Abstract
Objectives
T-cells are considered to play an important role in the inflammatory response causing arthroplasty failure. The study objectives were to investigate the composition and distribution of CD4+ T-cell phenotypes in the peripheral blood (PB) and synovial fluid (SF) of patients undergoing revision surgery for failed metal-on-metal (MoM) and metal-on-polyethylene (MoP) hip arthroplasties, and in patients awaiting total hip arthroplasty.
Methods
In this prospective case-control study, PB and SF were obtained from 22 patients (23 hips) undergoing revision of MoM (n = 14) and MoP (n = 9) hip arthroplasties, with eight controls provided from primary hip osteoarthritis cases awaiting arthroplasty. Lymphocyte subtypes in samples were analysed using flow cytometry.
Results
The percentages of CD4+ T-cell subtypes in PB were not different between groups. The CD4+ T-cells in the SF of MoM hips showed a completely different distribution of phenotypes compared with that found in the PB in the same patients, including significantly decreased CD4+ T-central memory cells (p < 0.05) and increased T-effector memory cells (p < 0.0001) in the SF. Inducible co-stimulator (ICOS) was the only co-stimulatory molecule with different expression on CD4+ CD28+ cells between groups. In PB, ICOS expression was increased in MoM (p < 0.001) and MoP (p < 0.05) cases compared with the controls. In SF, ICOS expression was increased in MoM hips compared with MoP hips (p < 0.05).
Conclusions
Increased expression of ICOS on CD4+ T-cells in PB and SF of patients with failed arthroplasties suggests that these cells are activated and involved in generating immune responses. Variations in ICOS expression between MoM and MoP hips may indicate different modes of arthroplasty failure.
Cite this article: Professor P. A. Revell. Increased expression of inducible co-stimulator on CD4+ T-cells in the peripheral blood and synovial fluid of patients with failed hip arthroplasties. Bone Joint Res 2016;5:52–60. doi: 10.1302/2046-3758.52.2000574
Article focus
-
Many metal-on-metal hip arthroplasty designs have failed earlier than expected due to adverse reactions to metal debris (ARMD), whilst metal-on-polyethylene hip arthroplasties traditionally fail later from aseptic loosening.
-
T-cells are considered to play an important role in the cellular reaction related to arthroplasty failure. This study investigated the composition and distribution of CD4+ T-cell phenotypes in the peripheral blood and synovial fluid of patients undergoing revision surgery for failed metal-on-metal and metal-on-polyethylene hip arthroplasties, and in a control group of patients awaiting total hip arthroplasty.
-
The phenotypes recognised were naïve or memory cells, the latter being of different effector types with distinctive activities in inflammatory immune processes.
Key messages
-
Increased expression of Inducible Co-stimulator on CD4+CD28+ T-cells in the peripheral blood and synovial fluid of patients with failed arthroplasties compared with controls suggests these cells are activated and may generate immune responses to implants.
-
Increased expression of Inducible Co-stimulator by lymphocytes in the synovial fluid of metal-on-metal hips compared to metal-on-polyethylene hips may explain the different modes of failure observed (adverse reaction to metal debris versus aseptic loosening) with these two different bearing surfaces.
-
This study suggests that peripheral blood examination using these T-cell markers has limited potential to detect immune responses occurring in relation to artificial joint arthroplasty failure.
Strengths and limitations
-
Strengths – This is the first study to perform simultaneous analysis of lymphocyte subtypes in both the peripheral blood and synovial fluid of patients with different types of failed hip arthroplasties.
-
Limitations – (1) It was not possible to obtain sufficient quantities of synovial fluid for analysis in all revised hips. (2) Inevitably, the number of patients available for a detailed prospective single centre study of this type is small, and the ability to perform such specialised experimental procedures in a standardised way across laboratories so as to combine results meaningfully in a multi-centre study is limited.
Introduction
The cellular reaction to wear particles in the periprosthetic tissues of artificial joint arthroplasties has long been considered a significant contributor to implant loosening in the absence of infection.1 Accumulating evidence demonstrates that in aseptic loosening of arthroplasties there are lymphocytes present in the interface fibrous tissue admixed with macrophages and foreign body multinucleate giant cells (MNGC).2-5 While controversy exists as to whether these lymphocytes are instrumental in implant failure, either in aseptic loosening or adverse reaction to metal debris (ARMD),6,7 several lines of evidence suggest that T-cell mediated type IV hypersensitivity to metals may play a role in some cases of aseptic loosening. Blood vessels at the interface show increased expression of P-selectin and E-selectin, cell adhesion molecules associated with migration of lymphocytes in immune-mediated inflammation.8 The T-cells at the interface tissue are activated and proliferating as evidenced by the expression of human leukocyte antigen (HLA) class II, proliferation nuclear antigen, and presence of the cytokines interleukin-2 (IL-2) and -15 (IL-15).9,10 Finally, lymphocytes and macrophages interact with active antigen presentation occurring in the periprosthetic tissue, as demonstrated by increased expression of the co-stimulatory molecules CD80, CD86, CD40, and intracellular adhesion molecule-1 (ICAM-1) on macrophages, with the counter-ligands CD28, CD40L, and lymphocyte function-associated antigen-1 (LFA-1) present on T-cells.11-13
Studies examining peripheral blood from hip arthroplasty patients have demonstrated evidence of antigen-presenting cells (APCs) in those individuals with metal-on-metal (MoM) bearing surfaces, but not in those with metal-on-polyethylene (MoP) bearings or in former MoM patients after revision to MoP articulations.14 The question arises as to whether more detailed analysis of T-cell subtypes might provide further information with respect to the differentiation of pathogenetic mechanisms as well as aiding in diagnosis. Following antigen exposure, CD4+ and CD8+ T-cells can differentiate from naïve cells (TN) through central memory (TCM), then effector memory (TEM), and finally to terminally differentiated effector memory (TEMRA) states.15,16
These phenotypes of T-cells can be distinguished on the basis of their expression of the lymphoid homing chemokine receptor CCR7 and the phosphatase CD45RA as follows: TN cells (CCR7+CD45RA+), TCM cells (CCR7+CD45RA−), TEM cells (CCR7−CD45RA−) and TEMRA cells (CCR7-CD45RA+).15,16 Central memory T-cells have the ability to home to secondary lymphoid organs but have little or no effector function.15,16 By contrast, TEM and TEMRA cells display immediate effector function by rapid entry into a site of inflammation and secretion of large amounts of cytokines.15,16 Although some studies have analysed lymphocyte subtypes in the peripheral blood (PB) of patients with different types of hip arthroplasty, none performed simultaneous analysis of the synovial fluid (SF).14,17-21
The study aims were to investigate the composition and distribution of CD4+ T-cell phenotypes in the PB and SF of patients undergoing revision surgery for failed MoM and MoP hip arthroplasties, and in patients with primary osteoarthritis awaiting total hip arthroplasty (THA).
Materials and Methods
Study design and inclusion criteria
This prospective case-control study was undertaken at one specialist arthroplasty centre. Ethical approval for this study was granted (REC Reference 09/H1010/75) with all subjects providing written informed consent.
Patients scheduled for revision of MoM or MoP hip arthroplasties (cases) and patients with primary hip osteoarthritis awaiting THA (controls) were recruited for this study over a one-year period (August 2011 to August 2012). The study exclusion criteria barred patients with suspected or confirmed deep prosthetic infection, those with known inflammatory arthritis, and any individual on immunosuppressive medications. There were 31 hips in 30 patients eligible for study inclusion (Table I). All cases had initially undergone their index hip arthroplasty for primary osteoarthritis. A total of six surgeons performed all the revision operations. A diagnosis of ARMD was confirmed only after histopathological examination and was graded as previously recommended.7
Table I.
Summary of clinical details of cohort (n = 31).
| MoM hip revisions | MoP hip revisions | Control group | |
|---|---|---|---|
| Hips (patients) | 14 (13) | 9 (9) | 8 (8) |
| Male (%):female (%) hips | 6 (43):8 (57) | 3 (33):6 (67) | 1 (13):7 (87) |
| Mean age (range) at sampling (yrs) | 59.8 (48.3 to 69.4) | 72.7 (38.8 to 85.3) | 60.4 (40.1 to 76.2) |
| Mean body mass index (range) (kg/m2) | 29.3 (23.8 to 41.3) | 26.5 (19.0 to 36.0) | 24.7 (19.7 to 28.2) |
| Mean time implant in situ (range) (yrs) | 6.4 (1.8 to 13.7) | 18.9 (5.6 to 27.1) | N/A |
| Indication for revision surgery (%) | ARMD = 13 (93) Aseptic loosening = 1 (7) | Aseptic loosening = 9 (100) | N/A |
| Median (interquartile range) blood metal ion concentration in µg/l | Co = 10.0 (4.2 to 39.6) /Cr = 7.0 (3.0 to 42.0) | N/A/N/A | N/A/N/A |
| Implant revised (%) | THA = 10 (71) /HR = 4 (29) | THA = 9 (100) | N/A |
| THA revision bearing used (%) | OxP = 7 (50) /MoP = 3 (21) /CoC = 2 (14)/CoP = 2 (14) | MoP = 8 (89) /CoP = 1 (11) | N/A |
-
ARMD = adverse reaction to metal debris; CoC, ceramic-on-ceramic; CoP, ceramic-on-polyethylene; Cr, chromium; Co, cobalt; HR, hip resurfacing; MoP, metal-on-polyethylene; N/A, not applicable; OxP, oxinium-on-polyethylene; THA, total hip arthroplasty
Whole blood was collected from patients with failed MoM (n = 14) and MoP (n = 9) hip arthroplasties immediately prior to revision surgery and in patients from the control group prior to primary THA (n = 8). It was not always possible for the revision surgeon to obtain sufficient quantities of SF for analysis to enable the extraction of mononuclear cells, although it was attempted in all cases. Suitable SF samples were available from 11 MoM and six MoP revisions. All PB and SF samples were immediately taken to the laboratory and processed within 24 hours of collection.
Sample validation and processing
Sample processing to isolate peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) was performed according to the standard protocols. To validate sample processing and flow cytometry, unstained PBMCs and SFMCs, as well as PBMCs and SFMCs spiked with BD fluorescence-activated cell sorting (FACS) seven-colour setup beads, were used. PBMC in Ethylenediaminetetraacetic acid (EDTA) and SFMC were isolated by density gradient centrifugation on Ficoll-Paque PLUS (GE Healthcare, Amersham, United Kingdom), washed twice with Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, Dorset, United Kingdom), counted using a Neubauer haemocytometer and re-suspended at 1 × 106 cells/ml in fresh medium.
Flow cytometry
Mononuclear cells were stained for surface antigens using multiple fluorescent labelled monoclonal antibodies: CD3-PerCP Cy5.5 (eBioscience, Hatfield, United Kingdom), CD4-Pacific blue (BD Biosciences, Oxford, United Kingdom), CCR7-FITC (R&D Systems, Minneapolis, Minnesota), CD45RA-APC (Biolegend, San Diego, California), ICOS-PE (BD Biosciences, Oxford, United Kingdom), CD28-APC (BD Biosciences, Oxford, United kingdom), CD14-Pacific Blue (Biolegend), CD86-FITC (BD Biosciences) at the dilutions recommended by the respective suppliers.
Analysis for Tregulator cells (CD25 high CD127 dim FOXP3+) was carried out by first surface staining the cells with CD3-FITC (BD Bioscience), CD4-Pacific blue (eBioscience), CD25-APC (BD Bioscience) and CD127-Pe-Cy7 (eBioscience), followed by fixation and permeabilisation using the FOXP3 Staining Buffer Set (Fix/Perm concentrate and diluent) (eBioscience), according to manufacturer’s instructions. Expression of FOXP3 was detected using FOXP3-PE antibody (eBioscience).
For cytokine expression, mononuclear cells were stimulated with 0.05 µg/ml phorbol myristate acetate (PMA) (Sigma-Aldrich) and 1µM ionomycin (Sigma-Aldrich) for four hours at 37°C in the presence of 10 µg/ml of brefeldin A (Sigma-Aldrich). Immunophenotyping of samples was performed with CD3-FITC (BD Biosciences) and CD4-Pacific blue (eBioscience). After this staining for surface antigens, intracellular staining was performed using fixation and permeabilisation kit (Invitrogen, Loughborough, United Kingdom) according to manufacturer’s instructions. Intracellular cytokines were detected using different colour fluorescent conjugated antibodies: IL-17A-PE (eBioscience), IL-4-Pe-Cy7 (BD Bioscience, Oxford, United Kingdom), and interferon (IFN)-γ-APC (BD Biosciences). All stained cells were run on a CyAn ADP Analyzer (Beckman Coulter Inc., Fullerton, California), and the data analysed using Summit v4.3 software (Beckman Coulter Inc.).
Statistical analysis
All statistical analysis was performed using GraphPad Prism 3.0 (GraphPad Software Inc., La Jolla, California). Depending on data distribution, either the mean and range or median and interquartile range have been provided. Analysis of paired samples (PB and SF from the same patient) was performed using the Wilcoxon signed-rank test. Analysis of independent samples (all comparisons between case and control samples) was performed using the Mann-Whitney U test. The level of statistical significance was set at p < 0.05.
Results
Revision indication
The indication for revision in 13 of 14 MoM hips was ARMD, with all patients with ARMD having raised whole blood metal ion levels and/or cross-sectional imaging confirming the diagnosis. The specific histopathological findings in the ARMD cases were: tertiary lymphoid organs with T-cells and B-cells (four hips), T-cell lymphocytic aggregates (four hips), diffuse lymphocytic infiltration with no aggregates (one hip), and macrophage response with variable amounts of intracellular metal wear debris but no lymphocytic component (four hips). All nine MoP revisions were performed for aseptic loosening, which was confirmed after histopathological and microbiological analysis.
Analysis of memory phenotypes of CD4+ T-cells
The memory phenotypes of CD4+ T-cells were differentiated by investigating the expression of lymphoid homing chemokine receptor CCR7 and the phosphatase CD45RA (Fig. 1a). There were no significant differences in the percentages of CD4+ T-cell subtypes in PBMCs between the three study groups (Fig. 1b: controls, Fig. 1c: MoP, Fig. 1d: MoM).

Fig.
Graphs showing naïve and memory CD4+ T-cell subpopulations in two groups of revised hip arthroplasty patients and a control group with osteoarthritis: (a) Mononuclear cells gated for CD3+ and CD4+ were divided into naïve and memory subpopulations using CCR7 and CD45RA markers, (b) Pooled data for subpopulations of CD4+ T-cells in peripheral blood of the control group with osteoarthritis (OA) (n = 8). Horizontal lines indicate medians, (c) Pooled data for blood CD4+ T-cell subpopulations in patients with metal-on-polyethylene (MoP) hips (n = 9). Horizontal lines indicate medians,(d) Naïve and memory CD4+ T-cell populations in peripheral blood (n = 14) and synovial fluid (n = 10) of patients with metal-on-metal (MoM) hips (D). Horizontal lines indicate medians. (TCM =central memory T-cell; TEM=effector memory T-cell; TN=naïve T-cell; TEMRA=terminally differentiated effector memory cell).
The CD4+ T-cells in the SF of the MoM hips showed a completely different distribution of phenotypes compared with PBMCs in the same patients (Fig. 1d). There was a significant decrease in the CD4+ TN cells (p < 0.0001) and CD4+ TCM cells (p < 0.05), together with a significant increase in TEM cell (p < 0.0001) populations in the SFMCs compared with the PBMCs of the MoM cases (Fig. 1d). Too few SF samples were available from MoP revision cases to perform a similar paired analysis to compare T-cell memory phenotypes in the PB and SF, and analysis of SF from controls was not part of the study protocol.
Expression of co-stimulatory molecules
To assess T-cell activation and antigen presentation, we examined the expression of co-stimulatory molecules, CD86, CD80, CD40 and inducible co-stimulator ligand (ICOSL) on CD14+ monocytes and CD28, ICOS, CD40L on CD4+ T-cells (Fig. 2a). The percentage of CD4+CD28+ cells expressing ICOS did not differ in the PB between the revision and control groups (Fig. 2b). A significant increase in the expression of ICOS in PB, measured by median fluorescent intensity, was detected in the MoM (p < 0.001) and MoP (p < 0.05) revision groups compared with the control group (Fig. 2c). The percentage of CD4+CD28+ T-cells was higher in the SF of MoM revisions compared with that of MoP revisions, though this was not statistically significant (Fig. 2d). However, a significant increase in the ICOS expression of CD4+CD28+ cells in the SF of the MoM revision group was observed compared with the MoP group (p < 0.05) (Fig. 2e). There were no other significant differences in any of the other co-stimulatory molecules between the groups. FACS analysis of PBMCs revealed no significant difference between the groups in the expression of CD28 and CD86 on the surface of CD4+ T-cells and CD14+ monocytes, respectively. No significant difference in CD28 expression was detected in the SFMCs between MoM and MoP revisions. Little or no expression of CD80, CD40 and ICOS-L on PB monocytes or CD40L on PB CD4+ T-cells was detected.
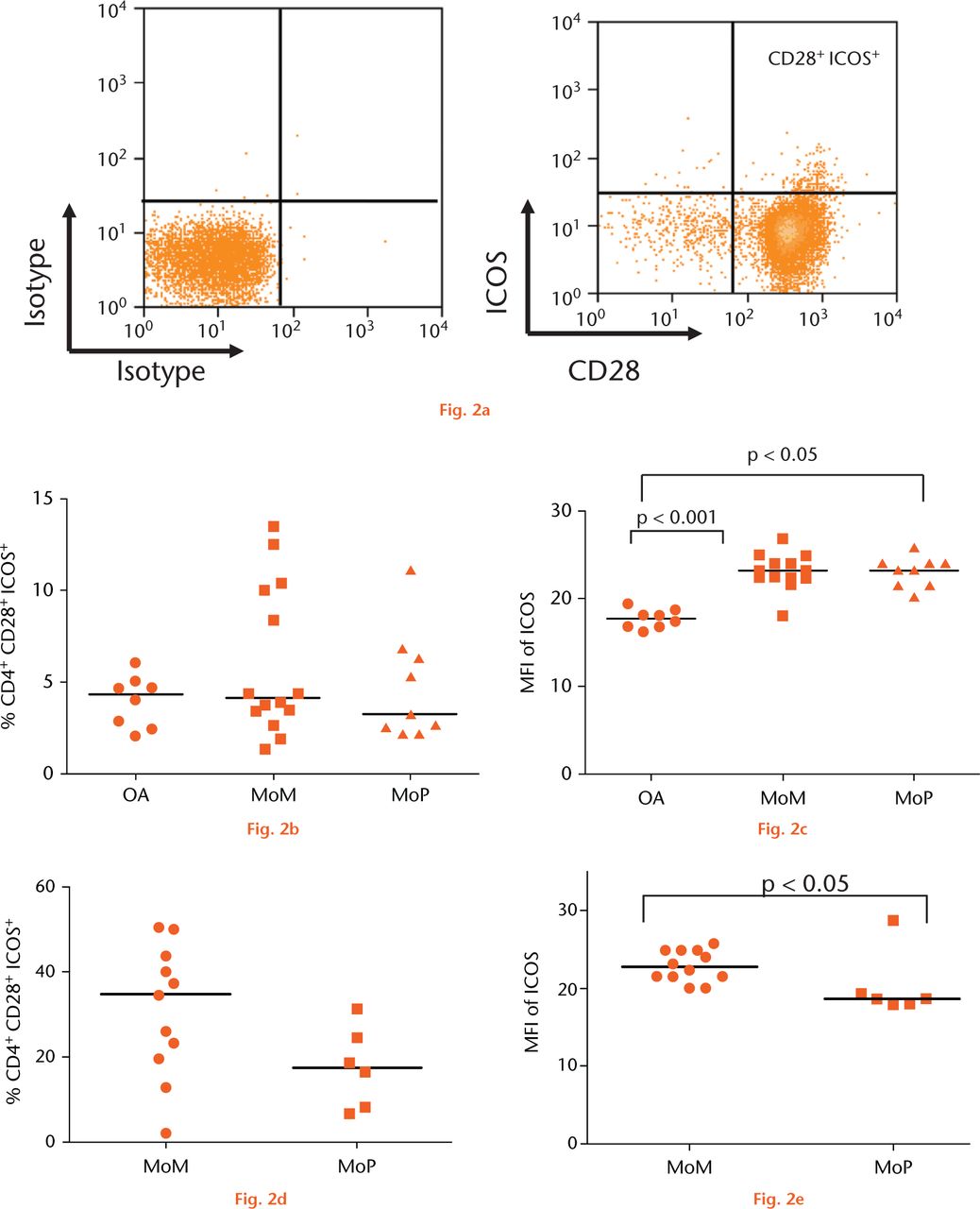
Fig.
Graphs showing the expression of inducible co-stimulator (ICOS) on CD4+ T cells: (a) Expression of CD28 plotted against ICOS on CD3+ CD4+ T-cells. b) Pooled data of frequency of CD4+ CD28+ T-cells expressing ICOS in the peripheral blood of a control group with osteoarthritis (OA) (n = 8), metal-on-metal (MoM )hips (n = 14) and metal-on-polyethylene (MoP) hips (n = 9). Horizontal lines indicate medians. c) Median fluorescence intensity (MFI) of ICOS in the peripheral blood of a control group with osteoarthritis (OA) (n = 8), MoM hips (n = 14) and MoP hips (n = 9). Horizontal lines indicate medians. d) The frequency of CD4+CD28+ICOS+ T-cells in the synovial fluid of patients with MoM (n = 11) and MoP hips (n = 6). Horizontal lines indicate medians. e) MFI of ICOS on CD4+CD28+ T-cells in the synovial fluid of patients with MoM (n = 11) and MoP hips (n = 6). Horizontal lines indicate medians.
Analysis of CD4+ T-cell subpopulations and cytokine expression
To further understand the immune response to MoM and MoP hips, the percentages of individual CD4+ T-helper (TH) subsets, namely TH1, TH2, TH17 and Tregulator, were analysed in the PB. Cells are cultured in the presence of PMA and ionomycin. TH1 cells are marked by their production of IFN-γ, TH2 by IL-4 production and TH17 cells by the presence of IL-17A/F and IL-22, while Tregulator cells specifically express the transcription factor FOXP3. No significant differences were observed in the profile of these CD4+ T-helper subtypes with respect to the expression of any of these markers by the PBMCs of the two revision groups compared with the control group on stimulation in culture with PMA and ionomycin. There were higher percentages of TH1 IFNγ-producing cells than the other types in all three groups. Due to insufficient sample volumes it was not possible to perform a similar analysis of SF-derived cells between patients with MoM and MoP hips.
Discussion
This study aimed to characterise in detail the systemic and local lymphocyte and monocyte responses by examining cell subpopulations in the PB and SF, respectively, of patients with failed hip arthroplasties at revision surgery using a group with hip osteoarthritis requiring primary arthroplasty as controls. Differences were sought between sets of individuals undergoing revision of MoM hips compared with MoP hips to determine whether there were detectable distinctions in the immune responses between these groups.
No significant differences in the distribution of T-cell subtypes were found in the PB between the MoM, MoP and control groups. However, there were clear differences in the distribution of CD4+ T-cell subtypes in the SF compared with the PB of patients with failed MoM hips, there being a significant increase in the percentage of effector memory T-cells in the SF. Furthermore, there was a decrease in the percentages of naïve and central memory cells in SF compared with PB. In respect of cellular activation and antigen-presenting function, there was a significantly increased expression of co-stimulatory molecule ICOS on CD4+ cells in the PB of failed MoM and MoP hips compared with controls, as well as significantly increased expression of ICOS in the SF of MoM hips compared with MoP hips. These findings support the concept that immune responses relating to the implant are present where there is joint arthroplasty failure of MoP and MoM hips for aseptic loosening and ARMD.
Memory phenotypes of CD4+ T-cells in blood and synovial fluid
The demonstrated differences in CD4+ T-cell subtypes present in the SF compared with PB in failed MoM hips may be important in understanding the pathogenesis of ARMD. The SF contained significantly increased CD4+ TEM cells compared with PB, and cells of this type typically show rapid entry into sites of inflammation with the secretion of large amounts of cytokines.15,16 While a previous report observed activated memory T-cells in the periprosthetic tissues of MoP hips,22 there were insufficient SF samples available from MoP hips to perform this analysis in the present study. As substantial amounts of metal wear debris can be generated locally from MoM hips with poor implant design and/or suboptimal component positioning,23 it is hypothesised that this debris causes active engagement of the CD4+ TEM cells locally. This recruitment may drive local immunological and inflammatory responses which manifest clinically as bone and soft-tissue destruction requiring early implant revision.23,24
Relationship between peripheral blood changes and local adverse tissue reactions
One study demonstrated that MoM hip patients with ARMD lesions had a significant increase in activated T-cells in PB compared with those without such lesions.20 By contrast, a recent report shows patients with MoM hips revised for pseudotumour have lower levels of TM (memory T-cells) of both TH (helper) and TC (cytotoxic) type in PB compared with failed MoM hips not having pseudotumours or those with well-functioning MoM hips.21 This is in line with previous studies showing lower PB levels of T-lymphocyte subpopulations in patients with MoM hips failing for ARMD.19,25-27 Sequestration of T-cells at the local site of an immune reaction, namely the pseudotumour, has been suggested as one explanation.14,21 Alternatively, there may be activation of an homeostatic mechanism in the presence of active antigen presentation, involving a regulator of T-cell activation (cytotoxic T-lymphocyte antigen 4; CTLA-4) which is an alternative counterligand to CD28 and causes inhibition of T-cell activation.28,29 There is good evidence for active antigen presentation in the cellular reaction to wear debris in the local tissues and PB provided in various studies,11-14,30,31 as well as from in vitro cell culture experiments.32,33 The correlation between elevated blood metal ions, particularly cobalt, and decreased T-cells has also been suggested as indicating a direct toxic and depressive effect on these cells.19,27 Paradoxically, increased T-cells have been found in those with well-functioning MoM hips in both studies and this was correlated with elevated blood metal ion levels.17,18,20 The present study of MoM hip patients requiring revision surgery is in line with those showing no significant increase in T-cells, and it may be that the difference is simply the progression from normal functioning asymptomatic cases to those with failure.
Synovial fluid as a reflection of local adverse tissue reactions
Examination of SF would seem to provide a likely means of assessing local rather than systemic effects, but it is noteworthy that there are no investigations in the literature in which the cellular changes in SF are reported. The present findings in which SF has also been investigated support the notion that examination of PB may have limited value for assessing immune responses in relation to joint arthroplasties given that: (a) the profile of lymphocytic phenotypes present in the SF was not reflected systemically in the PB of MoM hip patients, (b) there were no differences between lymphocyte subtypes in the PB between failed hips and controls, (c) apart from ICOS, there were no differences in the expression of co-stimulatory molecules in the PB between groups, and (d) there were no differences in the CD4+ TH-cell subpopulations between groups as assessed by cytokine or transcription factor expression. This may explain why previous studies attempting to detect immune responses in relation to artificial joint arthroplasties using PB have been inconclusive.6
Expression of co-stimulatory molecules as evidence of antigen presentation
In the only previous study of the co-stimulatory molecules produced by PB monocytes, the expression of CD86 and HLA-DR, but not CD80 or CD40, was significantly higher in patients with MoM hips compared with MoP hips, or in those who previously had a MoM joint revised to MoP.14 Paradoxically, CD28 expression by T-cells in the same samples showed higher levels in the MoP cases and those with MoM hips revised to MoP, though there were CD28-expressing cells also present in the MoM samples.
In the present study, the co-stimulatory molecule ICOS, which is functionally related to CD28 and important in T-cell activation and antigen presentation,34 was demonstrated to have significantly increased expression on the surface of CD4+CD28+ T-cells in the PB of patients with failed MoM and MoP hips compared with patients with osteoarthritis. This suggests that the local activation of T-cells in relation to artificial joint arthroplasties has the potential to generate immune responses.
The presence of ICOS was confirmed in the SF of patients with both failed MoM and MoP hips. This substantiates the activation of T-cells locally in relation to both types of implant. However, there was a significantly increased expression of ICOS in the SF of MoM hips compared with MoP hips. Activated T-cells may play an important role in the aggravated immune responses generated against MoM implants seen in some studies.7,35,36 Although immune responses may contribute to failure of both MoM and MoP hips, the increased expression of ICOS and propensity for antigen presentation in MoM hips may explain the clinical differences seen in the modes of failure between devices. ARMD can occur early after MoM bearing implantation and may be aggressive,37,38 whereas failure of MoP joints with aseptic loosening occurs more slowly over a number of years and is clinically less dramatic.39,40 Given that ARMD has recently been observed in patients with non-MoM bearing surfaces, namely those with modular junctions and dual-taper designs,41,42 it would be interesting to perform similar SF analyses to those of the present study in patients with these devices.
The presence of B-cells and a follicular lymphoid response with T-cell and B-cell interactivity has not been noted in MoP joints.1,3,5,43-46 However, recent findings for MoM joints have shown B-cells present in some MoM hips revised for ARMD, and these cases may represent a distinct subset of patients with features of tertiary lymphoid organs in the periprosthetic tissues.7
Analysis of CD4 and T-cell subpopulations and cytokine expression
Evidence has been presented elsewhere that the response at the bone-implant interface for MoP joints is TH1 cell-related, with activation of macrophages and T-cytotoxic/suppressor cells in a cell-mediated immune reaction with cytokine production and antigen presentation.1,2,4 That different CD4+ T-cell subsets (TH1, TH2, TH17 and T regulator) were present in the PB of MoM cases was evident in the current study, though significant differences from MoP or controls were not found. Detailed examination of peri-implant tissues with respect to the markers used here has not been performed for MoM joints. Analysis of CD4+ T-cell subpopulations by means of cell culture studies and cytokine expression on stimulation of isolated cells is a relatively complex process in which reproducibility between laboratories may be difficult to achieve.
Future studies of cellular reactions to implanted joint arthroplasty devices
Although there is a large body of evidence with respect to the tissue changes around MoP joints which should be reflected in the SF, parallel studies of this fluid and peri-prosthetic tissues have not been performed. That active antigen presentation occurs as part of the inflammatory response near the implant in MoP cases was proposed for the first time by Al-Saffar et al,12 with subsequent confirmation of the expression of co-stimulatory molecules CD80 and CD86 on macrophages and MNGCs, and CD28 on the related T-lymphocytes at the implant interface.11,30 The presence of CD40 on APCs and its counter-ligand, CD40L, on bone-implant interface lymphocytes has also been shown,13 while ICAM-1 and LFA-1 are present on interface macrophages and T-cells.31 Various functional studies in cell culture have revealed the expression of these co-stimulatory molecules in the context of particle phagocytosis.11,32,33 The production of the NFκB family of molecules (RelA, RelB, c-rel, p50, p52) by both a monocytic cell line and normal PB monocytes on phagocytosis of metal particles has been demonstrated in cell culture experiments as well as in tissue samples of MoP implant interface using immunohistochemistry, quantitative RT-PCR and FACS analysis.32,33 The presence of Rel B in interface inflammatory tissue and by cells phagocytosing wear debris in vitro is of some importance since this molecule is expressed by APCs only during their activation.33 There is no information available with respect to MoM joints having ARMD either for the expression of any of these markers or that of over 20 cytokines found in aseptic loosening of MoP joints1 so there is a large scope for future investigation and clarification of the immunopathological processes occurring.
Examination of large numbers of cases (preferably in the thousands) might reveal differences, but this is clearly not feasible from single centres. Future subtleties may only be revealed by meta-analytical methods, and these analyses in turn may be difficult to achieve where there is lack of conformity between laboratories, and even lack of consistency in the definition of the pathological entities being studied (pseudotumour, ARMD, and aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL)). It is recommended that future studies use both SF and peri-prosthetic tissue samples to investigate the immune responses occurring in relation to the implants, and that more attention is paid to the definitions of the terms used.
There are limitations to this study. As expected, there were differences in the baseline characteristics of the cohort, including age, gender, and time to revision (Table I). This is related to the inherent selection bias for the different types of arthroplasty, as well as obvious differences in the mechanism of failure.37-40 Although it is recognised that such differences could affect the analysis performed, it is unlikely that these confounders can be overcome in future studies. It is acknowledged that the number of SF samples obtained was small, especially in MoP patients, therefore our conclusions must be interpreted with this in mind. However, this practical issue of obtaining insufficient samples for analysis would also be encountered in subsequent studies. Although blood metal ion and histopathology results have been presented, explant analysis was not performed which may have provided more details regarding the ARMD failures. Finally, it is recognised that no patients with well-functioning hip arthroplasties were included in this analysis. This was because we were investigating mechanisms relating to implant failure, hence revised patients were recruited in preference. Although we therefore do not have baseline findings from samples in patients with well-functioning implants, it must be remembered that SF sampling in such patients is likely to raise significant ethical issues given the risk of inadvertently introducing infection. We do, however, have a baseline group of patients with primary hip osteoarthritis.
In conclusion, increased expression of the co-stimulatory molecule ICOS on the surface of CD4+CD28+ T-cells was observed in the PB and SF of patients with failed hip arthroplasties compared with patients with hip osteoarthritis. This suggests that immune responses with antigen presentation play an important role in the failure of both MoM and MoP hip arthroplasties. The increased expression of ICOS in the SF of MoM compared with MoP hips may explain the different modes of failure (ARMD versus aseptic loosening) observed with these two bearing surfaces. Differences in lymphocyte profiles in MoM hip patients in the SF compared with PB, and the lack of expression of other co-stimulatory molecules or cytokines in the blood suggest that PB examination has limited potential to detect immune responses occurring in relation to artificial joint arthroplasties. It is therefore recommended that future studies use SF samples to investigate the immune responses which occur in relation to artificial joint arthroplasties, and that this should be performed in parallel with studies of peri-prosthetic tissue.
Funding Statement
Funding has been received from Smith & Nephew by the University of Birmingham to fund study costs.
Funding has also been received by The Royal Orthopaedic Hospital NHS Foundation Trust from Smith & Nephew for hip-related research outside of this study. One author also received funding from Arthritis Research UK, The Royal Orthopaedic Hospital Hip Research and Education Charitable Fund, The Royal College of Surgeons of England and The Arthritis Research Trust during the course of this study for other research.
ICMJE conflict of interest:
None declared
References
1. Revell PA . Biological causes of prosthetic joint failure. In: RevellPA, ed. Joint Replacement Technology. Second ed. Cambridge: Woodhead, 2014:298-369. Google Scholar
2. Hercus B , RevellPA. Phenotypic characteristics of T lymphocytes in the interfacial tissue of aseptically loosened prosthetic joints. J Mater Sci Mater Med2001;12:1063-1067.CrossrefPubMed Google Scholar
3. Lalor PA , RevellPA, GrayAB, et al.. Sensitivity to titanium. A cause of implant failure?J Bone Joint Surg [Br]1991;73-B:25-28.CrossrefPubMed Google Scholar
4. Weyand CM , GeislerA, BrackA, BolanderME, GoronzyJJ. Oligoclonal T-cell proliferation and interferon-gamma production in periprosthetic inflammation. Lab Invest1998;78:677-685.PubMed Google Scholar
5. al-Saffar N , RevellPA. Pathology of the bone-implant interfaces. J Long Term Eff Med Implants1999;9:319-347.PubMed Google Scholar
6. Kwon YM , ThomasP, SummerB, et al.. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res2010;28:444-450.CrossrefPubMed Google Scholar
7. Mittal S , RevellM, BaroneF, et al.. Lymphoid aggregates that resemble tertiary lymphoid organs define a specific pathological subset in metal-on-metal hip replacements. PLoS One2013;8:e63470.CrossrefPubMed Google Scholar
8. al-Saffar N , MahJT, KadoyaY, RevellPA. Neovascularisation and the induction of cell adhesion molecules in response to degradation products from orthopaedic implants. Ann Rheum Dis1995;54:201-208.CrossrefPubMed Google Scholar
9. Saeed S , RevellPA. Production and distribution of interleukin 15 and its receptors (IL-15Ralpha and IL-R2beta) in the implant interface tissues obtained during revision of failed total joint replacement. Int J Exp Pathol2001;82:201-209.CrossrefPubMed Google Scholar
10. Revell PA , JellieSE. Interleukin 15 production by macrophages in the implant interface membrane of aseptically loosened joint replacements. J Mater Sci Mater Med1998;9:727-730.CrossrefPubMed Google Scholar
11. Bainbridge JA , RevellPA, Al-SaffarN. Costimulatory molecule expression following exposure to orthopaedic implants wear debris. J Biomed Mater Res2001;54:328-334.CrossrefPubMed Google Scholar
12. Al-Saffar N , RevellPA, KobayashiA. Modulation of the phenotypic and functional properties of phagocytic macrophages by wear particles from orthopaedic implants. J Mater Sci Mater Med1997;8:641-648.CrossrefPubMed Google Scholar
13. Bhatt R , SaeedS, AltafH, RevellPA. In vitro assessment of interactions between T-cells and antigen-presenting cells (APCs) when challenged with biomaterials: the CD40–CD40L interaction. In Proceedings of the 7th World Biomaterials Congress. Sydney: Australia, 2004. Google Scholar
14. Whittingham-Jones PM , DunstanE, AltafH, et al.. Immune responses in patients with metal-on-metal hip articulations: a long-term follow-up. J Arthroplasty2008;23:1212-1218.CrossrefPubMed Google Scholar
15. Sallusto F , GeginatJ, LanzavecchiaA. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol2004;22:745-763.CrossrefPubMed Google Scholar
16. Sallusto F , LenigD, FörsterR, LippM, LanzavecchiaA. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature1999;401:708-712.CrossrefPubMed Google Scholar
17. Hailer NP , BlahetaRA, DahlstrandH, StarkA. Elevation of circulating HLA DR(+) CD8(+) T-cells and correlation with chromium and cobalt concentrations 6 years after metal-on-metal hip arthroplasty. Acta Orthop2011;82:6-12.CrossrefPubMed Google Scholar
18. Hallab NJ , CaicedoM, McAllisterK, et al.. Asymptomatic prospective and retrospective cohorts with metal-on-metal hip arthroplasty indicate acquired lymphocyte reactivity varies with metal ion levels on a group basis. J Orthop Res2013;31:173-182.CrossrefPubMed Google Scholar
19. Penny JØ , VarmarkenJE, OvesenO, NielsenC, OvergaardS. Metal ion levels lymphocyte counts: ASR hip resurfacing prosthesis vs. standard THA: 2-year results from a randomized study. Acta Orthop2013;84:130-137. Google Scholar
20. Hailer NP , BengtssonM, LundbergC, MilbrinkJ. High metal ion levels after use of the ASR™ device correlate with development of pseudotumors and T cell activation. Clin Orthop Relat Res2014;472:953-961.CrossrefPubMed Google Scholar
21. Catelas I , LehouxEA, HurdaI, et al.. Do patients with a failed metal-on-metal hip implant with a pseudotumor present differences in their peripheral blood lymphocyte subpopulations?Clin Orthop Relat Res2015;473:3903-3914.CrossrefPubMed Google Scholar
22. Revell PA , al-SaffarN, KobayashiA. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng H1997;211:187-197.CrossrefPubMed Google Scholar
23. Langton DJ , JoyceTJ, JamesonSS, et al.. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg [Br]2011;93-B:164-171.CrossrefPubMed Google Scholar
24. Pandit H , Glyn-JonesS, McLardy-SmithP, et al.. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br]2008;90-B:847-851.CrossrefPubMed Google Scholar
25. Granchi D , CiapettiG, SteaS, et al.. Evaluation of several immunological parameters in patients with aseptic loosening of hip arthroplasty. Chir Organi Mov1995;80:399-408. (article in English and Italian)PubMed Google Scholar
26. Hart AJ , HesterT, SinclairK, et al.. The association between metal ions from hip resurfacing and reduced T-cell counts. J Bone Joint Surg [Br]2006;88-B:449-454.CrossrefPubMed Google Scholar
27. Hart AJ , SkinnerJA, WinshipP, et al.. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J Bone Joint Surg [Br]2009;91-B:835-842.CrossrefPubMed Google Scholar
28. Ellis JH , BurdenMN, VinogradovDV, LingeC, CroweJS. Interactions of CD80 and CD86 with CD28 and CTLA4. J Immunol1996;156:2700-2709.PubMed Google Scholar
29. Greenfield EA , HowardE, ParadisT, et al.. B7.2 expressed by T cells does not induce CD28-mediated costimulatory activity but retains CTLA4 binding: implications for induction of antitumor immunity to T cell tumors. J Immunol1997;158:2025-2034. Google Scholar
30. Altaf H , SaeedS, BhattR, RevellPA. The assessment of antigen presenting cells in the bone-implant interface. Biomaterialen2003;4:86-88. Google Scholar
31. al Saffar N , RevellPA. Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. Br J Rheumatol1994;33:309-316.CrossrefPubMed Google Scholar
32. Altaf H . The inflammatory response to particulate wear debris in the context of total hip replacement. PhD Thesis, Queen Mary University of London. 2007. Google Scholar
33. Altaf H , RevellPA. Evidence for active antigen presentation by monocyte/macrophages in response to stimulation with particles: the expression of NFκB transcription factors and costimulatory molecules. Inflammopharmacology2013;21:279-290.CrossrefPubMed Google Scholar
34. Hutloff A , DittrichAM, BeierKC, et al.. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature1999;397:263-266.CrossrefPubMed Google Scholar
35. Mahendra G , PanditH, KliskeyK, et al.. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop2009;80:653-659.CrossrefPubMed Google Scholar
36. Willert HG , BuchhornGH, FayyaziA, et al.. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg [Am]2005;87-A:28-36.CrossrefPubMed Google Scholar
37. Natu S , SidaginamaleRP, GandhiJ, LangtonDJ, NargolAV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol2012;65:409-418.CrossrefPubMed Google Scholar
38. Grammatopoulos G , PanditH, KwonYM, et al.. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg [Br]2009;91-B:1019-1024.CrossrefPubMed Google Scholar
39. Munro JT , MasriBA, DuncanCP, GarbuzDS. High complication rate after revision of large-head metal-on-metal total hip arthroplasty. Clin Orthop Relat Res2014;472:523-528.CrossrefPubMed Google Scholar
40. Allami MK , FenderD, KhawFM, et al.. Outcome of Charnley total hip replacement across a single health region in England. The results at ten years from a regional arthroplasty register. J Bone Joint Surg [Br]2006;88-B:1293-1298.CrossrefPubMed Google Scholar
41. Ling RS , CharityJ, LeeAJ, et al.. The long-term results of the original Exeter polished cemented femoral component: a follow-up report. J Arthroplasty2009;24:511-517.CrossrefPubMed Google Scholar
42. Cooper HJ , Della ValleCJ, BergerRA, et al.. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg [Am]2012;94-A:1655-1661.CrossrefPubMed Google Scholar
43. Lalor PA , GrayAB, WrightS, et al.. Contact hypersensitivity to titanium hip prosthesis? A preliminary report. Contact Dermat1990;23:193-194. Google Scholar
44. Salter DM , KrajewskiAS, RobertsonS. Lymphocytes in pseudomembranes of late prosthetic joint failure. J Pathol1992;166:271-275.CrossrefPubMed Google Scholar
45. Goodman SB , ChinRC, ChiouSS, et al.. A clinical-pathologic-biochemical study of the membrane surrounding loosened and nonloosened total hip arthroplasties. Clin Orthop Relat Res1989;244:182-187.PubMed Google Scholar
46. Revell PA . Characterization of the cells and immunological reactions adjacent to aseptically loosened orthopaedic implants. J Histotechnol2006;29:287-295. Google Scholar









