Abstract
Objectives
We wanted to investigate regional variations in the organisms reported to be causing peri-prosthetic infections and to report on prophylaxis regimens currently in use across England.
Methods
Analysis of data routinely collected by Public Health England’s (PHE) national surgical site infection database on elective primary hip and knee arthroplasty procedures between April 2010 and March 2013 to investigate regional variations in causative organisms. A separate national survey of 145 hospital Trusts (groups of hospitals under local management) in England routinely performing primary hip and/or knee arthroplasty was carried out by standard email questionnaire.
Results
Analysis of 189 858 elective primary hip and knee arthroplasty procedures and 1116 surgical site infections found statistically significant variations for some causative organism between regions. There was a 100% response rate to the prophylaxis questionnaire that showed substantial variation between individual trust guidelines. A number of regimens currently in use are inconsistent with the best available evidence.
Conclusions
The approach towards antibiotic prophylaxis in elective arthroplasty nationwide reveals substantial variation without clear justification. Only seven causative organisms are responsible for 89% of infections affecting primary hip and knee arthroplasty, which cannot justify such widespread variation between prophylactic antibiotic policies.
Cite this article: Bone Joint Res 2015;4:181–189.
Article focus
- Prophylactic antibiotics are often administered in the peri-operative period to reduce the risk of infection following joint arthroplasty surgery.
Key messages
- There is widespread variation in use of prophylactic antibiotics for elective lower limb arthroplasty. This variation is not justified by any regional variation in the organisms believed to have caused peri-prosthetic joint infections.
Strengths and limitations
- Strengths - data on organisms thought to have caused surgical site infections were extracted from a national surveillance database. A survey of antibiotic prophylaxis regimens achieved responses from every NHS organisation performing elective hip and knee arthroplasty.
- Limitations - this cross-sectional study has identified an important public health problem (unjustified variation in practice) but cannot prove a link between intervention (antibiotic choice) and population-level outcomes (organism distribution).
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are two of the most commonly performed orthopaedic procedures. Annually, over 86 488 THAs and over 90 842 TKAs are performed in the United Kingdom, and over 231 000 THAs and 542 000 TKAs in the United States.1,2 Although they are safe and effective operations, prostheses can fail due to aseptic loosening, dislocation, fracture, or infection.3 Surgical site infection (SSI), which includes prosthetic joint infection (PJI), has a prevalence of 0.7% to 2.1% in primary THAs and 0.6% to 1.8% in primary TKAs.4-6 With high-quality post-discharge surveillance, median infection rates were estimated to be 1.6% and 2.4% for THA and TKA, respectively, from 2011 to 2012 in England.7 PJI is the reason for 14.8% of THA revisions and the most common indication (25.2%) for revising TKAs.8 Each PJI is estimated to cost $30 000 to $40 000 (£18 374 to £24Â 500) and this complication will account for 50% of all hospital resources used for revision TKA by 2016.9 It is therefore necessary to optimise the use of safe, effective, and low-cost interventions to reduce the burden of PJI after lower limb joint arthroplasty.10
Over half of PJIs are caused by Staphylococcus species, particularly Staphylococcus (S.) aureus and coagulase-negative staphylococci (CoNS).4 Met(h)icillin-resistant S. aureus (MRSA) is isolated from 8% of infected prostheses, and anaerobes are isolated from 7% of infected prostheses. However, retrospective case series have shown that up to 36% of prostheses infections are polymicrobial.11 As most organisms are commensal skin flora, they are presumed to have inoculated the prosthesis at joint implantation.10 Less commonly, organisms can spread haematogenously from distant sites, for example from the urinary tract.
Interventions to reduce rates of PJI include MRSA decolonisation and met(h)icillin-sensitive S. aureus (MSSA) decolonisation, pre-operative nutritional optimisation, good diabetic control, careful hair removal, instrument sterilisation and skin decontamination, laminar flow theatres, body exhaust suits, and antibiotic-impregnated cement. Another key intervention is the use of peri-operative prophylactic antibiotics.12-14
In a pooled analysis of seven studies, the administration of prophylactic antibiotics reduced the relative risk (RR) of wound infection by 81% (RR 0.19; 95% confidence interval (CI) 0.12 to 0.31). This translates to an absolute risk reduction of 8%, meaning that one wound infection would be prevented for every 13 people treated compared with no administration of antibiotics.15 It is, however, difficult to recommend a particular regimen based on current studies, which vary in drug selection, dose, timing, and use of post-operative antibiotics. Antibiotic regimens might carry different risks and side-effect profiles, e.g., hypersensitivity reactions (including anaphylaxis), acute kidney injury, and Clostridium difficile infection (CDI).16-19
The aims of this paper are to report the bacterial spectrum of infections across England, to document national variation in antibiotic prophylaxis for primary THA and TKA, to identify emerging trends in the use of specific regimens, and to recommend an optimal regimen based on current evidence.
Materials and Methods
Current pathogens in hip and knee arthroplasty infections in England
We analysed 189 858 elective primary hip and knee arthroplasty procedures and 1116 inpatient or re-admission SSIs submitted by 184 NHS hospitals (representing 142 NHS Trusts and ten independent NHS treatment centres) to the Public Health England (PHE) national SSI database between April 2010 and March 2013. As the survey on surgical antibiotic prophylaxis was carried out in 2013, the PHE organism data available at the time were for April 2012 to April 2013. The dataset was therefore expanded to include data from the previous two years in order to increase the sample size. Although mandatory orthopaedic data were collected from April 2004, the inclusion of historical data that predated various national policies on healthcare-associated infections would have introduced bias and over-estimation of the burden of S. aureus. Participating hospitals follow a standard protocol of internationally-recognised case definitions for superficial, deep, and organ-space SSIs. Hospitals also undertake systematic prospective follow-up for the capture of cases.20-22 The standard follow-up period is 30 days for superficial SSIs and up to one year (365 days) for deep and/or organ-space SSIs.
Since April 2004, all NHS Trusts in England have been required to undertake mandatory surveillance in orthopaedic surgery. The four orthopaedic categories are hip arthroplasty, knee arthroplasty, repair of the neck of the femur, and reduction of long-bone fracture. PHE manages the SSI surveillance programme and publishes the rates of SSI by an NHS Trust on an annual basis for the orthopaedic modules. Since public reporting of orthopaedic SSI is at Trust level, the minimum requirement is participation by one hospital site for at least one surveillance quarter in one of the four mandatory orthopaedic categories.
Data are submitted via the PHE secure web-based portal. All data are checked for errors using an inbuilt automated validation system. For example inconsistencies in date values are identified and flagged to the user. SSIs with insufficient SSI criteria entered or superficial SSIs detected beyond 30 days are disallowed to avoid over-reporting of SSIs that do not meet the standard case definitions. Reporting on causative micro-organisms is optional, but must be based on clinically-significant isolates.
The SSIs included in this analysis were those detected during the inpatient stay, or on re-admission following initial hospital discharge, as these methods of detection are a requirement for all participating hospitals. Other forms of post-discharge surveillance (patient wound healing questionnaires or follow-up through review or outpatient clinics) are optional and used inconsistently, and thus were excluded from this study.
We analysed superficial, deep, and organ-space SSI isolates together then conducted a separate subgroup analysis restricted to deep and organ-space SSI isolates. All English NHS Trusts participated in this mandatory orthopaedic surveillance in 2010/2011, three failed to do so in 2011/2012, and two in 2012/2013.22
The proportion of participating hospitals undertaking continuous surveillance (all four quarters) increased year on year, from 51% in 2010/2011 to 56% in 2012/2013 for hip prosthesis and from 52% to 55% for knee prosthesis over the same time period.22
The four PHE ‘super regions’ used were London, Midlands and the East of England, the North of England, and the South of England.
Data on primary indications involving trauma/fracture or revision surgery (including revisions for aseptic loosening) were excluded from this analysis. Other indications for surgery were included (avascular necrosis, inflammatory joint disease, osteoarthritis, revision, and other).
Current regimens for prophylaxis in England
As a separate initiative from PHE’s routine surveillance activities, all 144 acute hospital Trusts performing primary hip and knee arthroplasty in England were contacted. Responses were received from 100% of these Trusts. Information governance leads at each Trust were emailed a standard questionnaire in October 2013 and those that did not respond within 30 days were contacted by telephone. Telephone calls were to the duty microbiologist, antibiotic pharmacist, medicines information line, or orthopaedic junior doctor on call. In all cases, sources were asked for information from their hospital’s antibiotic policy. Where the individual contacted was not aware of the local policy, another individual from the list of suitable contacts was contacted.
Contacts were asked ‘Which antibiotics are given prophylactically to patients undergoing elective primary THA or TKA at induction and/or post-operatively? What modifications are made for patients with a serious allergy to penicillin or a history of MRSA infection/colonisation? What doses are administered? What are the dosing intervals? Are there any special circumstances specified in their guidelines, e.g. repeated doses for prolonged surgery or excessive loss of blood?’.
Non-normally distributed continuous data were described using medians with interquartile ranges. Fisher’s exact test was used to compare differences in categorical outcomes between groups as there were small numbers in some cells. Statistical analyses were performed using Stata 13.0 (StataCorp, College Station, Texas) and p < 0.05 (two-tailed) was adopted as the threshold for significance.
Results
Patient characteristics of primary elective hip or knee procedures
The distribution of the patients’ age, gender and ASA score distribution were broadly similar across the two modules. Patients aged 65 to 74 years accounted for the largest proportion of procedures. Within this group, there were more patients receiving a knee prosthesis, compared with those receiving a hip prosthesis (38.1% and 34.4%, respectively). Patients aged < 45 years accounted for the smallest proportion across both populations, however, it was slightly higher in the THA group (4% and 1%, respectively). Female patients accounted for a slightly higher proportion in the THA than the TKA group (60.2% and 58.0%, respectively). Patients with ASA score of 3 or more were similar across the hip and knee modules (19.4% and 20.3%, respectively).
The median time from procedure date to onset of SSI was based on monomicrobial SSIs. Overall the median time to onset of SSI was 18 days (interquartile range (IQR) 11 to 29, minimum and maximum 1 to 363). The median time to onset of S. aureus SSIs was 19 days (IQR 12 to 29, 1 to 362); 16 days for CoNS SSIs (IQR 11 to 27, 1 to 345) and 17 days for Enterobacteriaceae SSIs (IQR 11 to 25, 2 to 267).
Pathogens reported to cause hip and knee arthroplasty infections in England
There were 1116 inpatient/re-admission SSIs, of which 73.3% (n = 818) included data on causative micro-organisms (Table I). 73.8% (n = 604) of these SSIs had a monomicrobial aetiology (n = 604) and 26.2% (n = 214) were polymicrobial. SSIs with organism data yielded a total of 1083 isolates and, of these isolates, 69.1% (n = 748) related to deep and/or organ-space SSIs.
Table I
Micro-organisms reported as causing surgical site infection (SSI) following hip or knee prosthesis surgery (Apr 2010 to Mar 2013)
| London | Midland and East of England | North of England | South of England | England (total) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | n | % | n | % | n | % | n | % | n | % |
| All SSI isolates | MSSA | 25 | 20.2 | 88 | 27.2 | 87 | 26.9 | 91 | 29.2 | 291 | 26.9 |
| MRSA | 3 | 2.4 | 24 | 7.4 | 8 | 2.5 | 10 | 3.2 | 45 | 4.2 | |
| CoNS | 39 | 31.5 | 73 | 22.5 | 85 | 26.3 | 79 | 25.3 | 276 | 25.5 | |
| Enterobacteriaceae | 20 | 16.1 | 52 | 16.0 | 58 | 18.0 | 39 | 12.5 | 169 | 15.6 | |
| Other bacteria* | 10 | 8.1 | 35 | 10.8 | 26 | 8.0 | 40 | 12.8 | 111 | 10.2 | |
| Enterococcus spp. | 11 | 8.9 | 20 | 6.2 | 31 | 9.6 | 17 | 5.4 | 79 | 7.3 | |
| Streptococcus spp. | 5 | 4.0 | 24 | 7.4 | 17 | 5.3 | 21 | 6.7 | 67 | 6.2 | |
| Pseudomonas spp. | 11 | 8.9 | 6 | 1.9 | 11 | 3.4 | 14 | 4.5 | 42 | 3.9 | |
| Fungi | 0 | 0.0 | 2 | 0.6 | 0 | 0.0 | 1 | 0.3 | 3 | 0.3 | |
| Total | 124 | 100 | 324 | 100 | 323 | 100 | 312 | 100 | 1,083 | 100 | |
| Deep or organ-space SSI isolates | CoNS* | 23 | 31.5 | 51 | 24.6 | 71 | 30.5 | 64 | 27.2 | 209 | 27.9 |
| MSSA | 18 | 24.7 | 50 | 24.2 | 62 | 26.6 | 54 | 23.0 | 184 | 24.6 | |
| MRSA | 1 | 1.4 | 13 | 6.3 | 3 | 1.3 | 8 | 3.4 | 25 | 3.3 | |
| Enterobacteriaceae | 12 | 16.4 | 34 | 16.4 | 40 | 17.2 | 35 | 14.9 | 121 | 16.2 | |
| Other bacteria† | 5 | 6.8 | 20 | 9.7 | 19 | 8.2 | 32 | 13.6 | 76 | 10.2 | |
| Enterococcus spp. | 5 | 6.8 | 18 | 8.7 | 18 | 7.7 | 13 | 5.5 | 54 | 7.2 | |
| Streptococcus spp. | 3 | 4.1 | 18 | 8.7 | 12 | 5.2 | 19 | 8.1 | 52 | 7.0 | |
| Pseudomonas spp. | 6 | 8.2 | 2 | 1.0 | 8 | 3.4 | 9 | 3.8 | 25 | 3.3 | |
| Fungi | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 1 | 0.4 | 2 | 0.3 | |
| Total | 73 | 100 | 207 | 100 | 233 | 100 | 235 | 100 | 748 | 100 | |
-
* The majority in this group comprised diptheroids/Corynebacterium spp. (40%) followed by unidentified organisms (31%) † The majority in this group comprised diptheroids/Corynebacterium spp. (42%) followed by unidentified organisms (28%) MSSA, met(h)icillin-sensitive S. aureus; MRSA, met(h)icillin-resistant S. aureus; CoNs,coagulase-negative staphylococci
MSSA was the predominant pathogen across England, accounting for 27.0% of isolates (n = 291) followed by coagulase-negative staphylococci (CoNS) at 25.5% (n = 276). MRSA accounted for 4.2% (n = 45) of total isolates. The seven most common causative organisms accounted for 89% of all SSI isolates following THAs and TKAs across the four PHE super regions.
At regional level, staphylococci (MSSA, MRSA, and CoNS) accounted for 57% of isolates with a similar distribution across the PHE super regions. The burden of MRSA was, however, significantly higher in the Midlands and East of England compared with the other three regions (7.4% vs 2.8%; Fisher’s exact test; p = 0.001).
The burden of Pseudomonas spp. was significantly higher in London compared with the three regions combined (8.9% vs 3.2%; Fisher’s exact test: p = 0.005).
Sub-group analysis limited to deep and organ space SSIs (n = 748) found that CoNS were the predominant pathogens and accounted for 27% of isolates (n = 209) followed by MSSA at 25% (n = 184). MRSA accounted for 3.3% of these isolates. Overall, staphylococci accounted for 56% of isolates in this analysis. Stratified analyses by PHE super region showed that the burden of MRSA was also significantly higher in the Midlands and East of England region compared with the other three regions combined (6.3% vs 2.2%; Fisher’s exact test: p = 0.010). The burden of Pseudomonas spp. was also significantly higher in London compared with other three regions combined (8.2% vs 2.8%; Fisher’s exact test: p = 0.028).
The reasons for the regional differences in MRSA and Pseudomonas spp. are not entirely clear. However, laboratory data reported to PHE’s voluntary surveillance system (LabBase2) from 2010 to 2013 shows that the rate of infections in the bloodstream due to Pseudomonas spp. was consistently higher in London compared with the other three regions over his period even with the declining trend.23 The corresponding analysis for MRSA was not available although a separate report showing trends by smaller geographical units called Area Teams (ATs) showed that ATs within the Midlands and East England region did not show the highest rates of bloodstream infections due to MRSA than other ATs.24 The MRSA result from the SSI programme is perplexing and needs further study.
Current prophylaxis regimens in England
Routine prophylaxis
The three most common antibiotics or antibiotic combinations made up 126/146 (87%) of observed practice. Flucloxacillin in combination with gentamicin was the most common regimen, with 57/146 (39%) of Trusts using it as their preferred regimen. Cefuroxime was used as the preferred regimen by 44/146 (30%), with teicoplanin plus gentamicin being the third most popular 25/146 (17%). There were ten further preferred regimens used by the remaining 20 Trusts.
Figure 1 illustrates the range of routine prophylactic antibiotic regimens used throughout England. Two Trusts employed two different regimens in this category of patient based on age. Therefore the denominator is 146.
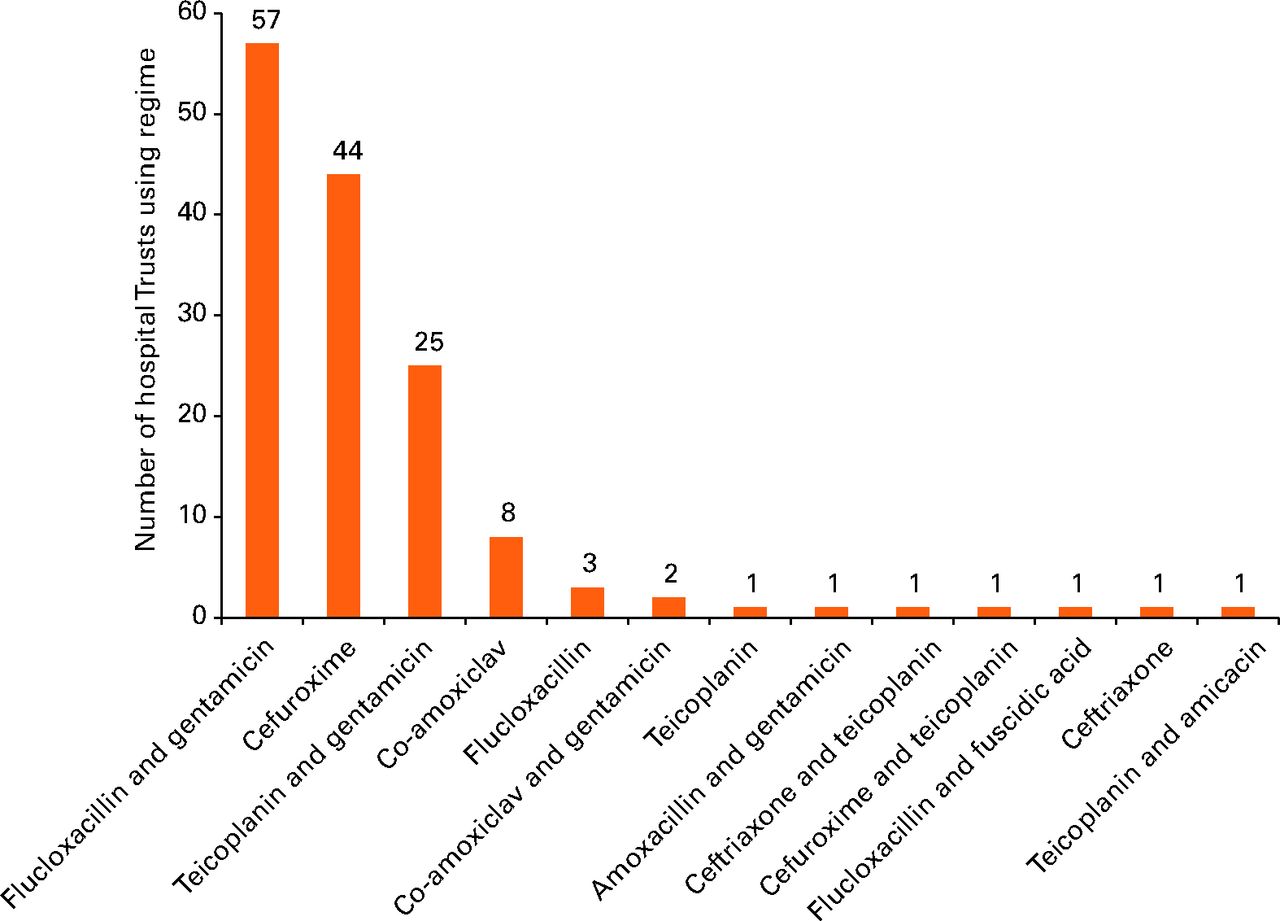
Fig. 1
Graph showing the prophylactic antibiotic regimens in general use for patients undergoing hip and knee arthroplasty.
Prophylaxis in patients with penicillin allergy
The two most common antibiotic/antibiotic combinations made up 128/145(88%) of observed practice. Teicoplanin in combination with gentamicin was the most common regimen, with 90/145 (62%) of Trusts using it as their preferred regimen. Teicoplanin alone was used as the preferred regimen by 36/145 (26%). There were 12 further preferred regimens used by the remaining 20 Trusts.
Figure 2 illustrates the spread of prophylactic antibiotic regimens employed throughout England for patients who are allergic to penicillin. One Trust employed two different regimens in this category. Therefore the denominator is 145.
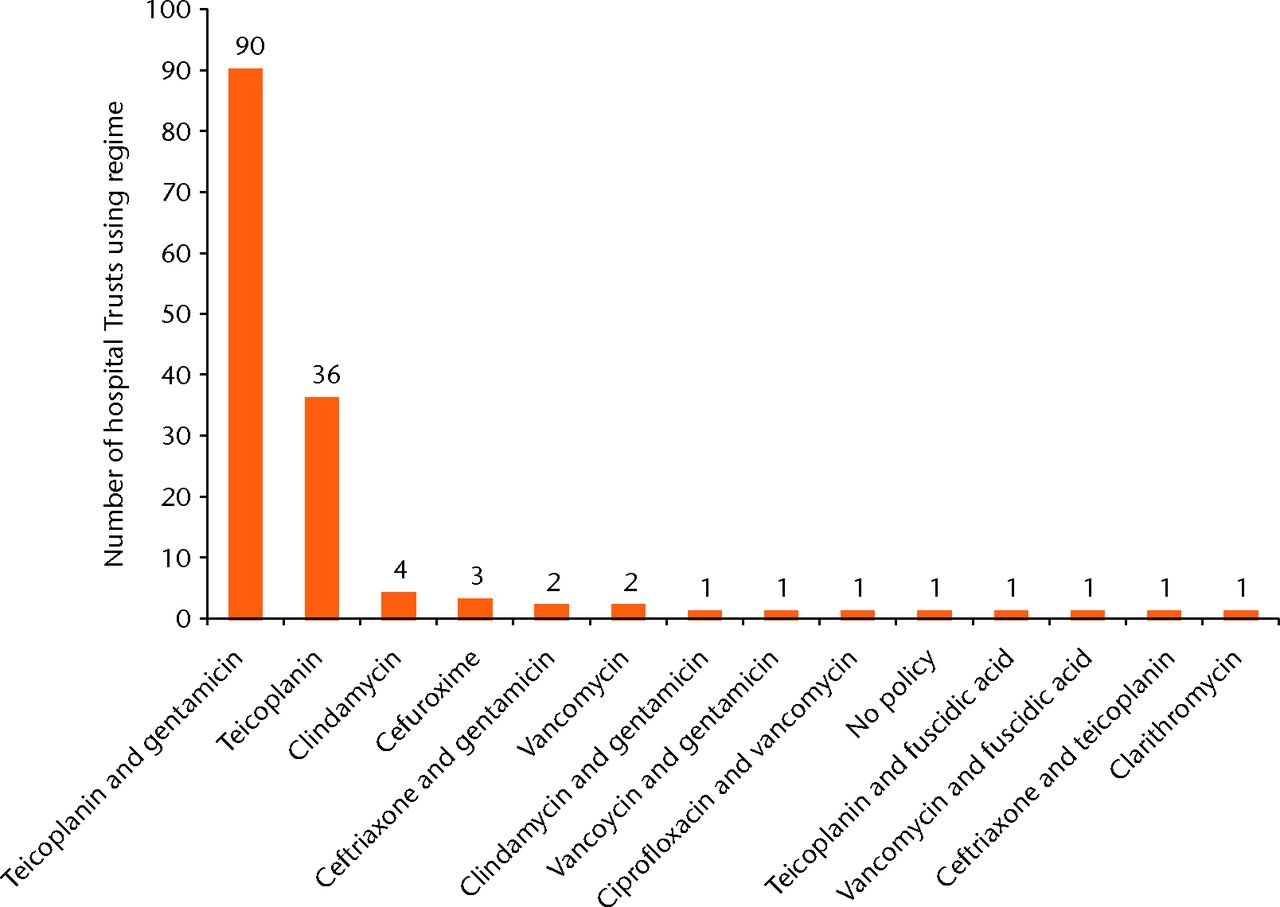
Fig. 2
Graph showing prophylactic antibiotic regimens in use for patients who are allergic to penicillin
Prophylaxis in patient with a high risk of developing MRSA SSI infection
The two most common antibiotic/antibiotic combinations made up 123/146 (84%) of observed practice. Teicoplanin, in combination with gentamicin, was the most common, with 87/146 (60%) of Trusts using it as their preferred regimen. Teicoplanin alone was used as the preferred regimen by 34/146 (24%). There were 11 further preferred regimens used by the remaining 20 Trusts.
Figure 3 illustrates the distribution of prophylactic antibiotic regimens employed throughout England for patients at high risk of developing MRSA SSI infection. Two Trusts employed two different regimens in this category, therefore the denominator is 146.
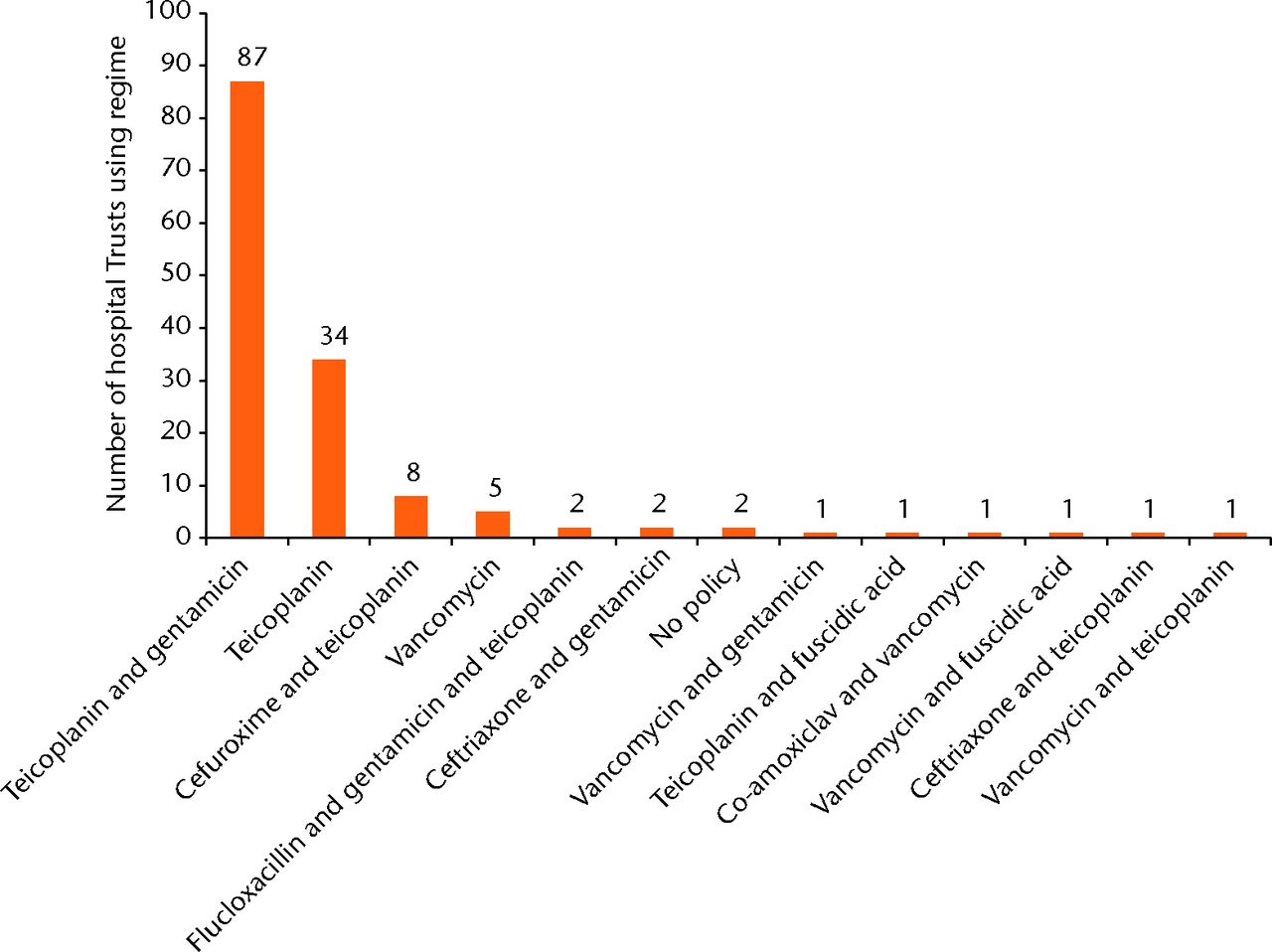
Fig. 3
Graph showing prophylactic antibiotic regimens in use for patients at a high risk of MRSA colonisation.
Discussion
Our study has shown that 89% of SSIs in hip and knee arthroplasty in England are reportedly caused by the same seven organisms. However, we also found higher rates of MRSA PJI in the Midlands and East of England and higher rates of Pseudomonas spp.PJI in London. Without further data, it is difficult to understand exactly why these trends exist. Centres reporting higher burdens of MRSA or pseudomonas may be tertiary referral centres for complex arthroplasties from elsewhere. These centres may therefore encounter larger volumes of patients with previous hospital admissions and antibiotic exposure that could render them more prone to infection with these organisms.
The low prevalence of SSI-related MRSA is against a background of falling numbers of SSIs caused by this organism, possibly related to national policies directed at reducing the MRSA.25 It is worth noting, however, that Trusts may choose to adopt these recommendations on an individual basis.
Although there is a large body of evidence for the use of prophylactic antibiotics in primary hip and knee arthroplasty, there is no clear benefit to using one particular agent/regimen.26,27 This is unsurprising, given that PJI is a rare event and that a randomised study would need over 3000 patients per group in order to demonstrate a reduction in the rate of infection from 2% to 1%, with a power of 90% at the 95% confidence interval.28 There are no randomised controlled trials available to guide the choice of any particular antibiotic regimen.
Current evidence
The evidence for different antibiotic regimens as prophylaxis for wound infections following joint arthroplasty surgery was last reviewed in 2005. This systematic review did not find any statistically significant difference in rates of infection when comparing cephalosporins with teicoplanin, cephalosporins with penicillin derivatives, or first-generation with second-generation cephalosporins. However, this review was based on poor-quality studies with variable follow-up and unsatisfactory definitions of infection.15
Dose and duration of therapy: cefuroxime
There is strong evidence for the use of 1.5 g cefuroxime at induction, however, two randomised controlled trials examining the effectiveness of post-operative doses of cefuroxime found no statistically significant difference in the prevention of SSIs.29,30
Dose and duration of therapy: flucloxacillin
Use of a single prophylactic dose of flucloxacillin (1g) is supported by one RCT in which it compared favourably with cefazolin in clean, semi-elective orthopaedic surgery involving the implantation of metal work.31
Dose and duration of therapy: gentamicin
There is no evidence for the use of systemic gentamicin as prophylaxis in primary elective THA and TKA surgery.
Dose and duration of therapy: teicoplanin
Four randomised controlled trials provide strong evidence for the use of a single dose of 400 mg of teicoplanin at induction.32-35 Although there is no evidence to suggest that higher doses or prolonged courses of treatment result in fewer SSIs, studies have shown that this dose may be inadequate for patients weighing over 70 kg.36
Complication profiles: cefuroxime
Although there is strong evidence for an association between cefuroxime and CDI in elderly inpatient populations and trauma patients receiving implantation of metal work, studies have not shown any association in the elective orthopaedic setting.18,37-39 Despite this, our analysis of PHE data showed that 25.5% of SSI isolates were reportedly due to CoNS and 4.2% to MRSA. Cefuroxime is ineffective against MRSA, and may not be effective against CoNS. Additional arguments against the continued use of cefuroxime include its lack of activity against enterococci and Pseudomonas spp, and the increasing number of infections caused by extended spectrum beta-lactamase-producing organisms.
Complication profiles: flucloxacillin with gentamicin
There has been an increase in the percentage of Trusts using flucloxacillin in combination with gentamicin – from 1.3% in 2005 to 38.4% in 2013.40The efficacy of gentamicin depends on local strains and sensitivities, but it is usually active against Enterobacteriaceae, Pseudomonas spp. and MRSA in the United Kingdom, although rates of resistance are increasing.
One large, good quality study showed that a single dose of gentamicin caused a significant increase in the number of patients suffering from transient acute kidney injury (AKI).18 Another non-randomised study found an association between combined high-dose flucloxacillin with single-dose gentamicin and renal impairment; including three patients that subsequently required short-term haemodialysis.41This was supported by a prospective study in 2013, which showed a highly significant increase in patients suffering AKI – from 1.7% with cefuroxime to 9.5% with combined flucloxacillin and gentamicin.41
Complication profiles: teicoplanin with gentamicin
Use of teicoplanin alone is not associated with significant complications, although it may cause AKI when combined with gentamicin. Advantages of teicoplainin over vancomycin include a reduced risk of nephrotoxicity and a quicker speed of pre-operative intravenous administration, despite increased time for reconstitution. It is administered as a five-minute intravenous bolus, rather than a one-hour infusion. Teicoplanin is highly active against both MRSA and MSSA, although resistance is increasing.28 This regime is also useful in those who are allergic to penicillin.
The limitations of this study include the absence of data on the use of antibiotic impregnated cement, and that microbial profiles provided by super regions may not directly correspond with causative organism distribution at Trust level, due to variation between Trusts in each region. Reporting on micro-organism is optional, and may have been a potential source of bias. However, the microbial aetiology among deep-seated SSIs was similar to the overall analysis, and so the possibility of bias in the type of organism being reported does not seem convincing. In addition, there is no guarantee that the antibiotics administered as prophylaxis always comply with Trust protocols.
The use of an ecological analysis to examine the correlation between interventions and outcomes at population level (prophylaxis choice and organism distribution) does not establish cause and effect. However, it has been useful in defining a problem in public health (variation in practice) for future investigation.
In conclusion, this survey outlines current practice with regard to antibiotic prophylaxis for elective primary TKA and THA in England. It reveals a disparity in the choice of antibiotic(s), duration of therapy and interpretation of the available evidence in the literature without clear justification. A number of regimens currently in use do not appear to take account of the most recent evidence, and could potentially result in avoidable complications and adverse events. The median time to onset of SSI suggests that a prophylaxis regimen is a relevant factor, hence practice changes remain indicated. Extremely late onset of SSI occurs much less frequently and, for these patients, surgical antibiotic prophylaxis practices may be less relevant, although this should not preclude optimisation of peri-operative practices.
Despite the lack of high-level evidence proving one antibiotic superior to others, there have been efforts in North America to establish a consensus. In Canada, a 2009 survey of antibiotic prophylaxis for total joint arthroplasty (TJA) surgery showed that 97.3% of surgeons surveyed routinely administered cefazolin as their first line prophylaxis.42 In the United States, the American Academy of Orthopaedic Surgeons recommend that cefazolin or cefuroxime are the preferred intravenous antibiotics to be used as prophylaxis in primary TJA.43 With rates of SSI reported as approximately 1% in the United States and Canada, their results are comparable with the United Kingdom and Europe. It is therefore difficult to understand why such variation exists in the United Kingdom. One possibility is that antibiotic choice is influenced by organisational aversion to certain antibiotics, e.g., following high-profile CDI outbreaks. For example, those that are averse to cephalosporins may be more likely to use the dual combination flucloxacillin and gentamicin. The 2014 English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report includes a national survey on antimicrobial stewardship in secondary care in 2014. A total of 99 (67.8%) of 146 contacted acute NHS Trusts responded to the survey. Of those, 98% of Trusts reported the use of surgical antibiotic prophylaxis policy. However, just over 80% of Trusts implemented audits of compliance to antibiotic guidelines (dose, route and duration). Although this is high further improvement in the process, monitoring is needed as this activity is key in order to influence changes in practice. The report also found that 24% of survey respondents had a written antimicrobial education and training strategy.23 These results may, in part, explain the variation in surgical prophylaxis that we observed in our study. Another explanation that could account for some of the variation in regimens is that it could reflect local analyses of infecting organisms. There is, however, no obvious reason why the United Kingdom should not aim to establish a consensus in the same way as has been achieved in North America.
It is arguable that the financial burden, morbidity, and mortality associated with SSIs following THA and TKA are sufficient to support efforts to undertake further research in this field. This research should focus on establishing the safest regimen/dose, by comparing complications such as SSI, AKI, CDI rates, and rates of MRSA infection. We would suggest that the National Joint Registry collect data on antibiotic(s) used including dose, route, duration, and timings. This data could then be combined with that already collected by PHE including rates of SSI, CDI and MRSA.
1 No authors listed. 10th Annual Report 2013.National Joint Registry for England, Wales and Northern Ireland. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/10th_annual_report/NJR%2010th%20Annual%20Report%202013%20B.pdf (date last accessed 16 Septemeber 2015). Google Scholar
2 DeFrances CJ , LucasCA, BuieVC, GolosinskiyANational Health Statistics Reports, Number 5. http://www.cdc.gov/nchs/data/nhsr/nhsr005.pdf (date last accessed 16 September 2015). Google Scholar
3 Hunt LP , Ben-ShlomoY, ClarkEM, et al.90-day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet2013;382:1097–1104.CrossrefPubMed Google Scholar
4 No authors listed. Public Health England - Surveillance of surgical site infections in NHS hospitals in England, 2012/2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/364319/SSI_annual_report_2012_to_13_final.pdf (date last accessed 13 November 2015). Google Scholar
5 No authors listed. European Centre for Disease Prevention and Control - Surveillance of surgical site infections in Europe, 2010-2011. Stockholm: European Centre for Disease Prevention and Control, 2013. http://ecdc.europa.eu/en/publications/Publications/SSI-in-europe-2010-2011.pdf (date last accessed 13 November 2015). Google Scholar
6 Lamagni T , ElgohariS, HarringtonP. Trends in surgical site infections following orthopaedic surgery. Curr Opin Infect Dis2015;28:125–132.CrossrefPubMed Google Scholar
7 Tanner J , PadleyW, KiernanM, et al.A benchmark too far: findings from a national survey of surgical site infection surveillance. J Hosp Infect2013;83:87–91.CrossrefPubMed Google Scholar
8 Bozic KJ , KurtzSM, LauE, et al.The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg [Am]2009;91-A:128–133.CrossrefPubMed Google Scholar
9 Kurtz SM , OngKL, SchmierJ, et al.Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg [Am]2007;89-A(suppl 3):144–151.CrossrefPubMed Google Scholar
10 Illingworth KD , MihalkoWM, ParviziJ, et al.How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg [Am]2013;95-A:50.CrossrefPubMed Google Scholar
11 Moran E , MastersS, BerendtAR, et al.Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect2007;55:1–7.CrossrefPubMed Google Scholar
12 Peersman G , LaskinR, DavisJ, PetersonM. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res2001;392:15–23.PubMed Google Scholar
13 Greene KA , WildeAH, StulbergBN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty1991;6:321–325.CrossrefPubMed Google Scholar
14 Johnson R , JamesonSS, SandersRD, et al.Reducing surgical site infection in arthroplasty of the lower limb: A multi-disciplinary approach. Bone Joint Res2013;2:58–65.CrossrefPubMed Google Scholar
15 AlBuhairan B , HindD, HutchinsonA. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg [Br]2008;90-B:915–919.CrossrefPubMed Google Scholar
16 Campagna JD , BondMC, SchabelmanE, HayesBD. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med2012;42:612–620.CrossrefPubMed Google Scholar
17 Challagundla SR , KnoxD, HawkinsA, et al.Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant2013;28:612–619.CrossrefPubMed Google Scholar
18 Sprowson A , SymesT, KhanSK, OswaldT, ReedMR. Changing antibiotic prophylaxis for primary joint arthroplasty affects postoperative complication rates and bacterial spectrum. Surgeon2013;11:20–24.CrossrefPubMed Google Scholar
19 Jenkins PJ , TeohK, SimpsonPM, et al.Clostridium difficile in patients undergoing primary hip and knee replacement. J Bone Joint Surg [Br]2010;92-B:994–998.CrossrefPubMed Google Scholar
20 No authors listed. Public Health England -Protocol for the surveillance of surgical site infection, version 6. London: Public Health England, 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/364412/Protocol_for_surveillance_of_surgical_site_infection_June_2013.pdf (date last accessed 13 November 2015). Google Scholar
21 No authors listed. National Healthcare Safety Network: surgical site infection (SSI) event. http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf (date last accessed 6 September 2015. Google Scholar
22 No authors listed. Health Protection Agency -surveillance of surgical site infection in NHS hospitals in England, 2010/11 and 2012/13. https://www.gov.uk/government/publications/surgical-site-infections-ssi-surveillance-nhs-hospitals-in-england (date last accessed 13 November 2015). Google Scholar
23 No authors listed. Public Health England - English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) Report 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/362374/ESPAUR_Report_2014__3_.pdf (date last accessed 13 November 2015). Google Scholar
24 No authors listed. Public Health England - annual epidemiological commentary: Mandatory MRSA, MSSA and E. coli bacteraemia and C. difficile infection data, 2014/15. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/442952/Annual_Epidemiological_Commentary_FY_2014_2015.pdf(date last accessed 13 November2015). Google Scholar
25 No authors listed. Public Health England -surveillance of surgical site infection in NHS hospitals in England and Wales 2013/14. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/386927/SSI_report_2013_14_final__3_.pdf (date last accessed 13 November 2015). Google Scholar
26 Glenny A , SongF. Antimicrobial prophylaxis in total hip replacement: a systematic review. Health Technol Assess1999;3:1–57.PubMed Google Scholar
27 Meehan J , JamaliAA, NguyenH. Prophylactic antibiotics in hip and knee arthroplasty. J Bone Joint Surg [Am]2009;91-A:2480–2490.CrossrefPubMed Google Scholar
28 Periti P , MiniE, MosconiG. Antimicrobial prophylaxis in orthopaedic surgery: the role of teicoplanin. J Antimicrob Chemother1998;41:329–340.CrossrefPubMed Google Scholar
29 Ritter MA , CampbellE, KeatingEM, FarisPM. Comparison of intraoperative versus 24 hour antibiotic prophylaxis in total joint replacement. A controlled prospective study. Orthop Rev1989;18:694–696.PubMed Google Scholar
30 Wymenga A , van HornJ, TheeuwesA, MuytjensH, SlooffT. Cefuroxime for prevention of postoperative coxitis. One versus three doses tested in a randomized multicenter study of 2,651 arthroplasties. Acta Orthop Scand1992;63:19–24.CrossrefPubMed Google Scholar
31 Van Meirhaeghe J , VerdonkR, VerschraegenG, et al.Flucloxacillin compared with cefazolin in short-term prophylaxis for clean orthopedic surgery. Arch Orthop Trauma Surg1989;108:308–313.CrossrefPubMed Google Scholar
32 Mollan RA , HaddockM, WebbCH. Teicoplanin vs cephamandole for antimicrobial prophylaxis in prosthetic joint implant surgery: (preliminary results). Eur J Surg Suppl1992;567:19–21.PubMed Google Scholar
33 Periti P , StringaG, DonatiL, et al.Teicoplanin--its role as systemic therapy of burn infections and as prophylaxis for orthopaedic surgery. Eur J Surg Suppl1992;567:3–8.PubMed Google Scholar
34 Suter F , AvaiA, FuscoU, et al.Teicoplanin versus cefamandole in the prevention of infection in total hip replacement. Eur J Clin Microbiol Infect Dis1994;13:793–796.CrossrefPubMed Google Scholar
35 Wall R , KlenermanL, McCulloughC, FyfeI. A comparison of teicoplanin and cefuroxime as prophylaxis for orthopaedic implant surgery: a preliminary report. J Antimicrob Chemother1988;21(suppl A):141–146.CrossrefPubMed Google Scholar
36 Tobin CM , LoveringAM, SweeneyE, MacGowanAP. Analyses of teicoplanin concentrations from 1994 to 2006 from a UK assay service. J Antimicrob Chemother2010;65:2155–2157.CrossrefPubMed Google Scholar
37 Jenkins PJ , TeohK, SimpsonPM, et al.Clostridium difficile in patients undergoing primary hip and knee replacement. J Bone Joint Surg [Br]2010;92-B:994–998.CrossrefPubMed Google Scholar
38 Al-Obaydi W , SmithCD, FoguetP. Changing prophylactic antibiotic protocol for reducing Clostridium difficile-associated diarrhoeal infections. J Orthop Surg (Hong Kong)2010;18:320–323.CrossrefPubMed Google Scholar
39 Challagundla S , KnoxD, HawkinsA, et al.Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant2012;0:1–7.CrossrefPubMed Google Scholar
40 Aujla RS , BrysonDJ, GuliharA, TaylorGJ. Trends in orthopaedic antimicrobial prophylaxis in the UK between 2005 and 2011. Ann R Coll Surg Engl2013;95:495–502.CrossrefPubMed Google Scholar
41 Bailey O , TorkingtonMS, AnthonyI, et al.Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J2014;96-B:395–398.CrossrefPubMed Google Scholar
42 Beer J , PetruccelliD, RotsteinC, et al.Antibiotic prophylaxis for total joint replacement surgery: results of a survey of Canadian orthopaedic surgeons. Can J Surg2009;52:229–234. Google Scholar
43 No authors listed. American Association of Orthopaedic Surgeons Information statement. Recommendations for the use of intravenous antibiotic prophylaxis in primary total joint arthroplasty. http://www.aaos.org/about/papers/advistmt/1027.asp (date last accessed 16 September 2015). Google Scholar
Funding statement:
M. R. Reed reports funding received by Newcastle University and Northumbria Healthcare NHS Foundation Trust from Ethicon, who funded this trial, and approved the trial’s design and manuscript. He also reports funding received from Zimmer, Heraeus cement, Convatec, AHSN, The Health Foundation, Stryker, Orthopaedic Research UK and AR-UK, none of which is related to this article.
Mr Sprowson sadly died in March 2015.
Author contributions:
C. J. Hickson: Study design, data collection, analysis and interpretation, drafted manuscript
D. Metcalfe: Study design, data collection, analysis and interpretation, critical revisions
S. Elgohari: Provided PHE data, data analysis and interpretation, critical revisions
T. Oswald: Study design, data interpretation, critical revisions
J. P. Masters: Study design, data interpretation, critical revisions
M. Rymaszewska: Undertook a feasibility study, data collection, and critical revisions
M. R. Reed: Study design, data interpretation, critical revisions
A. P. Sprowson† : Study design, data interpretation, critical revisions
ICMJE Conflict of Interest:
None declared
©2015 Hickson et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
Supplementary material. Further information and tables detailing antibiotic prophylaxis regimes, including dose, duration and most commonly used regimes, in routine, penicillin allergy, and cases presenting a high risk of MRSA, as well as a map demonstrating the ‘super regions’ can be found alongside the online version of this article at www.bjr.boneandjoint.org.uk









