying, with no obvious difference observed in recovery time after the surgical procedure. Animals were also closely monitored for wound healing and development of any inflammatory signs such as redness, swelling, abnormal gait and reduced spontaneous activity.26 The healing process of the incision and the inflammatory response to surgery were similar between sham-operated and experimental groups. Prior to sacrifice of animals at the different experimental points, joints were carefully examined, and there was no evidence of ulcerations, tenderness, swelling or redness in any of the animals. Similarly, on opening the joints, careful examination of the joint’s soft tissues was performed and no gross pathological changes were observed in the muscles, tendons, ligaments or menisci in the sham-operated or experimental animals.
Changes to cartilage following in vivo drying
Following visual inspection of exposed sham-operated joints, no macroscopic fibrillations were observed, however, in dried joints fibrillations were present in one of four joints following four weeks of recovery, and in three of four joints following eight weeks of recovery from surgery. On histological cartilage sections stained with H&E or with toluidine blue, sham controls showed no signs of degeneration or loss of surface congruity and were assessed with a modified Mankin score of 0 (Fig. 1(A)(a-c) and 1(B)). However, degeneration was observed in all recovered joints at four and eight weeks with examples shown in Figure 1(A)(d-f). After four weeks, the cartilage surface appeared uneven and there was loss of superficial zone (SZ) structure and the presence of empty lacunae, indicating chondrocyte loss. The modified Mankin score increased significantly to six (Fig. 1(B)). By eight weeks, the SZ had almost completely disappeared with additional loss of the mid zone (MZ), there were deep fibrillations, and there was a further significant increase in the modified Mankin score. No change to histological grading of the sham controls was observed over the eight-week period (Fig. 1(A)(a-c)); p > 0.05 by ANOVA; data not shown).
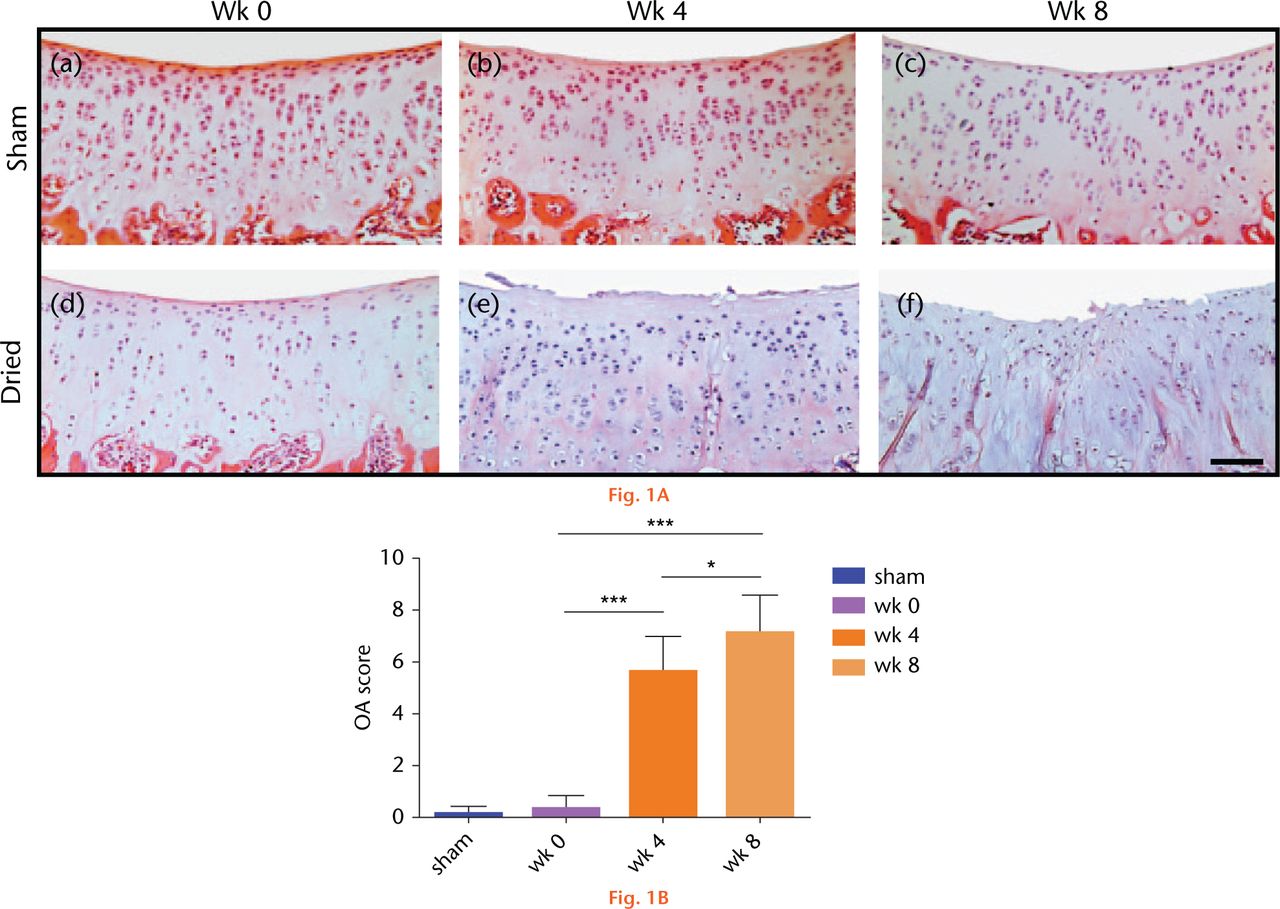
Fig. 1
Histological sections of sham-operated or dried in vivo articular cartilage and changes to the modified Mankin score following recovery from surgery. (A) Cartilage sections were obtained from sham-operated or dried joints at weeks 0, 4 and 8, respectively, and stained for H&E, and toluidine. (Bar = 50µm). (B) The modified Mankin score in H&E and toluidine-stained cartilage sections using criteria described in Materials and Methods. Data are means ± 95% confidence intervals for N(n) = 4(4). (Asterisks denote significance levels; *p < 0.05, **p < 0.01, ***p < 0.001).
There was a significant and progressive increase in cartilage thickness following the surgical procedure (Fig. 2). Thus at week four, thickness increased 1.6-fold, from week four to week eight it increased 1.3-fold, and there was a two-fold increase overall during the eight-week period (Fig. 2). There was no significant change to the cartilage thickness of the sham controls over this period (Fig. 2; p > 0.05 by ANOVA; data not shown).
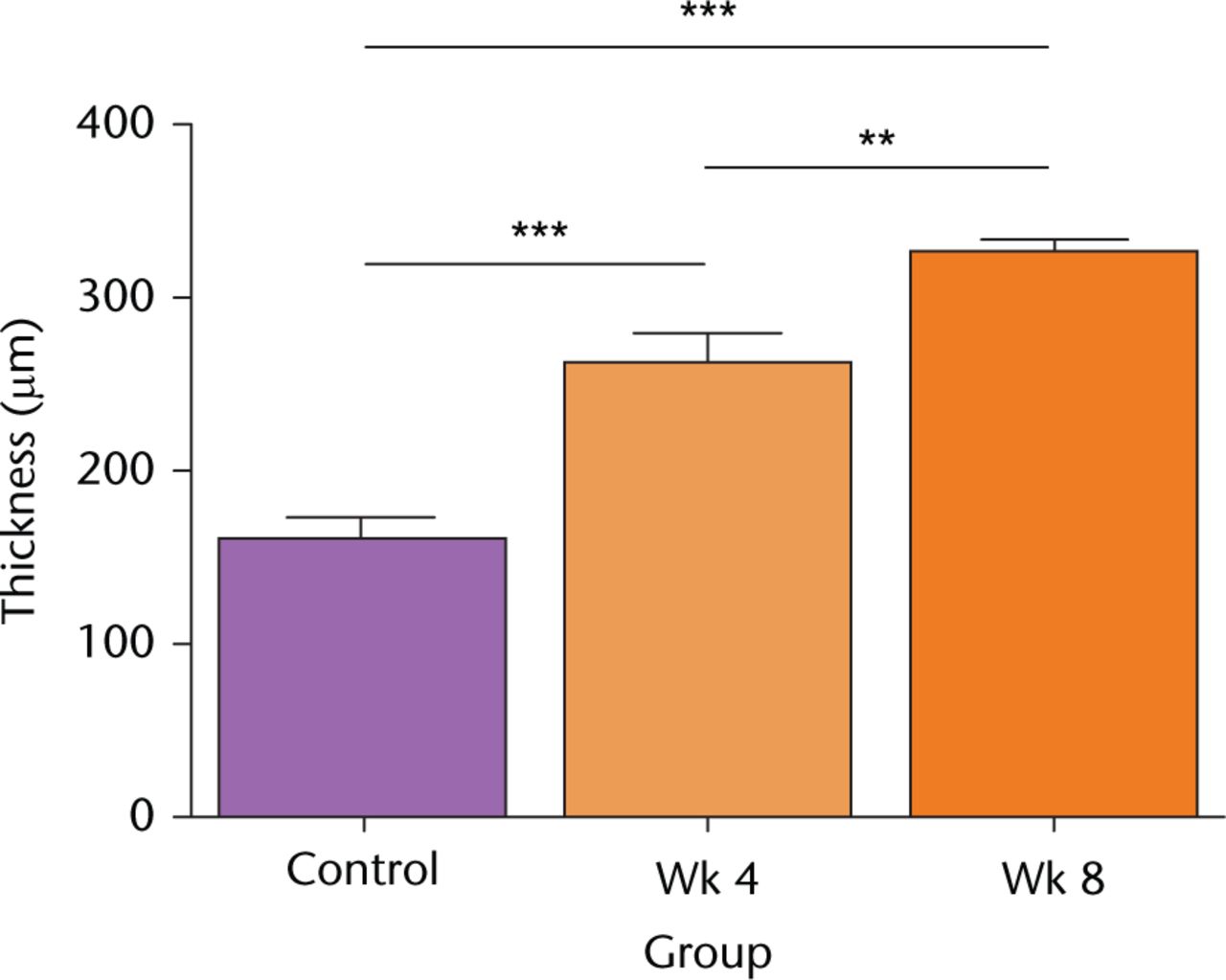
Fig. 2
Cartilage thickness following in vivo joint drying. Full-depth cartilage thickness was determined microscopically on histological sections obtained from decalcified joints as described in Materials and Methods. Data are means and 95% confidence intervals for N(n) = 8(12). (Asterisks denote significance levels; *p < 0.05, **p < 0.01, ***p < 0.001).
Viability and density of in situ chondrocytes following cartilage drying in vivo
Drying cartilage had marked effects on the viability and density of chondrocytes (Fig. 3(A)). For the sham control, no PI-labelled chondrocytes were observed (Fig. 3A(a)), chondrocyte density was approximately 170 000 cells/mm3 (Fig. 3(B)) and the percentage cell death (PCD) was negligible (Fig. 3(C)). There was no significant change to cell density or PCD of the sham controls over this period (p > 0.05 by ANOVA; data not shown). In contrast, following cartilage drying, there were no CMFDA-labelled chondrocytes, and the chondrocytes were labelled with PI, indicating that all the cells were dead (Fig. 3(A)b). By week four, some CMFDA-labelled cells were observed (Fig. 3(A)c), however, their density had declined significantly (more than fourfold) to approximately 35 000 cells/mm3. By week eight, more CMFDA-labelled chondrocytes were evident (Fig. 3(A)d), with a 2.8-fold increase in cell density to approximately 110 000 cells/mm3 (Fig. 3(B)) although noticeably the PCD had not changed significantly over the same time period (Fig. 3(C)).
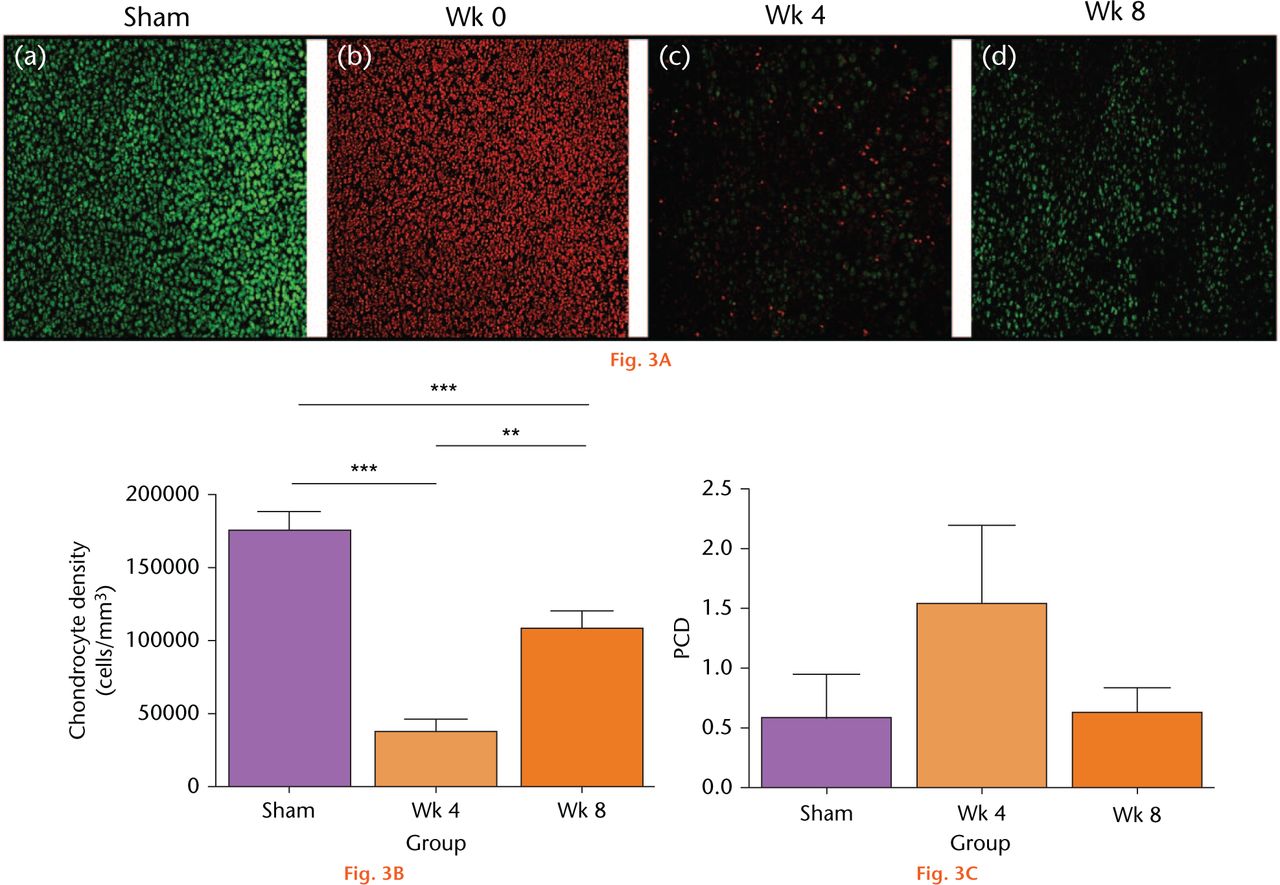
Fig. 3A
Changes to in situ chondrocyte viability and density following in vivo open joint drying. (A) Labelled in situ chondrocytes in cartilage explants from the patellar groove were imaged by CLSM (axial view) in sham controls (Sham), at the initial time point of the experiments (week 0), and then following surgical recovery after four or eight weeks. (Bar = 100 µm). (B) Cell density and (C) viability were assessed from CLSM images (see Methods). (Data are means and 95% confidence intervals for N(n) = 9(13)). (Asterisks denote significance levels; *p < 0.05, **p < 0.01, ***p < 0.001).
Effect of in vivo drying on the properties of in situ chondrocytes
Figure 4(A) shows axial images of fluorescently-labelled chondrocytes within the patellar groove in sham-operated (control) joints (Figs 4(A)(a-c)), and after eight weeks at either the non-fibrillated (Fig. 4(A)(d-f)) or fibrillated areas (Fig. 4(A)(g-i)). Chondrocyte volume and distribution appeared normal in cartilage of sham-operated joints, however, at eight weeks there were significantly fewer single chondrocytes but a significantly greater proportion of pairs and clusters (Fig. 4(B)). Chondrocyte volume also appeared to be larger compared with the images obtained in the sham control. As noted in histological sections (Fig. 1(A)), the cartilage became fibrillated, and these areas were devoid of chondrocytes (Fig. 4(A)(g-i)). Note that despite the dye being present, no PI-labelled chondrocytes were observed. Chondrocyte volume within dried cartilage eight weeks after recovery increased significantly (Fig. 5) compared with the sham controls at the same time point. However in samples from dried cartilage, there was no difference between the volume of chondrocytes at, or distant from, the fibrillations (1181 ± 256 vs 1379 ± 319 μm3, respectively; p > 0.05; N(n) = 4(1036); N(n) = 4(666), respectively).
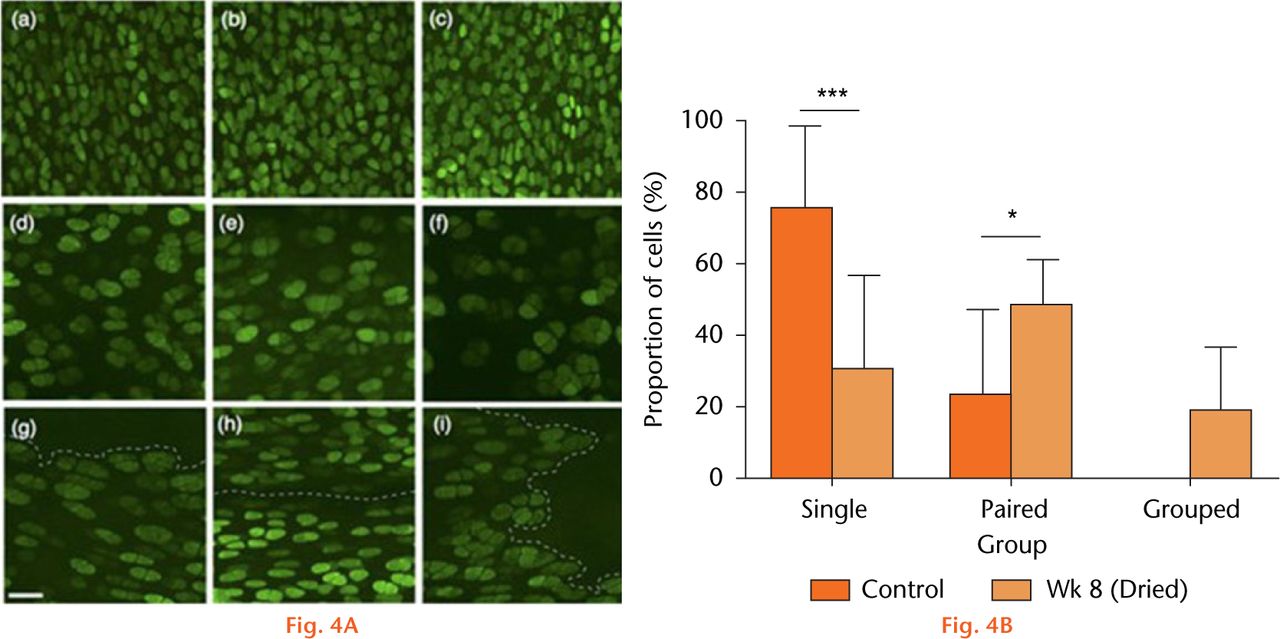
Fig.
Chondrocyte morphology and distribution. (A(a–c)), examples of CLSM images from sham-operated joints, (d-f), cartilage in vivo dried (60 minutes), then visualised after eight weeks in regions that were distant from, and (g-i) at fibrillated areas (broken lines). (Bar = 50 µm). (B) The proportion of chondrocytes either singly, in pairs, or in groups/clusters in sham-operated cartilage, or after drying and eight weeks post-surgery. Data are means ± 95% confidence intervals. (Control three joints; 870 cells), week 8 (four joints; 1702 cells). (Asterisks denote significance levels; *p < 0.05, **p < 0.01, ***p < 0.001).
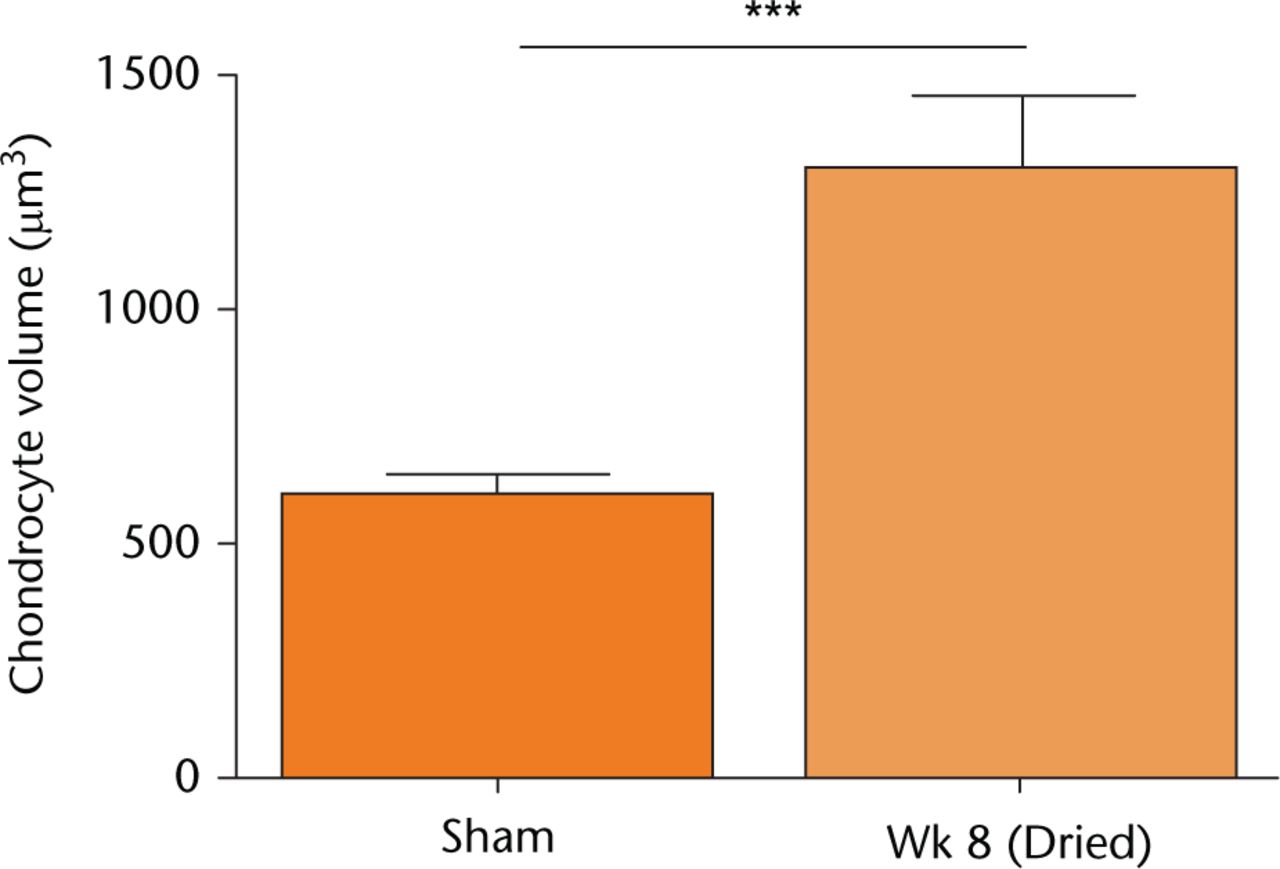
Fig. 5
The volume of in situ chondrocytes following in vivo cartilage drying. Sham-operated (control) data or that from joints dried following eight weeks of recovery, were obtained from confocal laster scanning microscopy (CLSM) images of 5-chloromethylfluorescein diacetate (CMFDA)-labelled chondrocytes as described (see Materials and Methods). Chondrocyte volumes shown are means ± 95% confidence intervals from cells visualised in Figure 4, with measurements performed on sham controls (three joints; 870 cells) and week 8 samples (four joints; 1702 cells). (Asterisks denote significance levels; *p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
In vivo drying of an exposed animal joint several weeks following surgery had profound effects on the joint cartilage and its chondrocytes. While we did not observe any obvious external changes to the animals, histology and cartilage grading showed that degeneration was evident (Figs 1(A) and 1(B)). The thickness of cartilage (Fig. 2) dried during surgery and then subsequently studied after four or eight weeks increased significantly, and chondrocyte density decreased by approximately four fold at four weeks (Fig. 3). There was then a significant increase at eight weeks, but cell density was still less than that for sham-operated joints (Fig. 3(B)). In addition, by week 8, the extent of chondrocyte pairing/clustering (Fig. 4(B)) and cell volume (Fig. 5) increased significantly. These results highlighted the damaging effect of laminar airflow and raise questions as to why joint drying had such a marked effect on cartilage and chondrocytes.
Swabbing the open joint to remove excess synovial fluid was performed in an attempt to limit variability and standardise the experimental conditions. However, this procedure would not occur during orthopaedic surgery and instead any protective properties of synovial fluid could be washed away by irrigating saline, potentially increasing the sensitivity of the cartilage and chondrocytes to subsequent drying. It is also important to note that the whole open joint was exposed to laminar airflow and it was not possible for this to be focused solely on the cartilage. Thus, neighbouring tissues (synovium, tendons, ligaments) would also have dried, perhaps in a way similar to that which occurs during orthopaedic surgery. However, we do not know if this had a permanent effect and influenced overall joint function, and cartilage properties in particular. While it is possible that these tissues may be capable of some repair and replacement of dead cells, this would not be the case for articular cartilage which has poor repair potential.14,15,27 However, the animals were monitored throughout, and no abnormal gait, external joint swelling or inflammation was observed. Additionally, during joint dissection, we did not observe any macroscopic deleterious changes to the soft tissues surrounding the joints or any evidence of inflammation. It may be that the whole exposed area was dried and damaged, but that the soft tissues recovered whereas the cartilage did not. Therefore it is possible that cartilage was the most sensitive tissue and that the other tissues were protected against the damaging effect of airflow, perhaps by their vascularised nature.
The data on chondrocyte density showed an initial decrease followed by a rise (Fig. 3(B)), whereas the results on chondrocyte viability showed no change over the same time course (Fig. 3(C)). This might be because the remains of dead chondrocytes were removed, e.g. by autophagy,28 and thus there was little labelled cellular debris. Additionally, enzymes in serum can degrade nucleic acids and the remains of any dead cells would be removed, also leading to a lack of DNA labelling by PI.29 Thus, the data on chondrocyte viability and density reflect the presence of viable chondrocytes, and any dead chondrocytes may not have been observed if no DNA material remained. It should also be noted that as these were axial CLSM images, it is possible that the reduced cell density at four weeks was due to the loss of chondrocytes within the SZ (Fig. 1(A)(e)). However, at eight weeks this zone may have disappeared (see Fig. 1(A)(f)) and chondrocytes in the mid zone were now near the damaged cartilage surface and would be imaged resulting in the increase in chondrocyte density.
It has been proposed that injuries causing chondrocyte death may stimulate the emergence and homing of chondrogenic progenitor cells (CPCs).30 We do not know what contribution to cartilage repair, if any, would be made by these cells following the death of chondrocytes by drying and the release of any ‘alarmins’.31 However, it is likely that as CPCs are located principally in the superficial zone,32 and since cells in this region are particularly sensitive to drying,12 the CPCs would be vulnerable. It is also possible that the rate of cartilage failure was too rapid for CPCs to play any effective role in repair.
Cartilage degeneration, as occurs in OA, is complex and results from risk factors such as age, genetic predisposition, obesity, anatomical abnormalities and excessive load or joint injury.14 The changes to cartilage and chondrocytes following in vivo drying reported here are, in some respects, similar to early OA. Our overall assessment of cartilage properties showed an increase in the modified Mankin score (Fig. 1(B)), which is characteristic of OA development.33 In addition, cartilage thickening and swelling (Fig. 2), chondrocyte loss followed by an increase in cell numbers (Fig. 3B), chondrocyte clustering (Fig. 4(B)) and increased chondrocyte volume (Fig. 5) were observed, and are all similar to the changes in OA.23,34-36 Cartilage swelling from damage to the collagen network leading to further water imbibition and over-hydration, is dramatic in early human femoral head OA where cartilage wet weight to dry weight increases by approximately 60%37 and this may markedly reduce load-bearing capacity.38 At four weeks, there was an initial decline in chondrocyte viability and density (Fig. 3(B)), but by eight weeks density had increased (Fig. 3(B)), with pairs and clusters and groups of cells being observed (Fig. 4(B)). This increase in chondrocyte death followed by a hyper-cellular response has also been reported during OA development where it may be present as part of a ‘repair’ response.23 Nonetheless, caution should be exercised when extrapolating these results to the situation that might occur during or after open orthopaedic surgery in humans. The effects we observed were very rapid compared with the time course of human OA development.14 Furthermore, the drying effect might be more marked because animal cartilage is thinner (200 μm to 400 μm) than human cartilage by an order of magnitude.6
At present, it is unknown how in vivo joint drying subsequently leads to these deleterious effects. Cartilage drying could cause chondrocyte damage or death, irreparable damage to the extracellular matrix, or a combination of these factors. The injury or death of chondrocytes is implicated in OA development.39,40 This could result in an increased release of degradative enzymes leading to cartilage breakdown,41 and/or the development of cartilage areas which are effectively dead, inevitably leading to matrix weakening or loss.42 Alternatively, drying could cause damage to matrix proteins, leading to surface roughness43 or glycosaminoglycan (GAG) depletion.9 While there is very little information on the effects of drying on matrix proteins and its recovery following rehydration, chondrocyte loss may ultimately lead to cartilage failure. Simon et al,44 investigating the effects of localised freezing on in vivo rabbit cartilage, reported that at six months the cartilage was apparently intact, but there were no stainable chondrocytes. However, at 12 months there was cartilage softening and fibrillations with chondrocyte clustering and other features resembling OA. While this does not rule out a role for freezing damage to the cartilage matrix, the injury is more focused than drying and suggests that chondrocyte death is important. It is firmly established that chondrocyte death, whether by apoptosis, necrosis, autophagy,45 chondroptosis,46 or a combination of these processes,28 is a crucial element in the process of cartilage degeneration. What is less clear is whether chondrocyte death precedes cartilage failure or is a consequence of it.
In summary, drying an exposed animal joint in vivo by laminar airflow leads to marked changes in cartilage and chondrocyte properties, the characteristics of which show some similarities to early OA. While the sequence of events following drying and its effects on cartilage integrity and chondrocyte viability are unclear, the results emphasise the critical importance of maintaining cartilage hydration during surgery.12
Funding Statement
This work was supported by Arthritis Research (UK) grant No: 19665. SP was funded by an Anatomy PhD Scholarship from the Deanery of Biomedical Sciences, University of Edinburgh. We thank Dr A. Kubasik-Thayil of the IMPACT imaging facility University of Edinburgh for assistance with confocal microscopy.
The Department of Orthopaedics and Trauma at the University of Edinburgh receives funding from Stryker, RCSEd, OTCF, ARUK, ORUK, and EPSRC, none of which is related to this article.
ICMJE Conflict of Interest
None declared.
References
1 No authors listed. Department of Health. Health Technical Memorandum 03-01: Specialised ventilation for healthcare premises. Part B - Operational management. 2007. https://www.gov.uk/government/organisations/department-of-health (date last accessed 15 February 2016). Google Scholar
2 No authors listed. Health Facilities Scotland. Ventilation for healthcare premises Part A - Design and validation. Scottish Health Technical Memorandum No: 03-01. 2013. http://www.hfs.scot.nhs.uk/ (date last accessed 15 February 2016). Google Scholar
3 Giannoudis PV , TzioupisC, PapathanassopoulosA, ObakponovweO, RobertsC. Articular step-off and risk of post-traumatic osteoarthritis. Evidence today. Injury2010;41:986-995.CrossrefPubMed Google Scholar
4 Scuderi GR , InsallJN, ScottNW. Patellofemoral pain after total knee arthroplasty. J Am Acad Orthop Surg1994;2:239-246.CrossrefPubMed Google Scholar
5 Mofidi A , LuB, PlateJF, et al.. Effect of arthritis in other compartment after unicompartmental arthroplasty. Eur J Orthop Surg Traumatol2014;24:805-812.CrossrefPubMed Google Scholar
6 Maroudas A , VennM. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis1977;36:399-406.CrossrefPubMed Google Scholar
7 Mitchell N , ShepardN. The deleterious effects of drying on articular cartilage. J Bone Joint Surg [Am]1989;71-A:89-95.PubMed Google Scholar
8 Han CD , KangHJ. Changes in the hyaline articular cartilage after air exposure. Yonsei Med J1990;31:53-59.CrossrefPubMed Google Scholar
9 Speer KP , CallaghanJJ, SeaberAV, TuckerJA. The effects of exposure of articular cartilage to air. A histochemical and ultrastructural investigation. J Bone Joint Surg [Am]1990;72-A:1442-1450.PubMed Google Scholar
10 Pun SY , TengMS, KimHT. Periodic rewetting enhances the viability of chondrocytes in human articular cartilage exposed to air. J Bone Joint Surg [Br]2006;88-B:1528-1532.CrossrefPubMed Google Scholar
11 Von Keudell A , SyedHM, CansecoJA, GomollAH. Efficacy of common surgical compounds in preventing articular chondrocyte death from desiccation. Knee Surg Sports Traumatol Arthrosc2015;23:1346-1350.CrossrefPubMed Google Scholar
12 Paterson SI , AminAK, HallAC. Airflow accelerates bovine and human articular cartilage drying and chondrocyte death. Osteoarthritis Cartilage2015;23:257-265.CrossrefPubMed Google Scholar
13 Lee DA , BentleyG, ArcherCW. The control of cell division in articular chondrocytes. Osteoarthritis Cartilage1993;1:137-146.CrossrefPubMed Google Scholar
14 Buckwalter JA , MankinHJ. Articular cartilage. 2: degeneration and osteoarthrosis, repair, regeneration and transplantation. J Bone Joint Surg [Am]1997;79-A:612-632. Google Scholar
15 Hunziker EB . Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable?Osteoarthritis Cartilage1999;7:15-28.CrossrefPubMed Google Scholar
16 Elsaid KA , JayGD, WarmanML, RheeDK, ChichesterCO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum2005;52:1746-1755.CrossrefPubMed Google Scholar
17 Tsuruoka H , SashoT, YamaguchiS, et al.. Maturation-dependent spontaneous healing of partial thickness cartilage defects in infantile rats. Cell Tissue Res2011;346:263-271.CrossrefPubMed Google Scholar
18 Seifer DR , FurmanBD, GuilakF, et al.. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage2008;16:1532-1538.CrossrefPubMed Google Scholar
19 Amin AK , HuntleyJS, BushPG, SimpsonAHW, HallAC. Osmolarity influences chondrocyte death in wounded articular cartilage. J Bone Joint Surg [Am]2008;90-A:1531-1542.CrossrefPubMed Google Scholar
20 Eltawil NM , HowieSEM, SimpsonAHRW, AminAK, HallAC. The use of hyperosmotic saline for chondroprotection: implications for orthopaedic surgery and cartilage repair. Osteoarthritis Cartilage2015;23:469-477.CrossrefPubMed Google Scholar
21 Bush PG , HallAC. The osmotic sensitivity of isolated and in situ bovine articular chondrocytes. J Orthop Res2001;19:768-778.CrossrefPubMed Google Scholar
22 Kiernan JA . Histological and histochemical methods: theory and practice. Oxford: Butterworth Heinemann, 1999. Google Scholar
23 Mankin HJ , DorfmanH, LippielloL, ZarinsA. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg [Am]1971;53-A:523-537.PubMed Google Scholar
24 van der Sluijs JA , GeesinkRG, vander, LindenAJ, et al.. The reliability of the Mankin score for osteoarthritis. J Orthop Res1992;10:58-61.CrossrefPubMed Google Scholar
25 Neugebauer V , HanJS, AdwanikarH, FuY, JiG. Techniques for assessing knee joint pain in arthritis. Mol Pain2007;3:8.CrossrefPubMed Google Scholar
26 Teeple E , JayGD, ElsaidKA, FlemingBC. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J2013;15:438-446.CrossrefPubMed Google Scholar
27 Hunziker EB . Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage2002;10:432-463.CrossrefPubMed Google Scholar
28 Almonte-Becerril M , Navarro-GarciaF, Gonzalez-RoblesA, et al.. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis2010;15:631-638.CrossrefPubMed Google Scholar
29 Zhou S , CuiZ, UrbanJ. Dead cell counts during serum cultivation are underestimated by the fluorescent live/dead assay. Biotechnol J2011;6:513-518.CrossrefPubMed Google Scholar
30 Seol D , McCabeDJ, ChoeH, et al.. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum2012;64:3626-3637.CrossrefPubMed Google Scholar
31 Houard X , GoldringMB, BerenbaumF. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep2013;15:375-385.CrossrefPubMed Google Scholar
32 Jiang Y , TuanRS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol2015;11:206-212.CrossrefPubMed Google Scholar
33 Pritzker KPH , GayS, JimenezSA, et al.. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage2006;14:13-29.CrossrefPubMed Google Scholar
34 McDevitt C , GilbertsonE, MuirH. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg [Br]1977;59-B:24-35. Google Scholar
35 Adams ME , BrandtKD. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol1991;18:428-435.PubMed Google Scholar
36 Bush PG , HallAC. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis Cartilage2003;11:242-251.CrossrefPubMed Google Scholar
37 Grushko G , SchneidermanR, MaroudasA. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res1989;19:149-176.CrossrefPubMed Google Scholar
38 Bank RA , SoudryM, MaroudasA, MizrahiJ, TeKoppeleJM. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum2000;43:2202-2210.CrossrefPubMed Google Scholar
39 Kühn K , D’LimaDD, HashimotoS, LotzM. Cell death in cartilage. Osteoarthritis Cartilage2004;12:1-16. Google Scholar
40 Del Carlo M Jr , LoeserRF. Cell death in osteoarthritis. Curr Rheumatol Rep2008;10:37-42.CrossrefPubMed Google Scholar
41 Goldring SR , GoldringMB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res2004;427(Suppl):S27-S36.CrossrefPubMed Google Scholar
42 Squires GR , OkouneffS, IonescuM, PooleAR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum2003;48:1261-1270.CrossrefPubMed Google Scholar
43 Smyth PA , RifkinR, JacksonRL, HansonRR. The average roughness and fractal dimension of articular cartilage during drying. Scanning2014;36:368-375.CrossrefPubMed Google Scholar
44 Simon WH , RichardsonS, HermanW, ParsonsJR, LaneJ. Long-term effects of chondrocyte death on rabbit articular cartilage in vivo. J Bone Joint Surg [Am]1976;58-A:517-526.PubMed Google Scholar
45 Lotz MK , CaramésB. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol2011;7:579-587.CrossrefPubMed Google Scholar
46 Roach HI , AignerT, KouriJB. Chondroptosis: a variant of apoptotic cell death in chondrocytes?Apoptosis2004;9:265-277.CrossrefPubMed Google Scholar









