Abstract
Objectives
Effective analgesia after total knee arthroplasty (TKA) improves patient satisfaction, mobility and expedites discharge. This study assessed whether continuous femoral nerve infusion (CFNI) was superior to a single-shot femoral nerve block in primary TKA surgery completed under subarachnoid blockade including morphine.
Methods
We performed an adequately powered, prospective, randomised, placebo-controlled trial comparing CFNI of 0.125% bupivacaine versus normal saline following a single-shot femoral nerve block and subarachnoid anaesthesia with intrathecal morphine for primary TKA. Patients were randomised to either treatment (CFNI 0 ml to 10 ml/h 0.125% bupivacaine) or placebo (CFNI 0 ml to 10 ml/h normal saline). Both groups received a single-shot femoral nerve block (0.25% 20 ml bupivacaine) prior to placement of femoral nerve catheter and subarachnoid anaesthesia with intrathecal morphine. All patients had a standardised analgesic protocol. The primary end point was post-operative visual analogue scale (VAS) pain score over 72 hours post-surgery. Secondary outcomes were morphine equivalent dose, range of movement, side effects, and length of stay.
Results
A total of 86 patients were recruited. Treatment and placebo groups were comparable. No significant difference was found in VAS pain scores, total morphine equivalent requirements, side effects, range of movement, motor block, or length of hospital stay.
Conclusion
No significant advantage was found for CFNI over a single-shot femoral block and subarachnoid anaesthesia after TKA.
Cite this article: Bone Joint Res 2015;4:11–16.
Article focus
Pain relief after primary total knee arthroplasty
Femoral nerve continuous infusion versus single nerve block in the context of spinal anaesthetic with morphine
Prospective randomised controlled trial
Key messages
No significant advantage for continuous femoral nerve block over a single injection
Strengths and limitations
Strength: Fully-powered, prospective, double-blinded, randomised controlled trial
Limitation: Lack of inclusion of anti-inflammatories and other analgesics in the pain protocol
Introduction
Optimal peri-operative analgesia after primary total knee arthroplasty (TKA) enhances patient satisfaction, minimises complications and expedites recovery. Subarachnoid anaesthesia is strongly advocated for TKA to reduce bleeding and thromboembolic risk.1 In our institution we use a combination of subarachnoid anaesthesia with intrathecal morphine, followed by a patient-controlled analgesic (PCA) morphine pump.
In recent years, post-operative analgesia has seen a decrease in opiate use and, thus, opiate side effects.2 Femoral nerve regional blocks have been shown not only to diminish opiate doses but also to give superior pain relief, knee mobility and to expedite discharge from hospital compared with epidurals or PCAs.3,4 The role of the sciatic nerve blockade, however, remains controversial.5-8 Major nerve blockade is not without its drawbacks and surgeons may be concerned about the perceived risk of motor weakness delaying mobilisation. Serious complications with femoral nerve blocks are rare and neurological injury is uncommon and estimated at < 0.2%.8
To our knowledge, no previous studies have reported the outcomes of subarachnoid anaesthesia with intrathecal morphine with a continuous femoral nerve infusion (CFNI) or single-shot femoral nerve block for TKA.
The aim of this study was to investigate whether a CNFI conveyed any additional benefit in terms of reduced pain and number of side effects over a single-shot femoral nerve block following a subarachnoid block with intrathecal morphine for primary TKA.
We tested the null hypothesis that in the first 72 hours after the operation, there is no difference in VAS pain scores, motor block and opiate-related side effects in patients receiving a femoral nerve infusion in comparison to a single-shot femoral nerve block in the context of subarachnoid anaesthesia with intrathecal morphine following primary TKA.
Materials and Methods
Pilot study and power calculation
We first performed a prospective, non-randomised, non-blinded observational pilot study in 76 patients who underwent a TKA under spinal anaesthesia at our institution. We examined VAS pain scores and opiate doses in patients receiving a CFNI compared with single-shot nerve block plus PCA. We found that on average there was a difference of 10 mm on the VAS for pain (sd 1.6). A 10 mm VAS difference is widely accepted as the minimum for clinical relevance. To detect this difference in a randomised controlled trial at any time point, using an unpaired t-test, with an alpha of 0.05 and a power of 80%, we required 40 patients per group.
Surgical technique
All TKAs were performed by one of four consultant orthopaedic surgeons using a medial parapatellar approach. The patella was everted and no patellar denervation was performed. A posterior-stabilised prosthesis was used without a mobile bearing and the patella was left unresurfaced.
Institutional review, ethical and trial registration approval
We obtained approval from the Institutional Review Board (Otago District Health Board) following Local Ethics Committee approval (LRS/08/08/030). We also registered the study as a clinical trial (ACTRN12609000406202).
Recruitment
From January 2010 until October 2011, patients listed for a primary unilateral TKA for osteoarthritis were invited to participate in the study via an information sheet posted at the same time as their appointment, to attend the pre-operative assessment clinic one week prior to the surgery date. Patients were approached by a research nurse at the clinic and those willing to participate were consented for the trial. The inclusion criterion was a unilateral primary TKA for osteoarthritis. Exclusion criteria are listed in Table I.9
Table I
Exclusion criteria
| Unable to give informed consent for any component of the study protocol |
| Outside of the study age range (< 18 and > 85 years) |
| BMI > 40 |
| Suffering from any of the following conditions: major psychiatric problems, previous drug dependency, ASA 4 or above, pregnancy, severe renal and hepatic disease, peripheral neuropathy |
| Allergic to any medications in the study protocol i.e. lignocaine, laxsol, paracetamol, bupivacaine, fentanyl, chlorhexidine, oxynorm, oxycontin, intrathecal morphine |
| Already on high-dose opiates (> 20 mg morphine/day) or on atypical analgesics (gabapentin, pregabalin, clonidine and mexiletine) |
| Unable to comprehend the visual analogue scale pain score |
| Unable to use a patient-controlled analgesia device |
| Judged inappropriate for the use of a femoral nerve infusion catheter on the basis of local infection, systemic sepsis, bleeding diathesis, anticoagulation, or pre-existing neurological abnormality of the lower limb |
| Judged as having a contraindication to subarachnoid blockade on the basis of medical comorbidity, localised infection, systemic sepsis, bleeding diathesis, anticoagulation,or pre-existing neurological abnormality of the lower limb |
-
ASA, American Society of Anesthesiologists9
Randomisation
The randomisation was by sealed envelope technique and independent third party:
50 cards for each study arm were placed in sealed envelopes by an independent nurse, shuffled and placed within a randomisation box.
Randomisation was undertaken in recovery by a nurse who was not involved in the study in any other way (she withdrew the envelope, prepared the drug infusion and completed the randomisation paperwork).
Blinding
Patients, surgeon, research nurse, medical statistician, ward nurses and physiotherapists were blinded to the intervention as the infusions were prepared by a recovery nurse on the day of surgery who had no further contact with the patient.
Intra-operative intervention protocol
Femoral nerve infusion catheter
The groin was shaved and prepared with a chlorhexidine solution followed by a local anaesthetic with 1% lignocaine. An 18 G Touhy Nerve block needle (Braun CONTIPLEX, Melsungen, Germany) was then introduced under ultrasound and nerve stimulator guidance (placement determined by adequate motor response of patellar twitch 0.6 mA) directly distal to the inguinal ligament. Once in position 15 ml 0.25% bupivacaine was injected around the femoral nerve in aliquots. The catheter was then advanced 3Â cm to 4 cm beyond the needle tip and an IV3000 dressing with surrounding tape applied, thus securing it to the lower abdominal wall. The catheter/block was placed prior to the subarachnoid anaesthetic to avoid masking the intra-neural injection.
Subarachnoid blockade and sedation/light general anaesthetic
A standard spinal technique was used with 0.5% bupivacaine plain/heavy (10 mcg to 20 mcg) plus intrathecal morphine 1.5 mcg/kg (max 150 mcg). The patient received sedation with a midazolam or propofol infusion titrated to effect. General anaesthesia was provided with fentanyl/propofol induction and sevoflurane maintenance and intra-operative analgesia with paracetamol 1 g IV after induction.
Surgery
Patients received a standard cemented condylar TKA via a medial parapatellar approach by a consultant orthopaedic surgeon.
Intervention
Randomisation was performed in the post-anaesthetic care unit (PACU). A femoral nerve infusion was prepared in PACU and commenced at 10 ml/hr with normal saline in the control group and 0.125% bupivacaine infusion at 10 ml/hr in the intervention group. The infusion continued for 48 hours. Patients were mobilised in the initial 48 hours under strict supervision either by a physiotherapist or a nurse. Complications of motor weakness, local anaesthetic toxicity, persistent pain from the infusion site, localised inflammation or signs of infection, haematoma and catheter dislodgment, were recorded. Leakages were dealt with by application of a firm dressing and IV3000, with a sterile swab placed over the insertion site.
Post-operative analgesia
Patients were prescribed paracetamol 1 g four times a day regularly, oxynorm 5Â mg to 10 mg as needed up to every three hours commencing on day 0, oxycontin SR 10 mg twice a day from day 1 and laxsol 2 tablets commencing on day 0. On the evening after surgery if a dose of more than 20 mg of oxynorm was required or the patient had severe pain not controlled by oral analgesia, a fentanyl PCA was started.
On the first post-operative day, if pain was not controlled, an increase of oxycontin to 20 mg twice a day with oxynorm 5 mg to 10 mg as needed every three hours, and fentanyl PCA available as a second line, was allowed. On the second post-operative day, a reassessment of oxycontin requirements was done based on the last 24 hours of use. If the pain remained poorly controlled the patient commenced a fentanyl PCA. To prevent potential confounding errors, anti-inflammatories, clonidine and tramadol were avoided.
Outcome measures
The primary end point was peak pain at rest over the first 72 hours after the operation. Pain was measured by a VAS 0 mm to 100 mm scale at four-hourly intervals during the first 24 hours, then at 30, 36, 42, 48, 54, 60, 66, and 72 hours thereafter. Participants were not woken to assess their pain, which explains some of the missing data. Secondary end points were range of movement (ROM), side effects (sedation, pruritus, nausea and vomiting, leakage, toxicity, bleeding, infection,inflammation, catheter blockage), cumulative narcotic dose in morphine equivalents, infusion rates and reasons for any changes, duration of in-patient stay and reason for any discharge delays.
Significance was set at the 5% level. No adjustment for multiple testing was done. The primary outcomes were tested by using a mixed model to allow for the repeated measurements of pain. For continuous data, Mann–Whitney tests were used to test for differences in non-normally distributed data (VAS and use of analgesia) and unpaired t-tests for normally distributed data (knee ROM and length of hospital stay.) Chi-squared tests were used to test for differences in proportions. All analysis was done using Stata 10 (StataCorp, 4905 Lakeway Drive, College Station, Texas), and with participants in the group to which they were randomised.
Results
Recruitment and demographics
Of the 226 patients who were invited to participate in the study, 34 declined and 75 were excluded for the following reasons: comorbidities (33), age > 85 years (11), exceeded daily morphine dose (11), BMI > 40 (9), declined spinal anaesthetic (5), morphine allergy (5) and unable to comprehend the VAS score (1). In total, 113 were found to be eligible for inclusion in the study. Of these, 31 were excluded prior to randomisation for the following reasons: anaesthetist not trained in femoral nerve catheter technique (27), failed spinal anaesthetic (2) and failed femoral nerve catheter insertion (2). The remaining 86 patients were randomised and 42 of the 43 completed the study protocol in each group. One patient in each group was withdrawn from the study as a result of a cardiac event and excessive uncontrolled pain, i.e. requiring more adjunct analgesia than that prescribed in the protocol.
With the exception of a predominance of males in the treatment group, there were no important differences between either group in terms of baseline demographics and clinical characteristics (Table II).
Table II
Demographics of treatment and control groups in the study
| Treatment (n = 43) | Control (n = 43) | |
|---|---|---|
| Mean age (yrs) (sd) | 68.2 (7.0) | 68.8 (8.2) |
| Gender (female/male) | 16/27 | 23/20 |
| Mean weight (kg) (sd) | 82.6 (16.3) | 77.6 (13.5) |
| Mean BMI (sd) | 28.5 (4.42) | 28.1 (4.96) |
-
sd, standard deviation; BMI, body mass index
Primary end point
There was no difference between the groups in pain experience over the first 72 hours after the operation (difference 1.5 mm, 95% confidence interval (CI) -3.4 to 6.4). The confidence interval did not reach the minimum clinically important difference (10 mm) so the differences are unlikely to be important. Adjusting these results for gender did not show any difference. At all stages there was no significant difference (p = 0.01) in the pain VAS score between the control and treatment groups (Fig. 1) -3.4 to 6.4). The confidence interval did not reach the minimum clinically important difference (10 mm) so the differences are unlikely to be important. Adjusting these results for gender did not show any difference. At all stages there was no significant difference (p = 0.01) in the pain VAS score between the control and treatment groups (Fig. 1). The confidence interval did not reach the minimum clinically important difference (10 mm) so the differences are unlikely to be important. Adjusting these results for gender did not show any difference. At all stages there was no significant difference (p = 0.01) in the pain VAS score between the control and treatment groups (Fig. 1) so the differences are unlikely to be important. Adjusting these results for gender did not show any difference. At all stages there was no significant difference (p = 0.01) in the pain VAS score between the control and treatment groups (Fig. 1) in the pain VAS score between the control and treatment groups (Fig. 1). At 24 and 48 hours there were no significant differences (p = 0.07) although at 24 hours, the treatment group had a pain score 10 mm less than the control group.
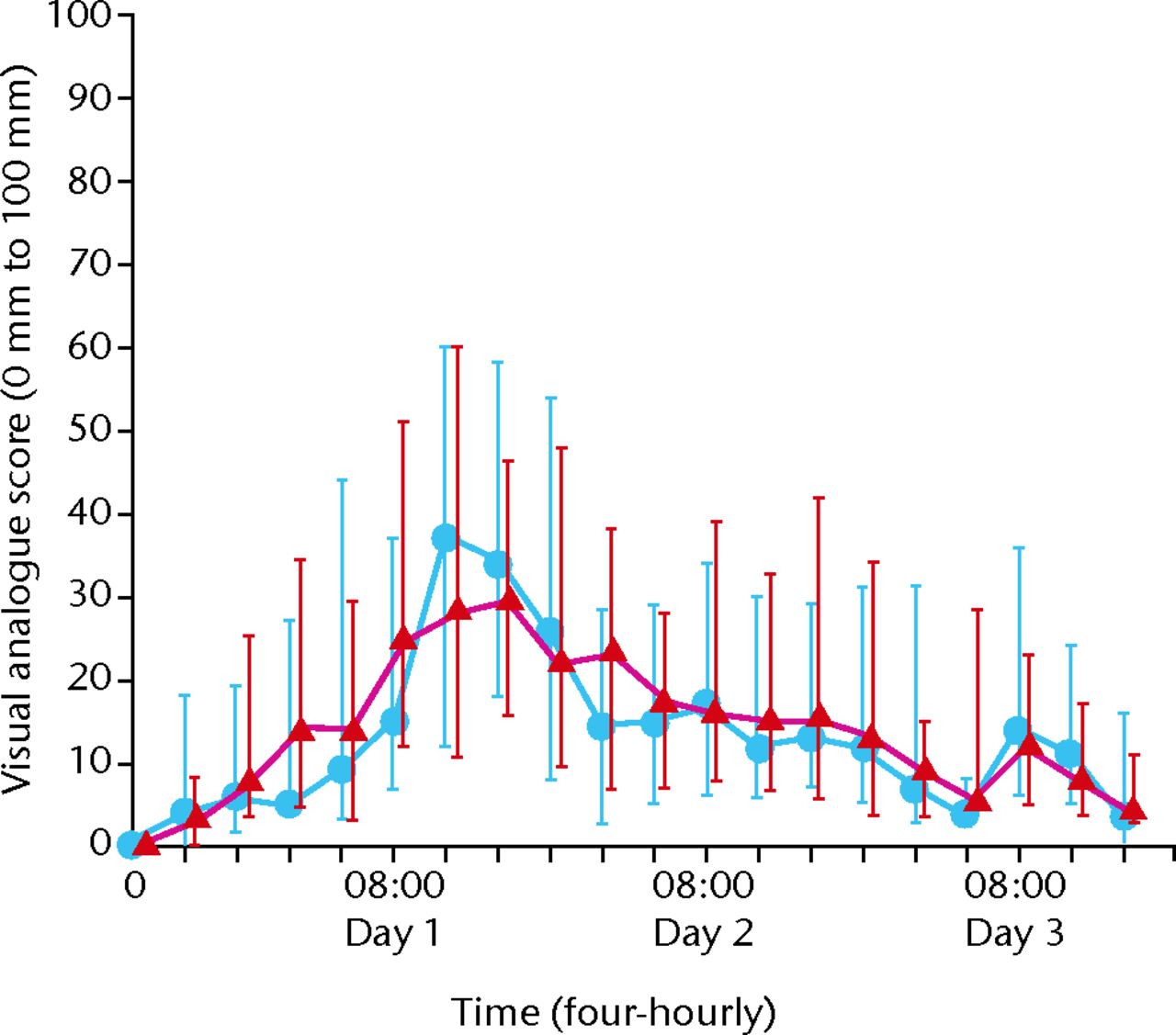
Fig. 1
Visual analogue pain scores for intervention and control group over time (blue dots, intervention group; red triangles, control group.
Secondary end points
There was no significant difference in total analgesia used in morphine equivalents at any time point during the study or overall. Furthermore, there were no significant differences in side effects between the two groups (p = 0.2 and p = 0.35, respectively) (Table III). There was no significant difference in the occurrence of motor block between the two groups (all p-values > 0.05) (Table IV) and no falls occurred. There were no infections and no haematomas at the catheter site. There was no significant difference in knee ROM between groups, on any day or at time of discharge (p = 0.45, 0.78, 0.52, 0.36 for days 1, 2, 3 and discharge, respectively) (Table V). There was no significant difference in length of hospital stay between groups (p = 0.87) (Table VI).
Table III
Occurrence of opiate-related side effects in control and treatment groups
| Treatment group (n = 42) | Control group (n = 42) | p-value | ||
|---|---|---|---|---|
| Yes (%) | Yes (%) | |||
| Day 0 | 37 (86) | 36 (84) | 0.75 | |
| Day 1 | 26 (60) | 23 (53) | 0.51 | |
| Day 2 | 19 (44) | 19 (44) | 1.0 |
Table IV
Occurrence of motor block in control and treatment groups
| Treatment group (n = 43) | Control group (n = 43) | p-value | ||
|---|---|---|---|---|
| Yes (%) | Yes (%) | |||
| Day 0 | 16 (37) | 18 (42) | 0.66 | |
| Day 1 | 14 (33) | 11 (26) | 0.47 | |
| Day 2 | Â Â 5 (12) | Â Â 8 (19) | 0.37 |
Table V
Post-operative knee range of movement
| Time period | Treatment group (n = 42) | Control group (n = 42) | Difference | p-value | |
|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | Mean (95% CI) | |||
| Day 1 | 56 (17) | 53 (19) | 3 (-4.8 to 10.8) | 0.45 | |
| Day 2 | 58 (18) | 57 (14) | 1 (-6.0 to 8.0) | 0.78 | |
| Day 3 | 67 (16) | 65 (12) | 2 (-4.1 to 8.1) | 0.52 | |
| Discharge | 79 (10) | 77 (10) | 2 (-2.3 to 6.3) | 0.36 | |
-
sd, standard deviation; CI, confidence interval
Table VI
Post-operative length of stay
| Treatment group (n = 42) | Control group (n = 42) | Difference | p-value | ||
|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | Mean (95% CI) | |||
| Length of stay (days) | 5.71 (1.47) | 5.76 (1.34) | -0.05 (-0.66 to 0.56) | 0.87 | |
-
sd, standard deviation; CI, confidence interval
Discharge criteria included independent transfers and activities of daily living, satisfactory wound healing, crutch walking for 20 metres and ability to walk up and down stairs. Four patients in each group had delayed discharge. The reasons for delay in discharge were variable but independent of the study protocol.
Discussion
We believe that there are no other studies directly comparing a single-shot femoral nerve block versus CFNI after a spinal with intrathecal morphine for primary TKA. Based on our findings, we would not advocate the routine use of femoral nerve infusion catheters in addition to a spinal anaesthetic and a single-shot femoral nerve block for analgesia after primary TKA.
A single-shot femoral nerve block is known to provide good analgesia with some properties persisting beyond the expected duration of the local anaesthetic alone. An infusion catheter, although appealing, is not without drawbacks. Possible motor block, risk of infection, or the presence of an infusion device attached to the patient may hamper patient mobilisation. A low concentration of bupivacaine to diminish the risk of motor block was used in this study and there were no significant differences in the occurrence of a motor block between the two groups in our study. As we did not observe any bleeding associated with the catheters, we do not recommend any change in thromboprophylaxis, nor any special vigilance concerning the timing of catheter removal. There are no current guidelines for the use of the new generation oral anticoagulants and peripheral nerve catheters/blocks.
In our study we did not observe a difference in the level of pain at any time. VAS pain scores were often < 10 mm. Therefore, it would be impossible to detect a difference in VAS of 10 mm between the treatment groups. It appears that in our study, the treatment without femoral nerve infusion was already so good that no improvement is possible. Interestingly, pain scores in the study group were worse on day one as patients began to mobilise. This most likely reflected a lower rate of administration of additional strong opiate analgesia and pain from the sciatic innervation of the knee. A greater amount of strong opiate was administered to the control group during the first 24 hours, presumably providing some benefit for this pain. A subsequent increase in analgesic requirements above the regular dose was seen in the study group to overcome this pain. Beyond this point, VAS and analgesia requirements converged with little difference seen.
The analysis of this study involved a great deal of multiple testing, for example of the pain scores at every time point. As there has been no adjustment for multiple testing, the results for these end points will have p-values that are too small. However, as all results were not statistically significant, any adjustment for multiple testing would increase the p-values and, thus, would not change the interpretation. There were missing values for the pain scores because participants were asleep at the recording time and were not woken. It could be argued that the pain scores for these people might have been lower because they were able to sleep. The results of mixed models are not biased by data that are missing at random.
The presence of a defined analgesic protocol most likely has the greatest effect on analgesia following TKA. We observed a marked improvement in pain recognition and delivery of analgesia in patients undergoing the study compared with traditional TKA patients in our institution. The protocol encouraged nurses to ask about pain (four-hourly) and gave clear guidelines on how to manage pain at any time, including backup plans for break-through analgesia.
Patients who were opiate-tolerant were excluded from the study for obvious reasons. More than 20 mg of morphine or equivalent would cause significant bias. Their inclusion and subsequent subgroup analysis would have most likely further reduced the power of the study.
This study was carried out before the introduction of enhanced recovery after surgery, which explains the slightly longer length of hospital stay.
We acknowledge that our chosen method of randomisation could have been improved by using an online method, and could be considered a weakness of the study, as could the delay from finishing the study in October 2011 to the review, almost three years later.
A major criticism of our investigation is the lack of inclusion of anti-inflammatories and other analgesics (such as tramadol or clonidine) in the pain protocol. Unfortunately, a significant proportion of our patients had relative contraindications to the use of NSAIDs and it was felt that this would have excluded a significant proportion of patients from our study. There is clear evidence that NSAIDs commenced at the time of surgery improves post-operative pain. Their inclusion in addition to intrathecal morphine and a femoral nerve block would likely result in further improvements in VAS pain scores and mobility. We experienced a larger than anticipated dropout of patients who had been identified as eligible prior to surgery. Many of these cases were excluded prior to randomisation because the anaesthetist did not have the skills to insert the femoral nerve catheter. The findings of our study have implications for the efficiency of theatre lists and have provided evidence for a change of practice in our institution, where continuous nerve infusions have been discontinued. With the exception of NSAIDs, we believe that our post-operative analgesia protocol was optimal.
Our study findings contrast with those of the retrospective study by Subramaniam and Sathappan,10 who found that CFNI achieved better pain relief and more rapid ambulation over the single-shot femoral nerve group. Our study findings are also different to those of the prospective study by Salinas et al.11 This study showed better pain relief in the CFNI group compared with the single-shot group. However, our study found, like that of Salinas et al,11 there was no difference in hospital length of stay.
Sciatic nerve block may provide additional analgesia in the first 24 hours after surgery. We elected not to explore this in addition to a CFNI. We were attempting to assess an approach that is easily implemented in any clinical department. Spinal anaesthesia with a femoral nerve block is easily performed in a timely manner in the operating theatre, whereas the addition of a sciatic block may introduce further time pressures with marginal improvement in patient analgesia or satisfaction. However, a future study could investigate the benefit of sciatic nerve blockade in TKA surgery.
In conclusion, CFNI has been reported as giving superior pain relief following TKA surgery. We have conducted a randomised placebo-controlled trial which has shown that there is no difference in VAS pain scores in the first 72Â hours when comparing continuous bupivacaine infusion versus saline in the context of a single-shot femoral nerve bloc and spinal anaesthesia. We also failed to demonstrate a difference in surgical outcomes including knee ROM and length of stay. As a result we do not recommend this technique as part of a post-operative TKA analgesia protocol.
Supplementary material
A copy of the CONSORT checklist and a CONSORT flow diagram are available with this article at www.bjr.boneandjoint.org.uk
1 Rodgers A , WalkerN, SchugS, et al.Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ2000;321:1493.CrossrefPubMed Google Scholar
2 Horlocker TT , KoppSL, PagnanoMW, HeblJR. Analgesia for total hip and knee arthroplasty: a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg2006;14:126–135.CrossrefPubMed Google Scholar
3 Capdevila X , BartheletY, BibouletP, et al.Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology1999;91:8–15.CrossrefPubMed Google Scholar
4 Singelyn FJ , DeyaertM, JorisD, PendevilleE, GouverneurJM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg1998;87:88–92.CrossrefPubMed Google Scholar
5 Duarte VM , FallisWM, SlonowskyD, KwartengK, YeungCK. Effectiveness of femoral nerve blockade for pain control after total knee arthroplasty. J Perianesth Nurs2006;21:311–316.CrossrefPubMed Google Scholar
6 Morin AM , KratzCD, EberhartLH, et al.Postoperative analgesia and functional recovery after total-knee replacement: comparison of a continuous posterior lumbar plexus (psoas compartment) block, a continuous femoral nerve block, and the combination of a continuous femoral and sciatic nerve block. Reg Anesth Pain Med2005;30:434–445.CrossrefPubMed Google Scholar
7 Allen HW , LiuSS, WarePD, NairnCS, OwensBD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesth Analg1998;87:93–97.CrossrefPubMed Google Scholar
8 Capdevila X , PiratP, BringuierS, et al.Continuous peripheral nerve blocks in hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1,416 patients. Anesthesiology2005;103:1035–1045.CrossrefPubMed Google Scholar
9 No authors listed. American Society of Anesthesiologists Physical Status Classification System. http://www.asahq.org (date last accessed 9 January 2015). Google Scholar
10 Subramaniam R , SathappanSS. The Effects of Single Shot versus Continuous Femoral Nerve Block on Postoperative Pain and Rehabilitation Following Total Knee Arthroplasty. Malaysian Orthopaedic Journal2010;4:19–25. Google Scholar
11 Salinas FV , LiuSS, MulroyMF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg2006;102:1234–1239.CrossrefPubMed Google Scholar
Funding statement:
We are privileged to have received funding from the following: Healthcare Otago Trust, The Wishbone Trust, University of Otago Medical School Bequest Fund, and The Richard Stewart Scholarship.
Author contributions:
M. C. Wyatt: Trial conception/instigation, Data analysis, Writing the paper
T. Wright: Performing anaesthesia
J. Locker: Trial instigation, Data collection
K. Stout: Data collection
C. Chapple: Data analysis
J. C. Theis: Data analysis, Writing the paper
ICMJE Conflict of Interest:
None declared
©2015 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









