Abstract
Objectives
The major problem with repair of an articular cartilage injury is the extensive difference in the structure and function of regenerated, compared with normal cartilage. Our work investigates the feasibility of repairing articular osteochondral defects in the canine knee joint using a composite lamellar scaffold of nano-ß-tricalcium phosphate (ß-TCP)/collagen (col) I and II with bone marrow stromal stem cells (BMSCs) and assesses its biological compatibility.
Methods
The bone–cartilage scaffold was prepared as a laminated composite, using hydroxyapatite nanoparticles (nano-HAP)/collagen I/copolymer of polylactic acid–hydroxyacetic acid as the bony scaffold, and sodium hyaluronate/poly(lactic-co-glycolic acid) as the cartilaginous scaffold. Ten-to 12-month-old hybrid canines were randomly divided into an experimental group and a control group. BMSCs were obtained from the iliac crest of each animal, and only those of the third generation were used in experiments. An articular osteochondral defect was created in the right knee of dogs in both groups. Those in the experimental group were treated by implanting the composites consisting of the lamellar scaffold of ß-TCP/col I/col II/BMSCs. Those in the control group were left untreated.
Results
After 12 weeks of implantation, defects in the experimental group were filled with white semi-translucent tissue, protruding slightly over the peripheral cartilage surface. After 24 weeks, the defect space in the experimental group was filled with new cartilage tissues, finely integrated into surrounding normal cartilage. The lamellar scaffold of ß-TCP/col I/col II was gradually degraded and absorbed, while new cartilage tissue formed. In the control group, the defects were not repaired.
Conclusion
This method can be used as a suitable scaffold material for the tissue-engineered repair of articular cartilage defects.
Cite this article: Bone Joint Res 2015;4:56–64
Article focus
Repair of articular osteochondral defects of the canine knee joint
Lamellar scaffold of nano-β-tricalcium phosphate/collagen I and II
Scaffold bone marrow stromal stem cells are complex
Key messages
The mean porosity was 92.3%
Excellent biocompatibility between bone marrow stromal stem cells and the scaffold
New cartilage was well integrated with peripheral normal cartilage
Strengths and limitations
Bone marrow stromal stem cells can be amplified without loss of multilineage differentiation potential
The collagen layer and nano-β-tricalcium phosphate degraded, and new trabecular bone grew inward
Improves the integration of the scaffold with excellent biocompatibility without cell toxicity
Introduction
Articular osteochondral defects caused by trauma or bone diseases are commonly seen in clinical practice, a trend which is set to increase year on year as the ageing population increases.1 Cartilage defects are often found to be accompanied by defects of the subchondral bone.2,3 When osteochondral defects extend deep into the subchondral bone, simple treatments such as debridement, grinding, drilling or chondrocyte transplantation, will lead to the formation of fibrous cartilage with inadequate mechanical properties, ultimately resulting in articular degeneration.4-9 Disadvantages such as incomplete integration and easy detachment of implants, are found in the healing of cartilage–bone formed between chondral material from in vitro culture and recipient tissue.10 Previous attempts at tissue engineering for articular defects have concentrated on the repair of the cartilage, while the repair of subchondral bone has been neglected.11 Furthermore, the design, manufacture and in vivo fixation of simple cartilaginous tissue engineering scaffolds is difficult, due to the thinness of the articular cartilage.12 Studies have shown that the binding between graft and recipient will be faster and firmer if the integration between the graft and the defect area is changed from a cartilage–bone interface to a bone–bone interface.13,14
Subchondral bone not only forms a certain outline shape of the joints, but it also provides the biomechanical environment for differentiation and development of cartilaginous tissue, suggesting that the subchondral bone has an important role to play in the repair of articular osteochondral defects. The bone–cartilage scaffolds currently being studied can be primarily categorised into monolayer and bilayer scaffolds.15-20 In bilayer scaffolds, which are currently more frequently studied, the bone and cartilage scaffold are usually constructed first, and then cultured in vitro separately. Finally, the tissue-engineered bone and cartilage are implanted as a tissue-engineered osteochondral complex, using biological adhesive and/or suturing.13,21 Stratification is easily observed at the bone–cartilage interface of osteochondral tissue, which is constructed by the above method. In our experiment, nano-β-tricalcium phosphate (β-TCP)/collagen I and II was used to create a lamellar scaffold, and a certain number of bone marrow stromal stem cells (BMSCs) were combined at the surface of the material. The osteochondral scaffolds consist of two layers: a mineralised type I collagen-β-TCP scaffold designed to regenerate the underlying subchondral bone, and a non-mineralised type II collagen-β-TCP scaffold designed to regenerate cartilage. The material was then pressed down slightly during implantation, focusing on repair of the subchondral bone, while the articular surface was reconstructed using stem cells at the material surface, and was primarily based on observing the results of repair of the articular osteochondral defect in the canine knee joint. At the same time, we used a hydroxyapatite nanoparticle (nano-HAP)/collagen I composite prepared in a preliminary test, constructed a bone–cartilage scaffold as a laminated composite, using a nano-HAP/collagen/copolymer of polylactic acid–hydroxyacetic acid as the bony scaffold, and sodium hyaluronate/poly(lactic-co-glycolic acid) (PLGA) as the cartilaginous scaffold. We assessed the biological compatibility and cytotoxicity of the scaffold in culture with rat BMSCs and evaluated cell proliferation using the MTT assay kit (Sigma-Aldrich, St Louis, Missouri). We observed these composite cultures of rat BMSCs combined with scaffold, with a scanning electron microscope (SEM).
Materials and Methods
These consisted of PLGA (100 000 Dalton molecular weight, Shangdong Jinan Research Institute of Medical Devices), DMEM/F12 culture medium (Gibco, Carlsbad, California), Fetal Bovine Serum (Gibco; PAA Company, Austria), Pancreatin (Sijiqing Biology Company, Hangzhou, China), Collagen I Immunohistochemical Detection Kit, Collagen II Immunohistochemical Detection Kit (Boshide company, Wuhan China), MTT (Sigma-Aldrich), Thermo Scientific 3110 CO2 Incubator (Thermo Fisher Scientific Inc., Waltham, Massachusetts), Inverted Phase Contrast Microscope (Olympus, Japan), Scanning Electron Microscope (Japan, NEC JC60X ), X-Ray Diffractometer (X’ Pert pro MPD), Fourier Transform Infrared Spectroscopy (EQUINOX-55).
All animals were provided by the experimental animal centre of the northern campus of Sun Yat-sen University. The animal provision license is Animals for Medical Use (Word) No. 13-012. Ten normal 12-month-old hybrid canines, female or male, weighing 12 kg to 15 kg, were randomly divided into the experimental group and the defect control group, and included five per group.
Wound healing, knee joint range of movement (ROM) and gait after operation were observed. Sample materials were collected at 12 weeks and 24 weeks after surgery, with a mass of osteochondral tissue at the articular defect of the right knee joint collected for general observation. Sample tissue was fixed with 10% neutral buffered formalin, decalcified for seven days in a mixed decalcifying fluid, then embedded in paraffin. Sections were cut for histological examination (Haematoxylin-eosin staining, Alcian blue staining, Safranin O staining) and collagen I and II immunohistochemical detection.
The nano-HAP/collagen I composite powder material and PLGA were mixed with sodium chloride (NaCl) at a certain proportion and dissolved in acetone as the substratum cartilage material. Meanwhile, PLGA was also mixed with NaCl at a certain proportion and dissolved in acetone as the upper stratum bony material. Each of the two mixtures was stirred separately and evenly. After a certain amount of the solvent of the mixed substratum material had evaporated, it was poured into a mould for shaping. After the substratum material had been shaped but before the solvent had completely evaporated, the mixed upper stratum material was rapidly poured to mix with the substratum material at the surface for shaping. Thus, the upper stratum would firmly adhere to the substratum. After the solvent had completely evaporated, the scaffold was soaked in de-ionised water for 48 hours, and a porous composite scaffold material comprising both upper stratum and substratum was obtained. After drying in a baking oven, the composite upper stratum scaffold was soaked in sodium hyaluronate solution for 24 hours, so that it was absorbed by the upper layer. After freezing and vacuum drying the mixture, an integrated bone–cartilage scaffold with a diameter of about 15 mm and thickness of 3 mm, was finally prepared.
The scaffold was gently broken open to display the transection structure. After metal spray plating, the upper and lower surfaces of the scaffold were observed, as well as the microstructure of the transection observed with a SEM.
The repaired tissue was scored in accordance with O’Driscoll’s improved repair standards for cartilage defects.10
The primary observation indices consisted of:
- Observation of wound healing, knee joint ROM and gait
- General observations
- Histological observations and scoring
Both the designer and evaluator was the first author, and the implementers of the intervention were all the authors, who were trained systematically. Blind evaluation was not used.
Statistical analysis
This was performed by the first author, and the t-test was used, with p < 0.05 representing a statistically significant difference.
Results
The TCP scaffold was provided by the Bioengineering Research Institute of Jinan University, with a porosity ≥ 83%. The scaffold of the upper stratum was made of collagen II, while the substratum scaffold was made of collagen I, and the interspace was the transition zone between collagen I and II. The size of the pore was 100 μm to 200 μm, and the compressive strength was about 4.30 MPa.
The appearance of the prepared osteochondral composite scaffold was ivory white, with a foamy structure. Observation under SEM showed that the composite scaffold possessed a porous structure with excellent pore–to–pore connectivity. As shown in Figure 1, the pore size of the scaffold was 100 μm to 300 μm, and the average porosity was 92.3%.
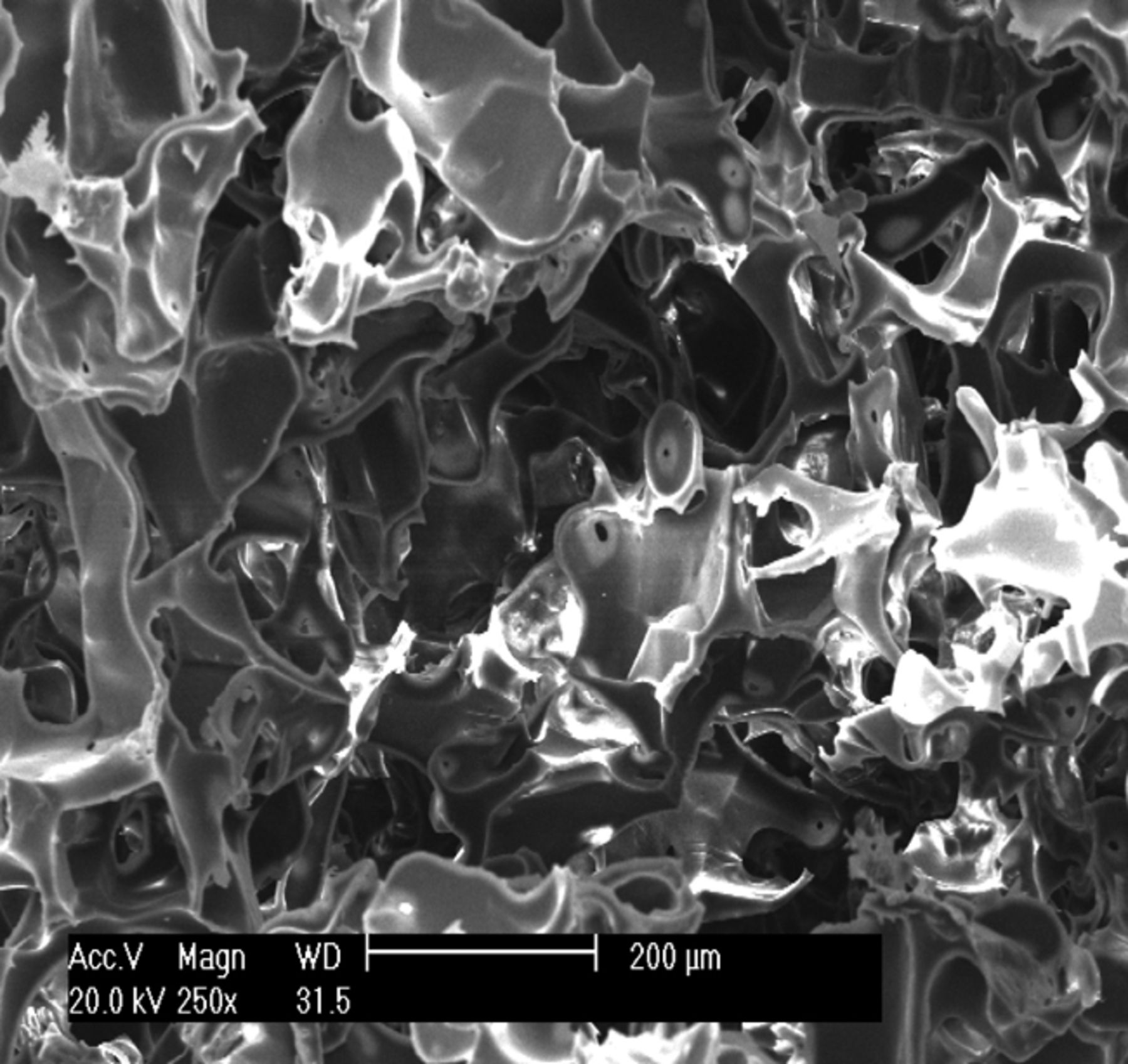


Figs. 1a - 1c
Scanning electron microscope micrographs of the osteochondral scaffold (× 250) showing a) the upper surface of the scaffold, b) the lower surface of the scaffold and c) a cross section of the scaffold.
It was observed under SEM that after one day in culture, BMSCs inoculated into the three-dimensional materials adhered and grew, forming protrusions and stretching out pseudopodia to attach to the surface of the material. Cells were observed to form interconnections (Figs 2a and 2b). After three days in culture, cells could be seen growing very well, tiling on the surface of the -material to which they were more closely adapted (Figs 2c and 2d). After seven days in culture, the number of cells had increased, and cells were found to have expanded and migrated into the micropores. The cells coalesced to form separate patches, and produced a large amount of extracellular matrix (Figs 2e and 2f). These observations suggest excellent biocompatibility between BMSCs and the scaffold.

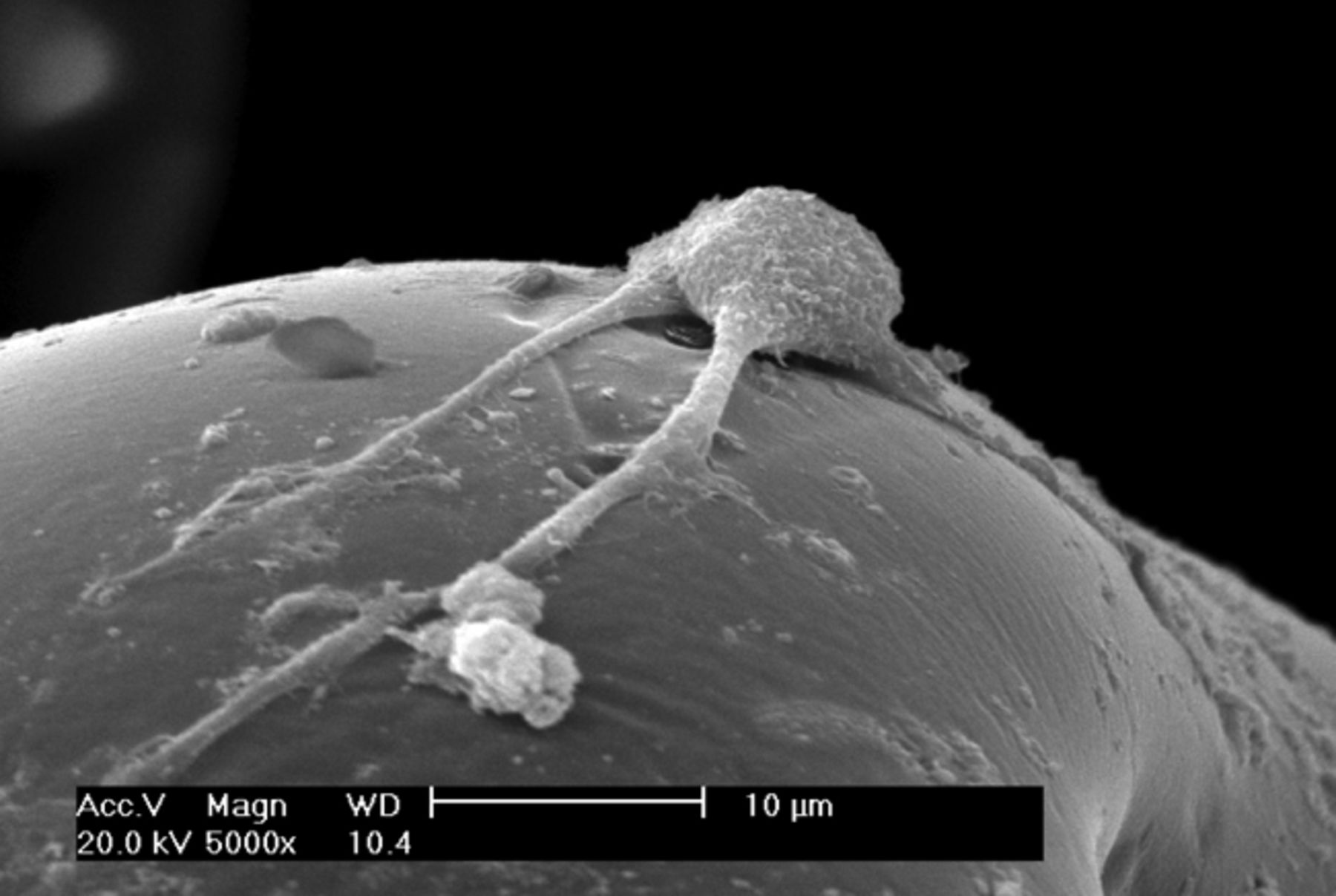




Figs. 2a - 2f
Scanning electron microscope micrographs of bone marrow stromal cells cultured on the scaffold showing a) the upper layer of the scaffold after one day in culture, b) lower layer of the scaffold after one day in culture, c) upper layer of the scaffold after three days of culture, d) lower layer of the scaffold after three days of culture, e) upper layer of the scaffold after seven days of culture and f) lower layer of the scaffold after seven days of culture.
Each dog was anaesthetised intravenously with 3% pentobarbital sodium. A bone marrow puncture at the posterior iliac spine was performed and approximately 10 mL of bone marrow was extracted. Karyocytes were separated by density gradient centrifugation, using a Percoll separating medium with a density of 1.073 g/mL. Cells were re-suspended in low sugar DMEM containing 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin. The cell suspension was inoculated into 25 cm2 plastic tissue culture flasks, and a primary cell culture was carried out in a 37°C incubator with 5% CO2 in air and 95% humidity. The culture medium was changed for the first time after 48 hours, then once every two or three days. Cells were passaged when they reached 90% confluence and canine BMSCs of the third generation were reserved for use in experiments.
Dogs of both groups were anaesthetised intravenously with 3% pentobarbital sodium (0.2 mg/kg to 0.4 mg/kg). The hair over the right knee joint was shaved, and the dog was positioned on the operating table in the supine position. The operating field was disinfected with tincture of iodine and ethanol, surrounded with aseptic drapes, and a medial incision of the knee joint was created. Incisions were made in the skin, subcutaneous tissues and articular capsule layer by layer, and the patella was dislocated laterally. With the knee joint at about 70° of genuflexion, a defect of 6 mm in diameter and 4 mm in depth was created in the subchondral bone at the femoral trochlea, as shown in Figure 3.
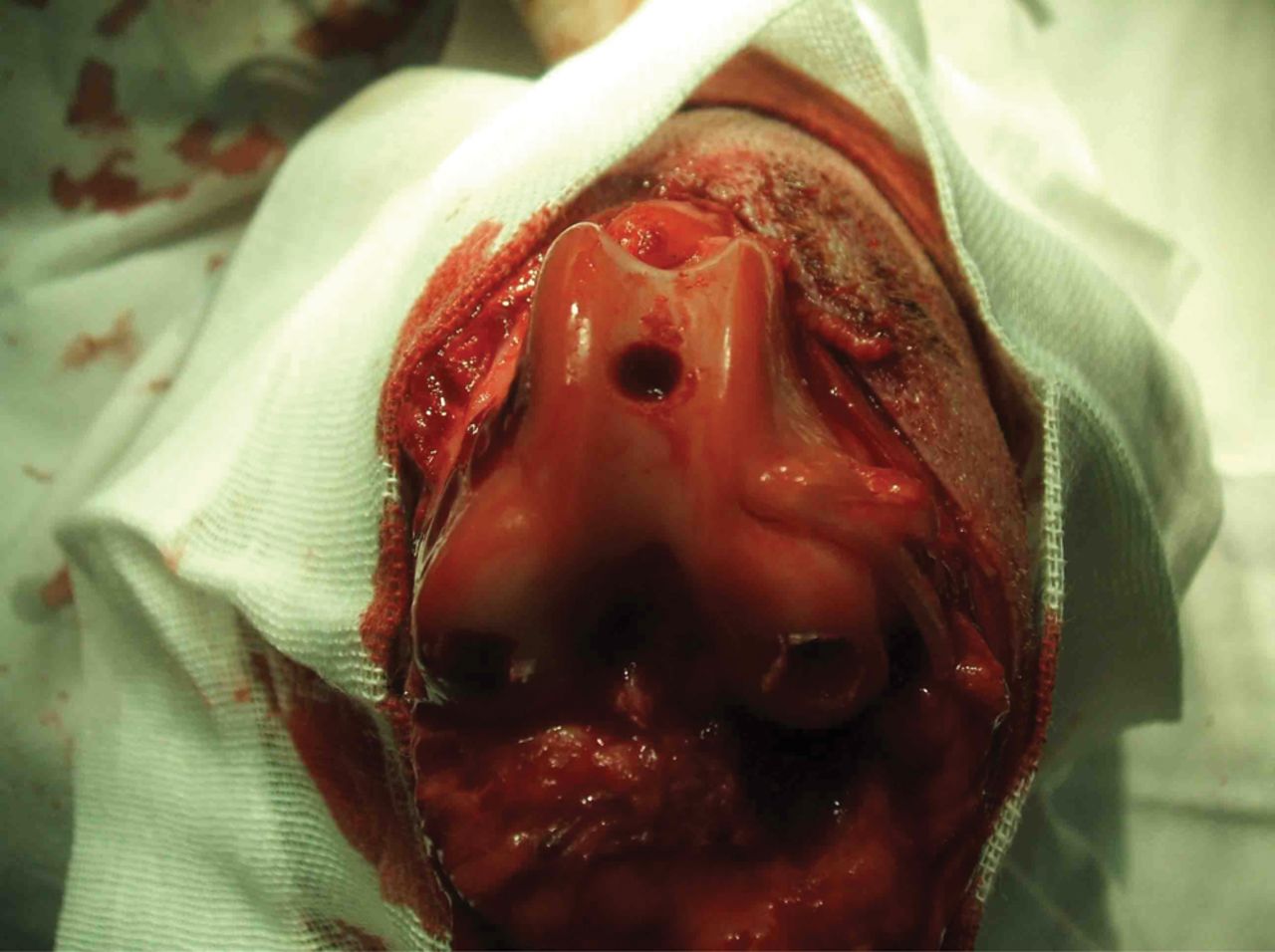
Fig. 3
Photograph of knee 6 mm × 4 mm artificial trochlea defects in the knee of all experimental animals.
A corneal trephine was used to make a lamellar scaffold of β-TCP/col I and II. This was formed into a cylinder-shaped column, which was packed and sealed after drying. Scaffolds were sterilised with ethylene oxide.
BMSCs were detached by trypsinisation, collected by centrifugation and the cell density was adjusted to 2×106 L. The cells were inoculated into the pre-prepared lamellar scaffold of β-TCP/col I and II and cultured for 24 hours in a 96-well plate. SEM micrographs of the cell/scaffold complex are shown in Figure 4.

Fig. 4
Scanning electron microscope micrographs showing canine bone marrow stromal stem cells adhering to the scaffold (Scanning electron microscopy, × 400)
After preparing the osteochondral defect in the right canine knee joint, the complex of the lamellar scaffold of β-TCP/collagen I and II and BMSCs was implanted at the site of the defect in animals of the experimental group, as shown in Figure 5. Animals in the control group were left untreated. After implantation, the incision was closed in layers. The animals were returned to the animal house for normal feeding post-operatively, and were allowed freedom of movement. Penicillin at 80 × 104 U was injected intra-muscularly twice a day, for three consecutive days.
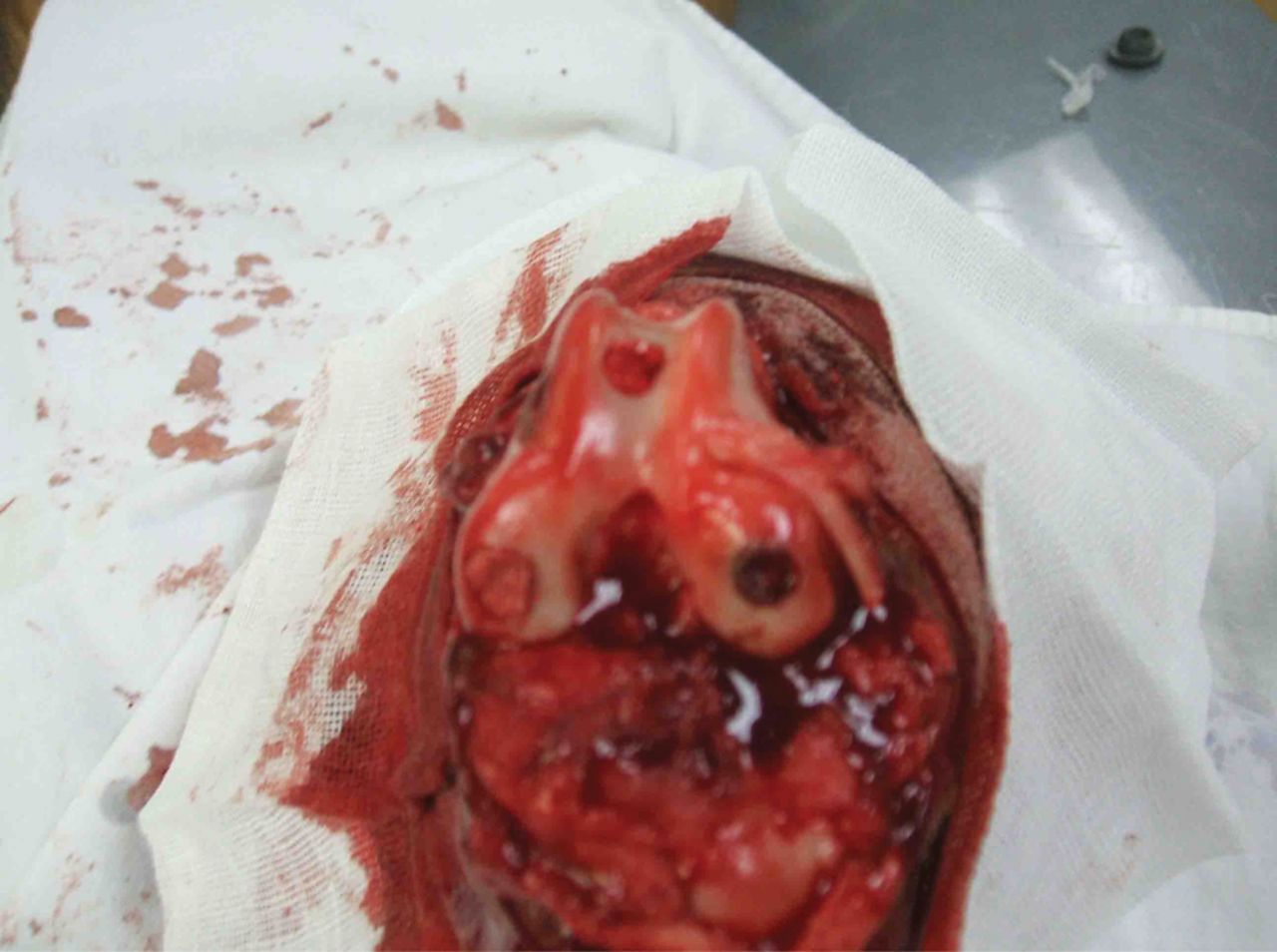
Fig. 5
Photograph of scaffold/cell composites implanted into the trochlea defects in the knee of the animals in the experimental group.
All ten animals were included in the analysis of results and there were no drop-outs during the experimental period.
Slight limping was observed in dogs of both groups after surgery, but all had returned to normal gait by day three. There were no cases of infection or death. No joint cavity adhesion or effusion, cartilage wear or osteophyte formation were observed in any specimen of all the experimental animals.
At week 12, the defects of the right knee joint in the experimental group were filled with white semi-translucent new cartilage tissue, of a colour similar to normal cartilage and tenacious in nature, slightly protruding over the peripheral cartilage surface, with no clear margin at the interface with normal cartilage, as shown in Figure 6. In the control group, a small amount of white membranous tissue had formed at the bottom of the defects, and the defect was depressed and exhibited a clear margin. For a brief period, it appeared similar to the composite as shown in Figure 5.

Fig. 6
Photographs of the surface of the cartilage at 12 weeks post-operatively in the experimental group (control group on the right). The regenerated tissues were slightly higher than the surrounding cartilage, with a colour and lustre similar to that of the surrounding normal tissue.
At week 24, the defects of the right knee joint in the experimental group were filled with a white semi-translucent new cartilage tissue, of a colour and nature similar to that observed at week 12. As it was not well demarcated from the surrounding cartilage surface, the margin where it joined the normal cartilage had almost disappeared, as shown in Figure 7. In the defect control group, the tissue formed in the defect space was found to be pale in colour, soft in nature, discontinuous, compressible and did not have a smooth surface. The border with the normal cartilage was clear, and the defect space was not fully filled. Some peripheral cartilage was found to be denatured, but no synovial hyperplasia was observed.

Fig. 7
Photographs of the surface of the cartilage at 24 weeks post-operatively in the experimental group (control group on the right). The regenerated tissues were smooth, and of a colour and lustre similar to that of the surrounding normal cartilage.
At week 12, the new cartilage in the experimental group was observed to be thick, with a continuous surface, and smooth and tenacious in nature. Cells in the deep lamella were arranged in a disorderly fashion, while cells in regional areas were found to cluster together. The matrix was extensively metachromatic and the new cartilage was well integrated with peripheral normal cartilage. Most of the scaffold materials had degraded, as shown in Figure 8. Immunohistochemical staining for collagen II stained the regenerated cartilaginous tissue a tan colour, as shown in Figure 9.
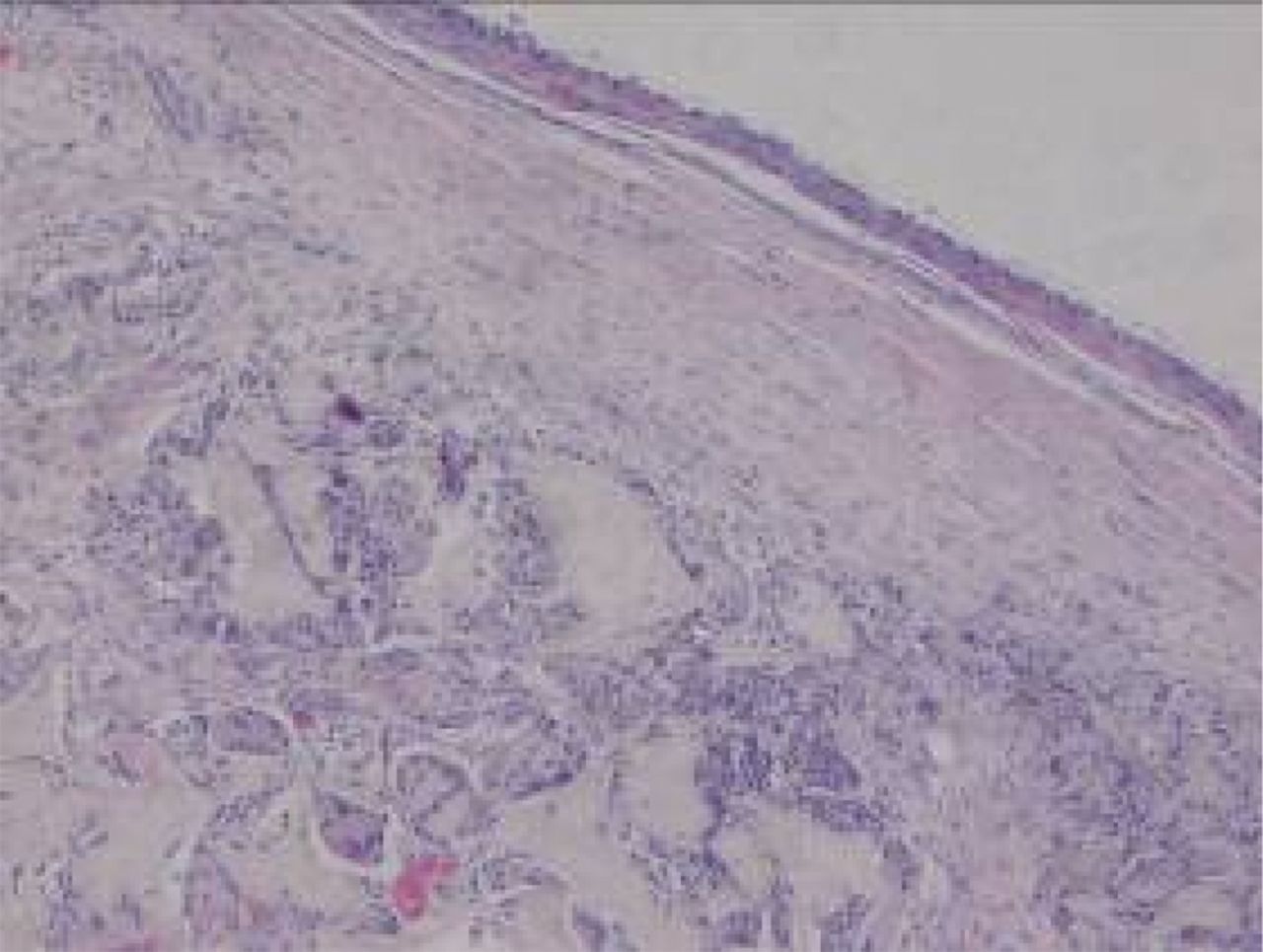

Figs. 8a - 8b
Haematoxylin-eosin staining in the experimental group 12 weeks post-operatively, observed using an optical microscope, which showed that a) the surface is smooth, and many inflammatory cells can be seen in the scaffold (× 100) and b) cells are arranged in a disorderly fashion, and the majority of the scaffold has degraded (× 400).

Fig. 9
Haematoxylin-eosin staining at 12 weeks after surgery in the experimental group, observed using an optical microscope, showing that the scaffold had partly degraded. Type II collagen in chondrocyte plasma and extracellular matrix was stained a browny-yellow colour (immunohistochemistry, × 200).
At week 24 in the experimental group, the new cartilage was found to be close to normal thickness, with a continuous, smooth surface. The cells on the surface were arranged parallel to the joint surface, while the cells in the deep layer were arranged in a disorderly manner with a trend towards a columnar arrangement. The cells were clustered together in relatively small groups, while the matrix was extensively metachromatic and the new cartilage was finely integrated with peripheral cartilage. The scaffold materials had basically degraded, as shown in Figure 10a. There were significant deposits of cartilage matrix, as shown in Figure 10b. Immunohistochemical staining of collagen II in the regenerated cartilaginous tissue was a tan colour, as shown in Figure 10c. As for subchondral bone, the upper stratum stained red is the cartilage matrix, while the middle part formed the osteochondral interface, and the tidal line was indistinct, as shown in Figure 10d.
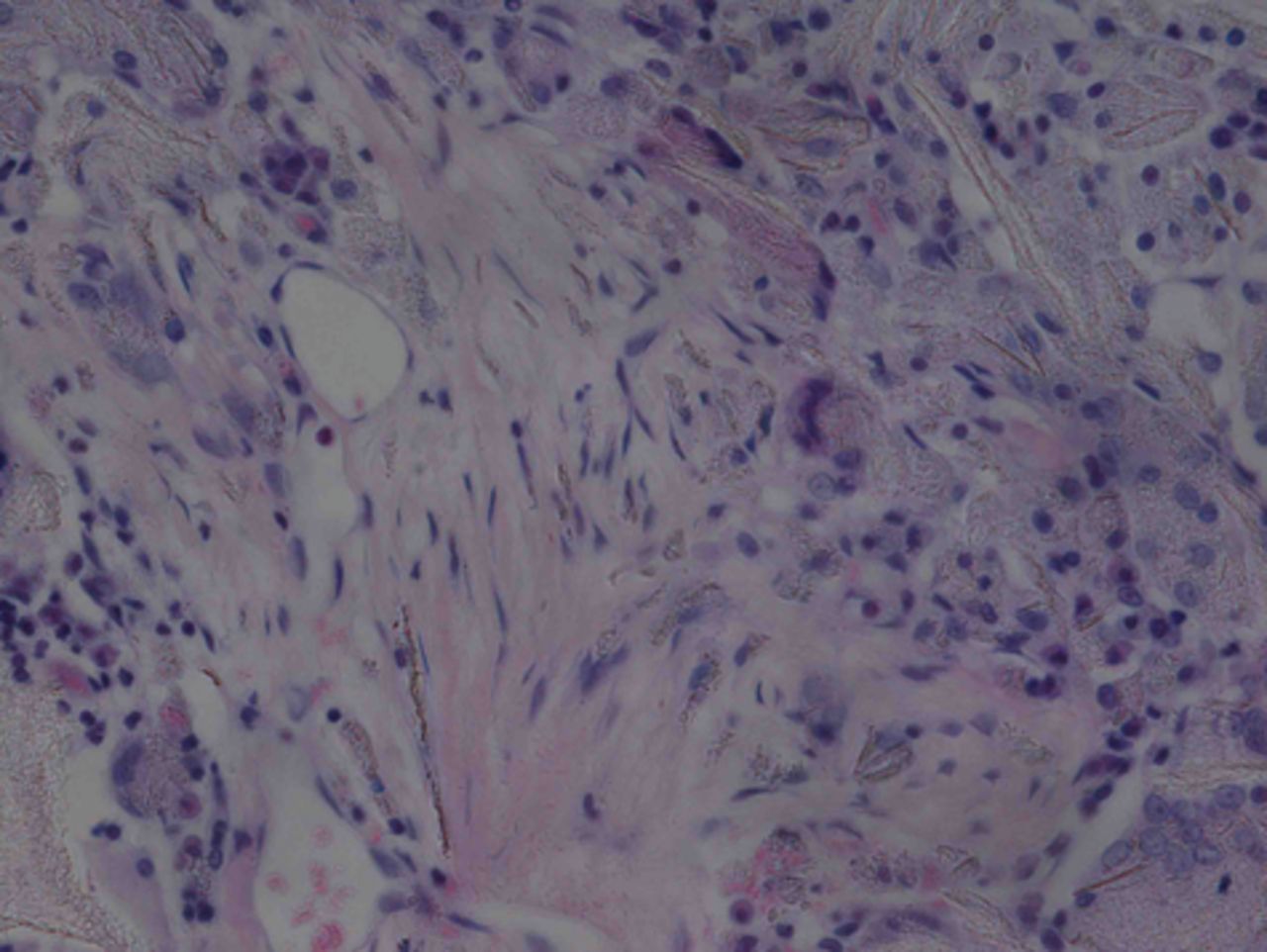
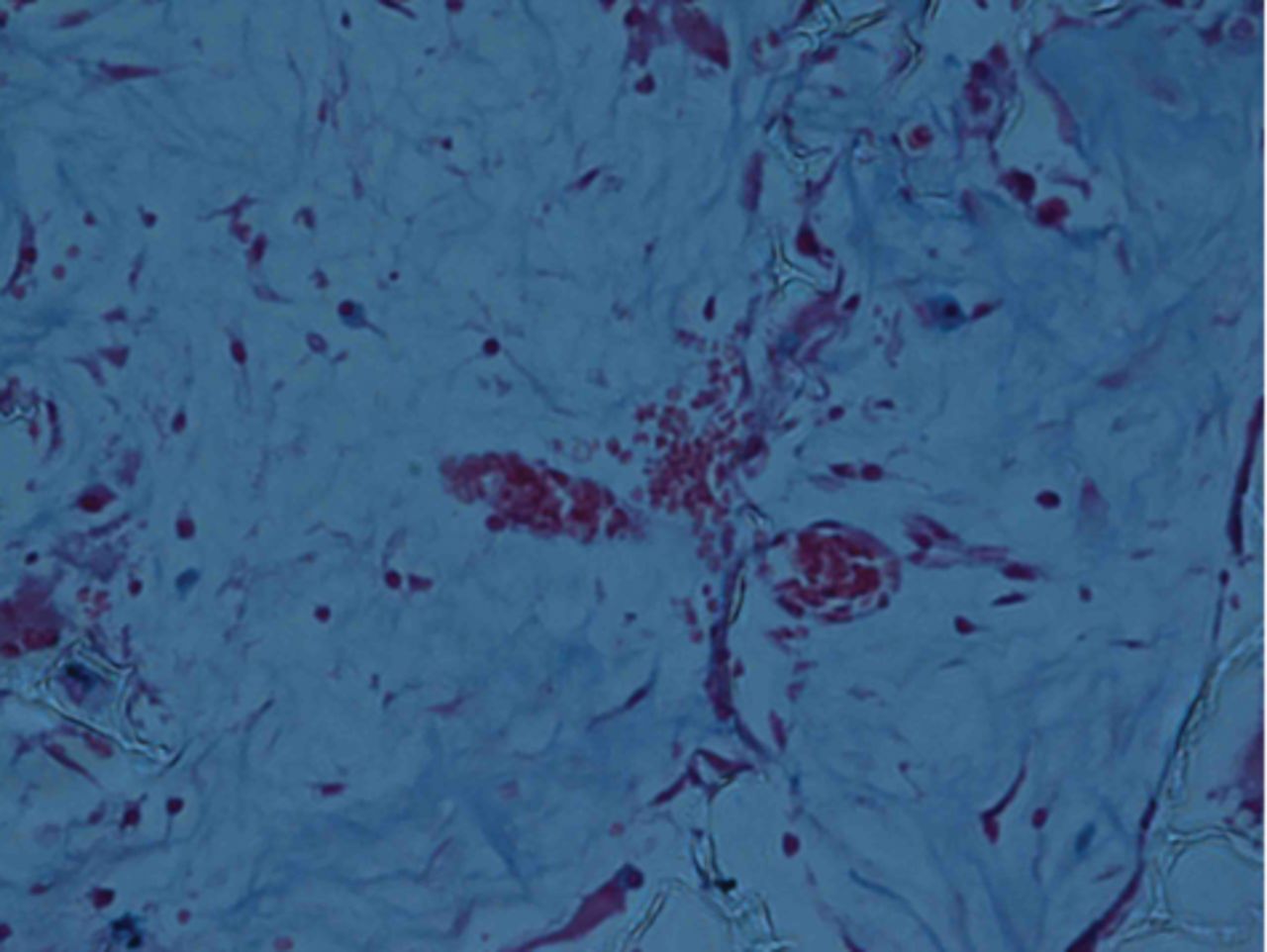
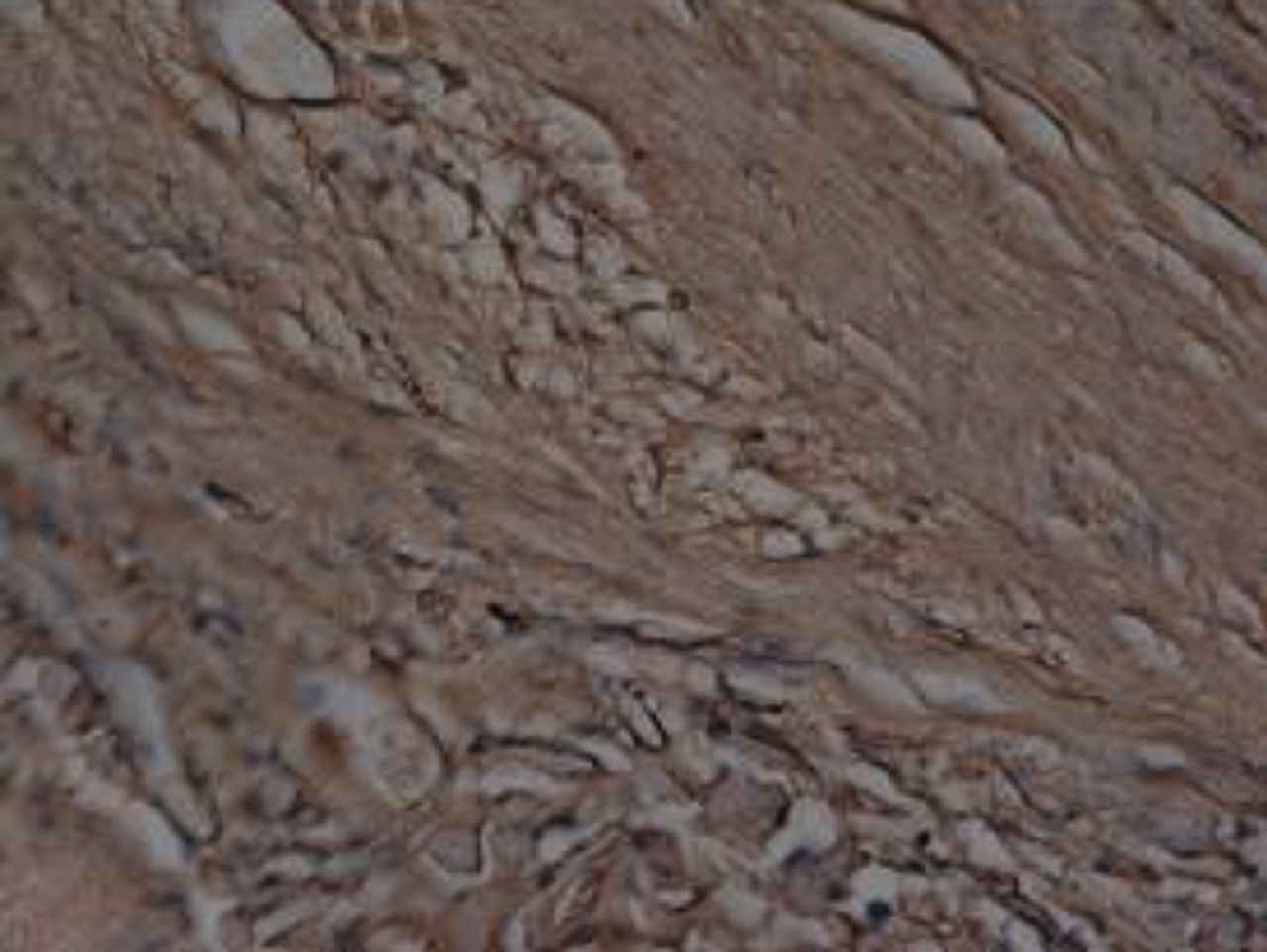
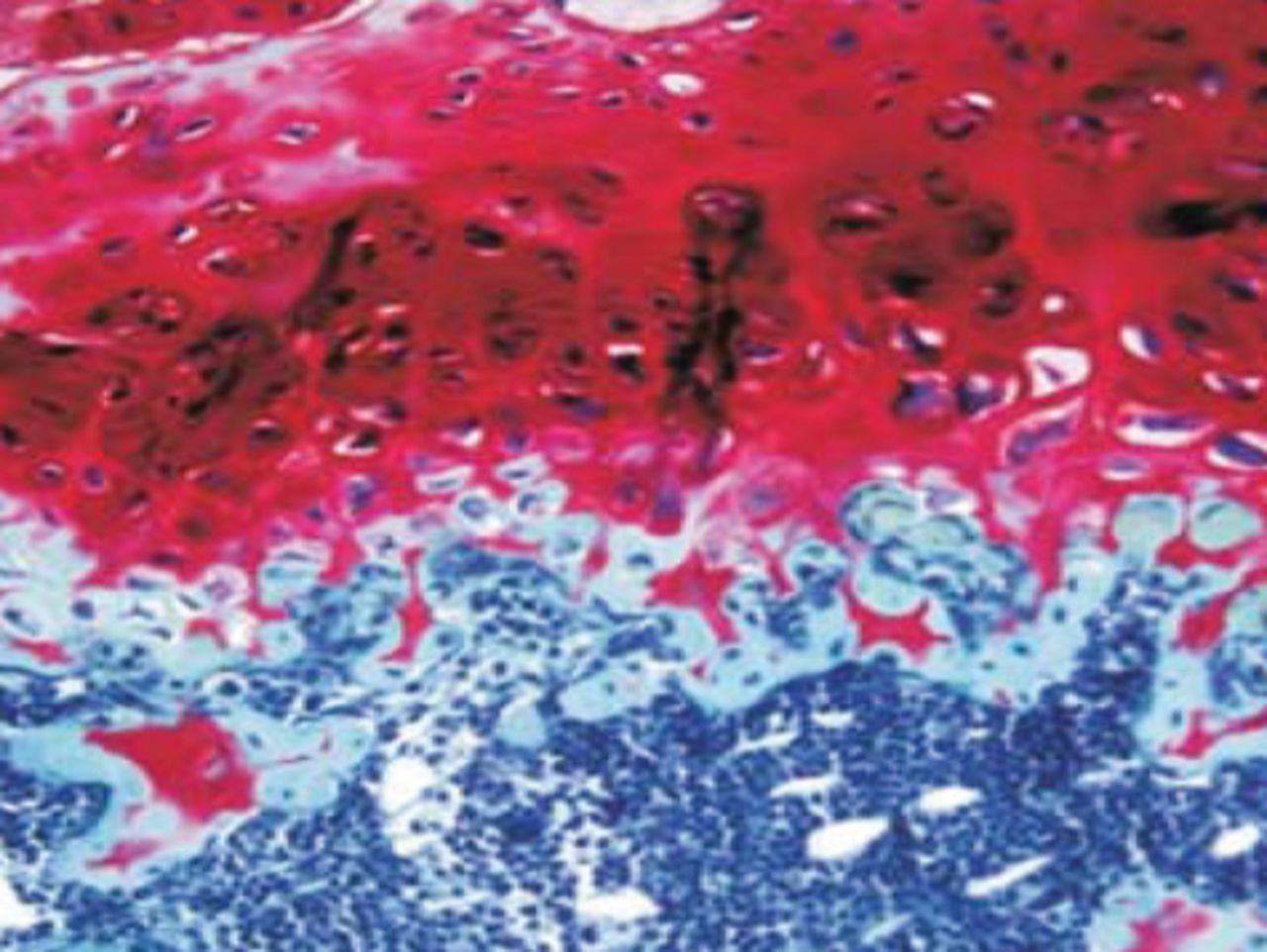
Figs. 10a - 10d
Histological observation in the experimental group 24 weeks post-operatively, observed using an optical microscope, showing a) cells arranged in a disorderly manner: the scaffold has degraded (Haematoxylin-eosin staining, × 400), b) cells arranged in a disorderly manner, the scaffold has degraded (Alcian blue, × 400), c) the scaffold has mostly degraded, type II collagen in chondrocyte plasma and extracellular matrix is stained a browny-yellow colour (Immunohistochemistry, × 100) and d) the upper layer (red) is cartilage matrix, the middle is the osteochondral interface, and the tidal line is unclear (Safranin O staining, ×40).
Discussion
Articular cartilage defects are frequently encountered in orthopaedic clinical practice, and the techniques currently used for repair do not give satisfactory results. Tissue engineering provides a new method for cartilage repair, but it is a complex process involving interactions between the scaffold, the seeded cells and various cytokines.10,14,22 The scaffold used to create a suitable repair is required temporarily in the reconstruction of the cartilage defect. This material should possess properties of a controllable degradation rate and non-toxic degradation products, which can be disposed of through metabolic pathways or normal physiological mechanisms.23,24,25 The β-tricalcium phosphate/collagen composite is one of the materials which meet such requirements.10,26 At present, alternative materials which can provide an artificial extracellular matrix with cell recognition signals and bionic design of the surface is a hot topic in the study of scaffold materials in tissue engineering. Preparations of bionic composite scaffolds appear to meet the requirements of tissue engineering technology for the scaffold.
TCP is a classic alternative filling material for bony defects, and has been shown to have excellent bony coherence, degradation and bony conductivity. It can be replaced by new bone after absorption.10,16 TCP nanoparticles are a new material manufactured at the nano-structure level or nanometer scale (1 nm to 100 nm), using nanotechnology, and exhibit the small-size and surface effects which confer many excellent qualities of performance along with completely new functions.26,27 Collagen is the primary organic component of bone in the human body, and can significantly enhance the interaction between cells in vivo, such as promoting cell chemotaxis and proliferation.28,29 Collagen implants possess excellent degradation performance in vivo, good stability and fine biocompatibility, as well as good plasticity and low antigenicity.28,30,31 Chinese researchers have prepared artificial composite bone using collagen and hydroxyapatite to implant into an experimental rat radius bone defect. The results showed that collagen possesses excellent biodegradation properties, confers bonding and shaping abilities on hydroxyapatite, and effectively inhibits particle dispersion, displacement and migration.26 Collagen is used as a bonding and shaping agent for β-TCP in this experiment due to its specific adhesion, with the aim of effectively preventing β-TCP powder from moving and displacing, and thus ensuring stability of position and shape after implantation.
Mauney et al32 first proposed use of a ‘double-phase’ carrier with histomorphology similar to normal articular osteochondral tissue. This consists of an open structure possessing large internal spaces to accommodate more cells, so that a cartilage layer at the articular surface can be formed during the interaction in this three-dimensional system after inoculation precursor cells, with a dense part of high strength to support the newly formed cartilage after the material is implanted into an articular osteochondral defect. This plays the role of the subcartilaginous osseous lamella. The ‘double-phase’ PLGA carrier has a relatively dense area of subchondral bone and a reasonably open area of cartilage. The results suggest that collagen I simulates formation of a bone-like layer, while collagen II simulates formation of a cartilage-like layer. When combined with autologous BMSCs, an excellent repair of the cartilage layer was obtained. Toluidine blue staining showed strong metachromasia. The repair primarily focused on regeneration of the transparent cartilage, while the layer of subchondral bone was partially repaired. This difference may be caused by lack of time or slow degradation of hydroxyapatite. However, the results in the experimental group were better than those in the control group, indicating that subchondral bone can be repaired with BMSCs, combined with a β-TCP/col I and II scaffold.
Based on ideas of bionic design, we chose the combination of nano-HAP/collagen I composite material and PLGA, with a similar composition to natural bone as the bony part of the scaffold, to induce formation of bone tissue, and we selected a combination of primary extracellular matrix of cartilage-hyaluronic acid with PLGA as the cartilaginous part of the scaffold to induce formation of cartilaginous tissue. A smaller particle size was observed under TEM in the nano-HAP/col I composite particles, reaching the nanometer level. Further tests of the physical and chemical properties using fourier transform infrared spectroscopy and x-ray diffraction analyses, indicated that the composition and crystallinity of the composite material was close to that of natural bone. Thus, this scaffold provided a good simulation of the extracellular micro-environment, and induced cell proliferation and differentiation. Observation of the surface morphology of the scaffold under SEM and measurement of the porosity showed that the pore size of the scaffold was moderate and even, at 100 μm to 300 μm, with a porosity > 90%, and that the pore-to-pore interconnectivity was unobstructed, promoting the growth of cells towards the interior of the scaffold, as well as the delivery of nutrients and the discharge of metabolites. The combination of hyaluronic acid and PLGA matrix material was directly adopted to form the cartilage part of the scaffold, to promote secretion of natural extracellular matrix by seed cells so as to construct new cartilaginous tissue. The two-part form of the laminated composite was adopted to create a bone–cartilage composite scaffold, the bone–to–bone combination was considered to be more conducive to rapid and strong integration with the subchondral bone, so as to complete the repair of the tissue defect.
The advantages of the use of BMSCs lie in the variety of sources, easy access and powerful proliferation ability. They can be amplified on a large scale in vitro, without loss of their multilineage differentiation potential. Under appropriate culture conditions and with the control of growth factors, they can be induced to differentiate into chondrocytes or osteoblasts with excellent biological activity.33-35 It was difficult to simulate the suitable induction environment fully in vitro, while the in vivo micro-environment was the natural environment for inducing the differentiation of BMSCs, as it included a variety of biological factors, including physical and chemical stimuli, and the appropriate mechanical environment. Therefore, some researchers have proposed the concept of an “in vivo bioreactor” that would make full use of local factors such as blood supply and the variety of physical and chemical factors, to stimulate seeded cells to form new target tissues more quickly and of a better quality.36,37 In this study, preliminary research on the biocompatibility between a composite scaffold material and seeded cells was performed by evaluating both aspects of cytotoxicity testing, and the ability of seeded BMSCs to grow on the scaffold, creating a good basis for further construction of osteochondral composites. Observations under SEM after one, four and seven days of culture of the BMSCs with the bone–cartilage scaffold showed that BMSCs could grow well in this composite scaffold and exhibited excellent proliferation, as well as being able to secrete large amounts of extracellular matrix. These results show that the scaffold material possessed excellent cellular affinity. This was attributed to the similarity of the composition and structure of both the upper stratum and the substratum of the scaffold material to natural bone–cartilage tissues, having the appropriate pore size and fine unobstructed pore-to-pore pathway structure that is conducive to the adhesion and growth of BMSCs.
In our experiments, a lamellar scaffold composed of β-TCP/col I and II was combined with autologous BMSCs. These cells could differentiate in the direction of cartilage under the combined effects of hypoxia, an appropriate three-dimensional structure, high density, a variety of growth factors and collagen II in the joint cavity, and could differentiate in the direction of bone under the effects of a rich blood supply, high oxygen levels, high density, a variety of growth factors and collagen I in the bone marrow cavity.
After surgery, the animals were allowed freedom of movement and given mechanical stimuli to promote differentiation of BMSCs. At 12 and 24 weeks post-operatively, the collagen layer was found to have degraded, and had been replaced with a layer of cartilage. The β-TCP was partially degraded, and new trabecular bone had grown inward, indicating that a laminated scaffold of β-TCP/col I and II could play the role of a carrier, which fosters repair of osteochondral defects. The combined double stratum of the bone–cartilage scaffold constructed of laminated composites possessed excellent microstructure, as it was less hierarchical and more firmly integrated at the implant site. The same matrix material could form the scaffold of both bone and cartilage implants, and was found to improve the integration of the scaffold into host cartilage defect without cell toxicity.
In conclusion, this construct possessed excellent biocompatibility, and was therefore suitable as a bone–cartilage scaffold for the repair of cartilage injury.
1 Choi WJ , KimBS, LeeJW. Osteochondral lesion of the talus: could age be an indication for arthroscopic treatment?Am J Sports Med2012;40:419–424.CrossrefPubMed Google Scholar
2 Westacott C . Interactions between subchondral bone and cartilage in OA. Cells from osteoarthritic bone can alter cartilage metabolism. J Musculoskelet Neuronal Interact2002;2:507–509.PubMed Google Scholar
3 Martin I , MiotS, BarberoA, JakobM, WendtD. Osteochondral tissue engineering. J Biomech2007;40:750–765.CrossrefPubMed Google Scholar
4 Barber FA , ChowJC. Arthroscopic osteochondral transplantation: histologic results. Arthroscopy2001;17:832–835.CrossrefPubMed Google Scholar
5 Beiser IH , KanatIO. Subchondral bone drilling: a treatment for cartilage defects. J Foot Surg1990;29:595–601.PubMed Google Scholar
6 Nebelung W , KayserR, RöpkeM, UrbachD, BeckerR. Autologous lateral patellar transplant as a reserve method for managing large osteochondral defects of the knee joint in young adults. Zentralbl Chir2000;125:505-508.(In German). Google Scholar
7 Koulalis D , SchultzW, HeydenM, KönigF. Autologous osteochondral grafts in the treatment of cartilage defects of the knee joint. Knee Surg Sports Traumatol Arthrosc2004;12:329–334.CrossrefPubMed Google Scholar
8 Krishnan SP , SkinnerJA, BartlettW, et al.Who is the ideal candidate for autologous chondrocyte implantation?J Bone Joint Surg [Br]2006;88-B:61–64.CrossrefPubMed Google Scholar
9 Goebel S , SteinertA, RuckerA, RudertM, BarthelT. Minimally invasive retrograde drilling of osteochondral lesions of the femur using an arthroscopic drill guide. Oper Orthop Traumatol2011;23:111-120.(In German). Google Scholar
10 Zou C , WengW, DengX, et al.Preparation and characterization of porous beta-tricalcium phosphate/collagen composites with an integrated structure. Biomaterials2005;26:5276–5284.CrossrefPubMed Google Scholar
11 Morihara T , HarwoodF, GoomerR, HirasawaY, AmielD. Tissue-engineered repair of osteochondral defects: effects of the age of donor cells and host tissue. Tissue Eng2002;8:921–929.CrossrefPubMed Google Scholar
12 Gratz KR , WongVW, ChenAC, et al.Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: tensile modulus of repair tissue and integration with host cartilage. J Biomech2006;39:138–146.CrossrefPubMed Google Scholar
13 Cao T , HoKH, TeohSH. Scaffold design and in vitro study of osteochondral coculture in a three-dimensional porous polycaprolactone scaffold fabricated by fused deposition modeling. Tissue Eng2003;9(Suppl 1):103–112.CrossrefPubMed Google Scholar
14 Cook SD , PatronLP, SalkeldSL, RuegerDC. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg [Am]2003;85-A(Suppl 3):116–123.CrossrefPubMed Google Scholar
15 Cherubino P , GrassiFA, BulgheroniP, RongaM. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong)2003;11:10–15.CrossrefPubMed Google Scholar
16 Eppley BL , PietrzakWS, BlantonMW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg2005;16:981–989.CrossrefPubMed Google Scholar
17 Eppley BL . Biomechanical testing of alloplastic PMMA cranioplasty materials. J Craniofac Surg2005;16:140–143.CrossrefPubMed Google Scholar
18 Jarman-Smith ML , BodamyaliT, StevensC, et al.Porcine collagen crosslinking, degradation and its capability for fibroblast adhesion and proliferation. J Mater Sci Mater Med2004;15:925–932.CrossrefPubMed Google Scholar
19 Podskubka A , PovýsilC, KubesR, SprindrichJ, SedlácekR. Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation on a hyaluronic Acid ester scaffolds (Hyalograft C). Acta Chir Orthop Traumatol Cech2006;73:251-263.(In Czech). Google Scholar
20 Jiang Y , ChenLK, ZhuDC, et al.The inductive effect of bone morphogenetic protein-4 on chondral-lineage differentiation and in situ cartilage repair. Tissue Eng Part A2010;16:1621–1632.CrossrefPubMed Google Scholar
21 Buma P , PieperJS, van TienenT, et al.Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects--a study in rabbits. Biomaterials2003;24:3255–3263.CrossrefPubMed Google Scholar
22 Louwerse RT , HeyligersIC, Klein-NulendJ, et al.Use of recombinant human osteogenic protein-1 for the repair of subchondral defects in articular cartilage in goats. J Biomed Mater Res2000;49:506–516.CrossrefPubMed Google Scholar
23 Yeganegi M , KandelRA, SanterreJP. Characterization of a biodegradable electrospun polyurethane nanofiber scaffold: mechanical properties and cytotoxicity. Acta Biomater2010;6:3847–3855.CrossrefPubMed Google Scholar
24 Stylios G , WanT, GiannoudisP. Present status and future potential of enhancing bone healing using nanotechnology. Injury2007;38(Suppl 1):S63–S74.CrossrefPubMed Google Scholar
25 Kon E , FilardoG, Di MatteoB, PerdisaF, MarcacciM. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res2013;2:18–25.CrossrefPubMed Google Scholar
26 Liao SS , CuiFZ, ZhangW, FengQL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater2004;69:158–165.CrossrefPubMed Google Scholar
27 Ozkan S , KalyonDM, YuX. Functionally graded beta-TCP/PCL nanocomposite scaffolds: in vitro evaluation with human fetal osteoblast cells for bone tissue engineering. J Biomed Mater Res A2010;92:1007–1018.CrossrefPubMed Google Scholar
28 Yang Z , ShiY, WeiX, et al.Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods2010;16:865–876.CrossrefPubMed Google Scholar
29 Kin S , HagiwaraA, NakaseY, et al.Regeneration of skeletal muscle using in situ tissue engineering on an acellular collagen sponge scaffold in a rabbit model. ASAIO J2007;53:506–513.CrossrefPubMed Google Scholar
30 Kanematsu A , YamamotoS, OzekiM, et al.Collagenous matrices as release carriers of exogenous growth factors. Biomaterials2004;25:4513–4520.CrossrefPubMed Google Scholar
31 Vijayan S , BentleyG, RahmanJ, et al.Revision cartilage cell transplantation for failed autologous chondrocyte transplantation in chronic osteochondral defects of the knee. Bone Joint J2014;96-B:54–58.CrossrefPubMed Google Scholar
32 Mauney JR , SjostormS, BlumbergJ, et al.Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int2004;74:458–468.CrossrefPubMed Google Scholar
33 Maeda S , FujitomoT, OkabeT, WakitaniS, TakagiM. Shrinkage-free preparation of scaffold-free cartilage-like disk-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng2011;111:489–492.CrossrefPubMed Google Scholar
34 Zhao L , LiG, ChanKM, WangY, TangPF. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif Tissue Int2009;84:56–64.CrossrefPubMed Google Scholar
35 Takagi M , UmetsuY, FujiwaraM, WakitaniS. High inoculation cell density could accelerate the differentiation of human bone marrow mesenchymal stem cells to chondrocyte cells. J Biosci Bioeng2007;103:98–100.CrossrefPubMed Google Scholar
36 Stojkovska J , BugarskiB, ObradovicB. Evaluation of alginate hydrogels under in vivo-like bioreactor conditions for cartilage tissue engineering. J Mater Sci Mater Med2010;21:2869–2879.CrossrefPubMed Google Scholar
37 Stevens MM , MariniRP, SchaeferD, et al.In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci U S A2005;102:11450–11455.CrossrefPubMed Google Scholar
Funding statement:
This work was partly supported by Guangdong Province Natural Science Foundation (grant number 07002690).
Author contributions:
Y. M. Lv: Data collection, Data analysis, Performed surgeries
Q. S. Yu: Data analysis, Writing the paper
ICMJE Conflict of Interest:
None declared
©2015 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









