Abstract
Objective
Dunkin Hartley guinea pigs, a commonly used animal model of osteoarthritis, were used to determine if high frequency ultrasound can ensure intra-articular injections are accurately positioned in the knee joint.
Methods
A high-resolution small animal ultrasound system with a 40 MHz transducer was used for image-guided injections. A total of 36 guinea pigs were anaesthetised with isoflurane and placed on a heated stage. Sterile needles were inserted directly into the knee joint medially, while the transducer was placed on the lateral surface, allowing the femur, tibia and fat pad to be visualised in the images. B-mode cine loops were acquired during 100 µl. We assessed our ability to visualise 1) important anatomical landmarks, 2) the needle and 3) anatomical changes due to the injection.
Results
From the ultrasound images, we were able to visualise clearly the movement of anatomical landmarks in 75% of the injections. The majority of these showed separation of the fat pad (67.1%), suggesting the injections were correctly delivered in the joint space. We also observed dorsal joint expansion (23%) and patellar tendon movement (10%) in a smaller subset of injections.
Conclusion
The results demonstrate that this image-guided technique can be used to visualise the location of an intra-articular injection in the joints of guinea pigs. Future studies using an ultrasound-guided approach could help improve the injection accuracy in a variety of anatomical locations and animal models, in the hope of developing anti-arthritic therapies.
Cite this article: Bone Joint Res 2015;4:1–5.
Article focus
Dunkin Hartley guinea pigs develop osteoarthritis spontaneously and have small knee joints.
Injections into the joint space of these animals are often performed without confirmation that the injection is delivered to the correct location.
Can high frequency ultrasound be used to improve intra-articular knee injections in small animals?
Key messages
High frequency ultrasound can be used to visualise intra-articular needle placement and injections in the knee joints of guinea pigs.
Separation of the fat pad showed fluid accumulation near the cartilage surface, confirming injections were in the correct location.
This imaging method could be used with other animal models to ensure the correct positioning of injections when developing anti-osteoarthritic therapies.
Strengths and limitations
Strength: Real-time visualisation of needle placement and intra-articular injections with ultrasound can be used to improve accuracy and confirm delivery location of anti-arthritic therapies.
Limitation: We were not able to visualise 25% of the injections due to the small size of the knee joint, difficulties with probe positioning, variation in the location of the needle tip and unanticipated animal limb movement during the injection. More practice and longer recordings could improve this rate of visualisation.
Introduction
Osteoarthritis is a chronic joint condition characterised by joint pain, crepitus, reduction of range of movement and variable degrees of inflammation, cartilage erosion and subchondral changes in bone. It is estimated that somewhere between 15% and 20% of the entire US population suffers from some form of arthritis.1 While osteoarthritis occurs at multiple locations, cartilage breakdown in the knee leads to significant comorbidities since these are weight-bearing joints.2 The development of locally injected anti-arthritic drugs to limit or even prevent cartilage breakdown is a current area of research,3-6 but it is difficult to ensure that injected material is deposited adjacent to cartilage in the synovial space. These false positives can lead to the question of whether a therapy is not working correctly or has simply not been delivered to the right location. Previous clinical studies in humans have demonstrated that ultrasound guidance will improve the accuracy of an intra-articular injection,7-10 but similar studies that focus on animal disease models have not been conducted. Indeed, the small size of many laboratory animals requires high frequency ultrasound capable of producing images with good image resolution at shallow depths.11-13
Dunkin Hartley guinea pigs develop spontaneous, age-related osteoarthritis, characterised by cartilage degeneration, osteophyte formation, subchondral bone changes and synovitis, making these animals a popular disease model for the human condition.14 These albino guinea pigs have histological evidence of osteoarthritis as early as three months old and disease severity continues to increase with age. Previous work has shown that proteoglycan content increases, collagen concentration in cartilage decreases, and radiological changes (including osteophyte formation, sclerosis of subchondral bone, femoral condyle cyst formation and calcification of collateral ligaments) present more in older animals.14 At the level of the tibial plateau, their knee joints are typically 2Â cm to 3 cm in the cranio-caudal direction and 1 cm to 2Â cm in the medio-lateral direction. The joint is < 1 cm below the skin’s surface in the parapatellar region. These features thus make visualisation with high frequency ultrasound possible.
The purpose of this study was to determine if high frequency ultrasound could be used to ensure intra-articular injections were correctly deposited in the knee joint. While we focused on the knees of Dunkin Hartley guinea pigs, the methods of ultrasound-guided injection described here could be used to improve accuracy of injection in a variety of small animal models. Indeed, joint visualisation should be possible as long as the anatomy allows for both needle insertion and placement of the ultrasound transducer head. We did not focus on direct visualisation of the needle with ultrasound, but rather the movement and changes within the intra-articular space in order to quantify the location of the injection. Future small animal studies using this ultrasound-guided approach can help confirm intra-articular deposition of injected fluid and aid in the development of anti-arthritic therapies.
Materials and Methods
A total of 216 injections in 36 Dunkin Hartley guinea pigs were imaged for this study: 12 animals received one injection bilaterally, 12 animals received three injections bilaterally, and 12 animals received five injections bilaterally. The right stifle joints were injected with 100 μl of phosphate-buffered saline and the left joints with 100 μl of a proteoglycan (aggrecan) mimetic. Animals were anaesthetised with 2% to 5% isoflurane in 2 L/min O2 using a chamber and a mask. The hair covering both knee joints was removed with clippers and the skin was scrubbed with chlorhexidine gluconate (2% dilution) and sterile saline. The rate of respiration was monitored visually and each animal was placed on a heated stage to maintain body temperature. Depth of anaesthesia was assessed with periodic toe pinches. Alcohol swabs were used to clean the transducer and stage between animals.
A high-resolution small animal ultrasound system was used for the image-guided injections (Vevo2100, FUJIFILM VisualSonics Inc., Toronto, Canada). A 256-element, 40 MHz linear transducer with a 7.0 mm geometric focus (MS550D) was clamped to an adjustable bench-mounted rail system designed for the positioning of a small animal. The axial, lateral and elevation image resolution were 40Â μm, 90 μm and 193 μm, respectively. Guinea pigs were positioned in dorsal recumbency on the heated stage such that the knee joint could be easily manipulated (Fig. 1). A 256-element, 40 MHz linear transducer with a 7.0 mm geometric focus (MS550D) was clamped to an adjustable bench-mounted rail system designed for the positioning of a small animal. The axial, lateral and elevation image resolution were 40Â μm, 90 μm and 193 μm, respectively. Guinea pigs were positioned in dorsal recumbency on the heated stage such that the knee joint could be easily manipulated (Fig. 1) was clamped to an adjustable bench-mounted rail system designed for the positioning of a small animal. The axial, lateral and elevation image resolution were 40Â μm, 90 μm and 193 μm, respectively. Guinea pigs were positioned in dorsal recumbency on the heated stage such that the knee joint could be easily manipulated (Fig. 1).
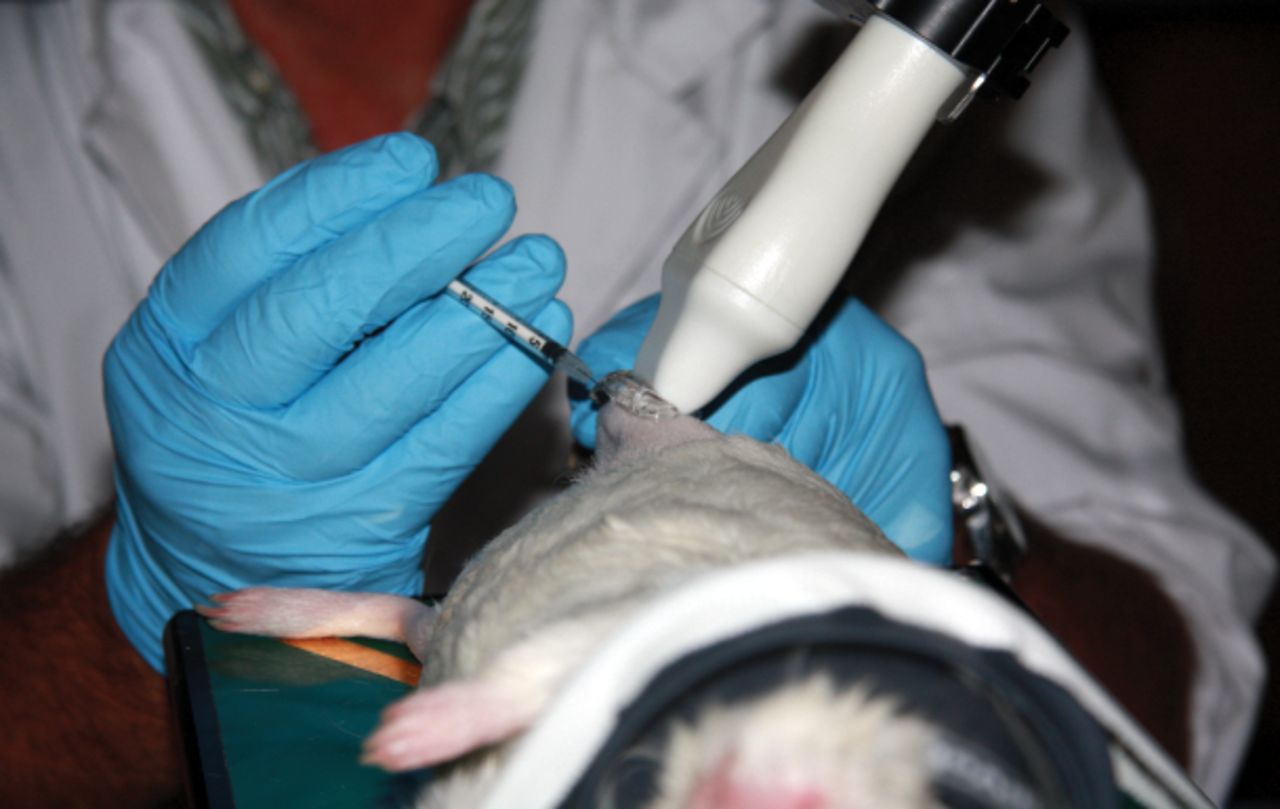
Fig. 1
Photograph of intra-articular injections in the right knee of a guinea pig. Image shows the ultrasound transducer, needle and knee during the injection. The right hand of the operator held the syringe that was inserted in the joint, while the left hand controlled the positioning of the limb of the guinea pig. The ultrasound probe, clamped to an adjustable rail system (not shown), was oriented in a craniolateral-caudomedial oblique direction.
A sterile 28-gauge needle was then inserted approximately halfway between the patella and tibial tuberosity, just medial to the patellar tendon. The needle was inserted into the knee joint until it was in direct contact with the medial condyle of the femur, without the benefit of ultrasound visualisation (Fig. 1). We then applied sterile ultrasound gel over the lateral joint area before lowering the ultrasound probe to the animal. Moderate extension of the joint and firmly pushing the back of the leg against the transducer surface is crucial to visualise the tibial plateau, medial femoral condyle and fat pad at the same time. We further refined the joint position until lucent lines separating the fat pad and tibia and fat pad and femur, representing joint space and articular cartilage, were clearly distinguishable (Fig. 2). The needle was inserted at an angle of 45° to the long axis of the ultrasound transducer in order to image the entire joint, including the femur, tibia and fat pad. Once an acceptable orientation was obtained, a 500-frame B-mode cine loop of approximately five seconds was acquired during the 100 μl injection of aggrecan mimic or PBS (control). For each animal, one knee received aggrecan mimic injections while the contralateral joint received PBS injections. We then assessed our ability to visualise 1) important anatomical landmarks, 2) the needle and 3) anatomical changes due to the injection.
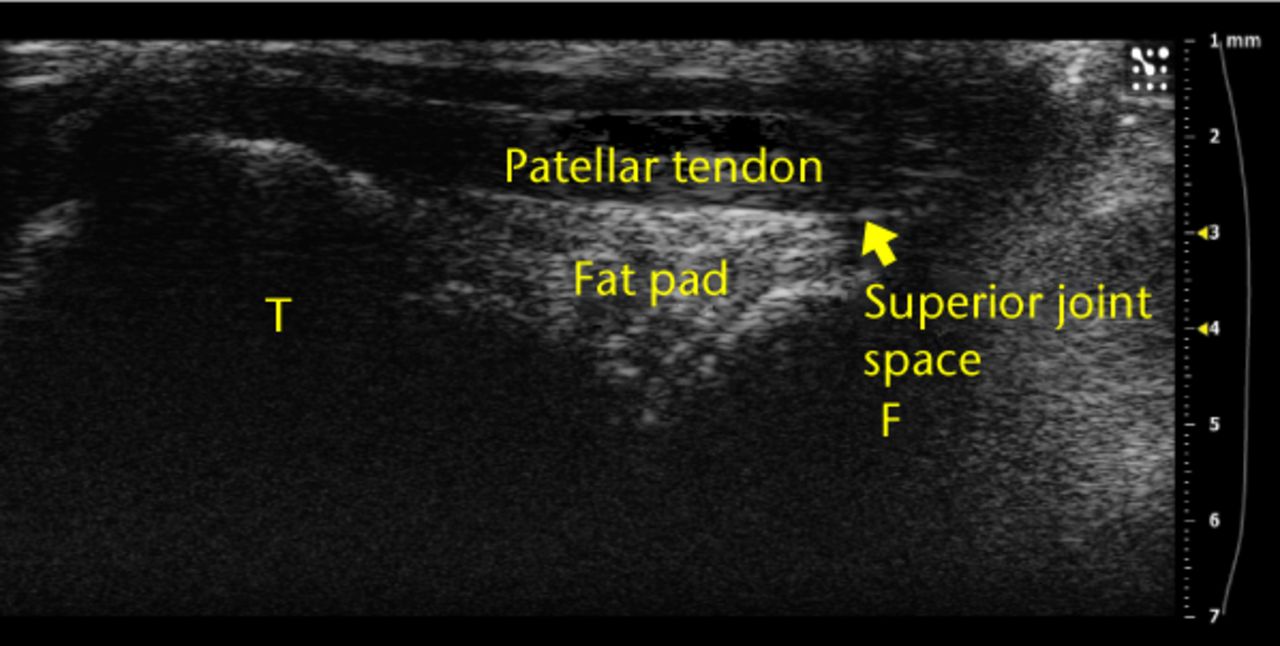
Fig. 2
Example ultrasound image of a knee joint with labels over the femur (‘F’), tibia (‘T’), superior joint space and fat pad. These anatomical landmarks were observed for each injection.
Results
Anatomical landmarks of the knee joint could be distinguished from the ultrasound images in all animals (Fig. 2) and clearly visualised in 75% of the injections (Table I). We defined ‘visualised’ injections as those where separation, movement, or expansion due to the build-up of intra-articular fluid was observed of the fat pad, of the joint space dorsal to the patella and trochlea, or of the patellar tendon. Separation of the fat pad was identified when the fat pad was physically pushed away from the femur or tibia (Fig. 3). We defined ‘visualised’ injections as those where separation, movement, or expansion due to the build-up of intra-articular fluid was observed of the fat pad, of the joint space dorsal to the patella and trochlea, or of the patellar tendon. Separation of the fat pad was identified when the fat pad was physically pushed away from the femur or tibia (Fig. 3). Dorsal joint expansion was identified when fluid or bubbles moved into the joint space proximal to the patella or trochlea. Finally, movement of the patellar tendon was identified as injections that led to a pressure increase that caused the tendon to bend or arch. No differences between injections of aggrecan mimic or PBS were observed. Table I summarises our results by identifying and characterising several anatomical features and observed movement due to the injections.
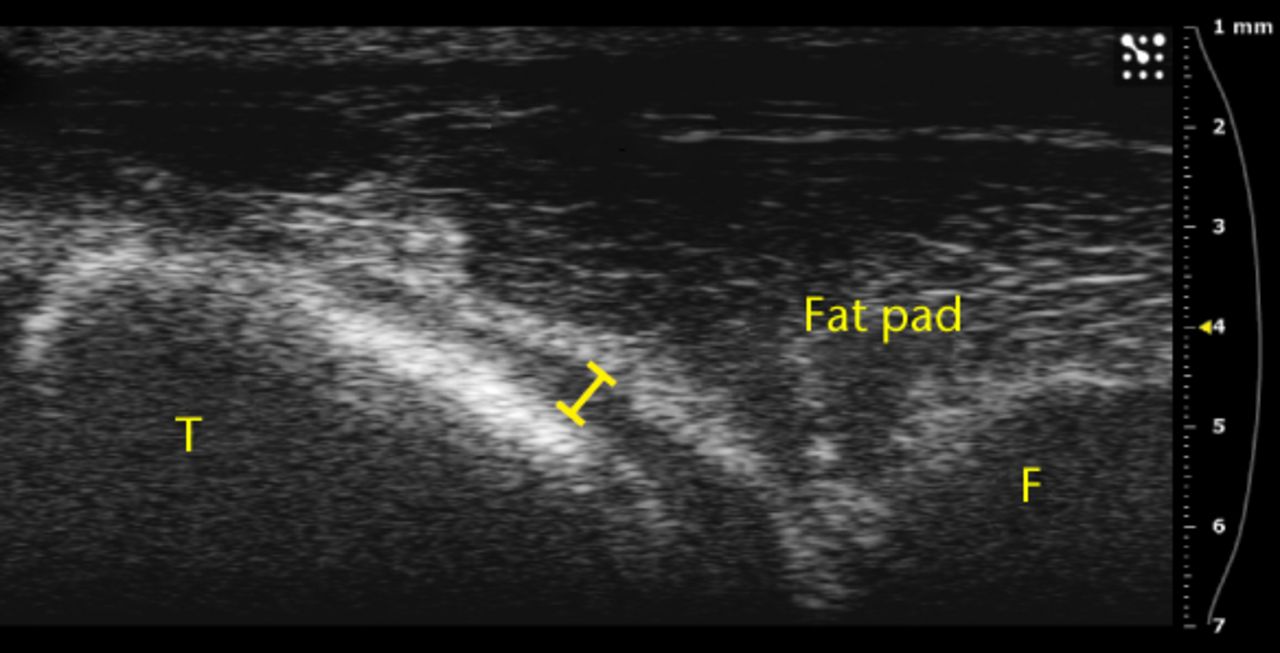

Figs. 3a - 3b
Ultrasound images showing substantial separation of the fat pad from the tibia a) before and b) after an injection.
Table I
Summary of total injections visualised and anatomical landmarks observed. We quantified the number and percentage of injections where the needle or movement of the fat pad, superior joint space and/or patellar tendon was clearly observed.
| Number of injections | Visualised | Needle observed | Fat pad observed | Separation of fat pad | Superior joint space observed | Superior joint space expansion | Patellar tendon observed | Patellar tendon movement | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 216 | 162 (75.0) | 21 (9.7) | 205 (95.0) | 145 (67.1) | 108 (50.0) | 50 (23.1) | 92 (42.6) | 22 (10.2) |
The vast majority of the ultrasound images where the injection was visualised also showed separation of the fat pad (145 of 162; 89.5%), providing confidence that the injected fluid was delivered to the correct location. Furthermore, proximal/superior joint expansion was observed both with and without separation of the fat pad. Only 12 of the 50 injections where dorsal joint space expansion was observed occurred without separation of the fat pad. We also observed small bubbles in 28 of the images (Fig. 4a), providing confidence that the injected fluid was delivered to the correct location. Furthermore, proximal/superior joint expansion was observed both with and without separation of the fat pad. Only 12 of the 50 injections where dorsal joint space expansion was observed occurred without separation of the fat pad. We also observed small bubbles in 28 of the images (Fig. 4a), an occurrence that increased echogenicity of the injected fluid and helped with visualisation. Finally, five injections led to subcutaneous expansion below the skin surface but above the patellar tendon (Fig. 4b).

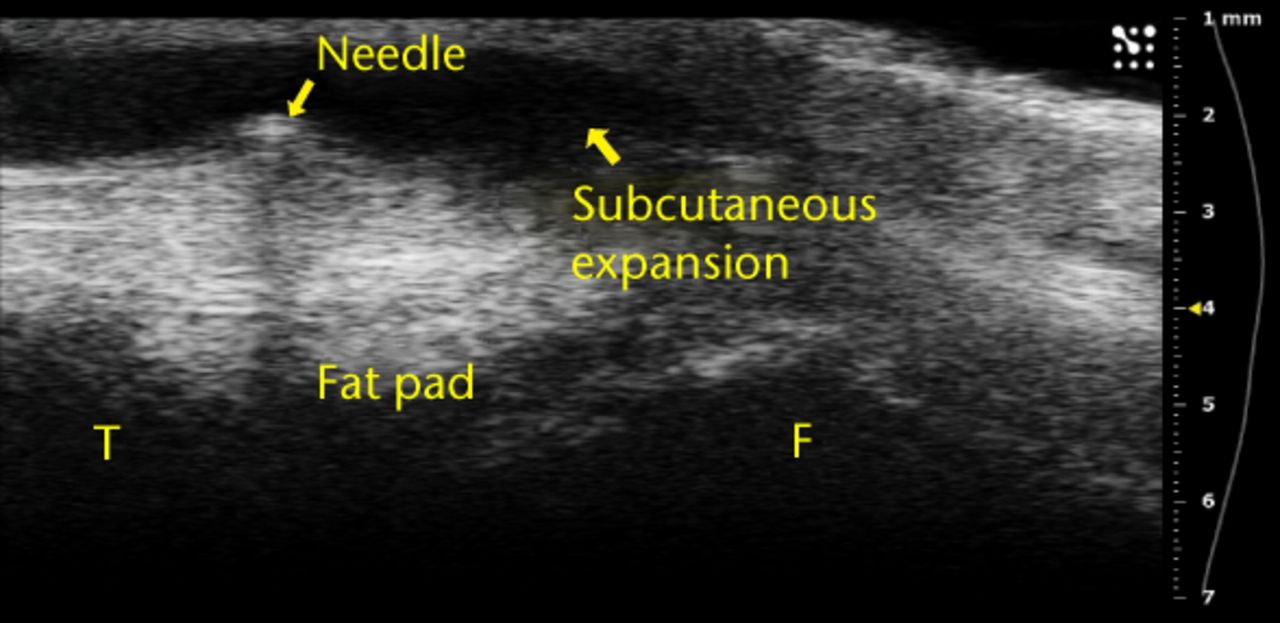
Figs. 4a - 4b
Ultrasound images showing a) that bubbles helped visualise the injections and were clearly observed in the joint space between femur and fat pad. The needle b) and subsequent metal shadow are clearly seen during an injection that created subcutaneous expansion.
Discussion
The results of this study suggest that high frequency ultrasound can be used to measure the accuracy of intra-articular injections in the knee joints of Dunkin Hartley guinea pigs. Because of the size of the stifle joint and the need to maintain aseptic technique, insertion of the needle under ultrasound guidance was not possible. However, we were able to demonstrate that 75% of the injections were deposited intra-articularly. The small knee joint size, difficulties with probe positioning, variation in the location of the needle tip and unanticipated animal limb movement during the injection are likely reasons why we were not able to visualise 25% of the injections. Furthermore, even though we made a strong effort to keep the ultrasound probe position consistent, the innate variation between animals and the fast injection times meant that visualisation was not always possible. In future studies, longer cine loops of more than 500 frames can be obtained to help ensure the exact time of injection is acquired with the ultrasound system.
We realised during this study that several small changes could be made to improve visualisation of the injection. First, we began adding a small amount of air into the syringes in order to create bubbles during the injection. These bubbles helped us distinguish exactly where the injected fluid appeared and spread. Similarly, contrast-enhanced ultrasound using gas-filled microbubbles has become common in clinical imaging.15 Second, the knee joints were also placed on the left side of the image such that both the fat pad and superior joint space could be visualised at the same time. This joint placement facilitated observation of injections that spread from the centre of the joint into the superior joint space. Finally, small movements of the needle, before the injection, allowed us to localise the tip location by either visualisation of the metal artefact caused by the needle, or from movement of adjacent tissue. While the 90° orientation difference between the probe and needle reduced our ability to directly visualise the tip, small amounts of movement of the needle helped to ensure tip placement near the cartilage surface. Our experience also suggests that both the success of the injection and quality of the image improves with practice.
This high frequency technique of ultrasound injection could be used to help guide intra-articular needle placement for studies that focus on developing treatments for osteoarthritis with a variety of animal models. While we focused on the knee in this study, the hip, shoulder, elbow, or any other joint where arthritis is common, could also benefit from the use of ultrasound to improve the accuracy of injections. This is particularly important as advancements in protein and tissue engineering have led to exciting new potential candidates that may one day slow, prevent, or even reverse cartilage damage.5,6
The choice of animal models and anatomy will influence the ideal ultrasound frequency and position of the probe. Larger animals or joints deep within the body would likely require transducer frequencies of < 40 MHz due to the inverse relationship between frequency and depth. In other words, lower frequency ultrasound can penetrate deeper into the body, but then produces images with a lower resolution. Furthermore, joint anatomy and positioning are important to consider as ultrasound does not easily penetrate calcified bone. The dense femur and tibia comprising the knee required us to place the joint in moderate flexion such that the intra-articular space could be visualised. Thus, ultrasound probe placement, in addition to needle insertion, may require some adjustments depending on the size, depth and orientation of the region of interest. If the desired location is not reached, an injection can always be repeated to ensure proper administration. Indeed, clinical studies have shown that the use of ultrasound improves the accuracy of injection in the knee7,10 and hip.9
In conclusion, these data suggest that high frequency ultrasound can be used to visualise intra-articular needle placement and injections in small animals. Separation of the fat pad from the cartilage surface showed that fluid accumulates in the correct location within the knee joint. This method could also be used with different frequency probes for other animal models to ensure the correct positioning of injections when developing anti-osteoarthritic therapies.
1 Lawrence RC , HelmickCG, ArnettFC, et al.Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum1998;41:778–799.CrossrefPubMed Google Scholar
2 Felson DT . Clinical practice. Osteoarthritis of the knee. N Engl J Med2006;354:841–848. Google Scholar
3 Boon AJ , SmithJ, DahmDL. Efficacy of intra-articular botulinum toxin type A in painful knee osteoarthritis: a pilot study. PM R2010;2:268–276.CrossrefPubMed Google Scholar
4 Kon E , BudaR, FilardoG, et al.Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc2010;18:472–479.CrossrefPubMed Google Scholar
5 Bernhard JC , PanitchA. Synthesis and characterization of an aggrecan mimic. Acta Biomater2012;8:1543–1550.CrossrefPubMed Google Scholar
6 Sharma S , PanitchA, NeuCP, et al.Incorporation of an aggrecan mimic prevents proteolytic degradation of anisotropic cartilage analogs. Acta Biomater2013;9:4618–4625.CrossrefPubMed Google Scholar
7 Daley EL , BajajS, BissonLJ, ColeBJ. Improving injection accuracy of the elbow, knee, and shoulder: does injection site and imaging make a difference? A systematic review. Am J Sports Med2011;39:656–662.CrossrefPubMed Google Scholar
8 Jackson DW , EvansNA, ThomasBM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg [Am]2002;84-A:1522–1527.CrossrefPubMed Google Scholar
9 Sanchez M , GuadillaJ, FizN, AndiaI. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford)2012;51:144–150.CrossrefPubMed Google Scholar
10 Sibbitt WL Jr , KettwichLG, BandPA. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee?Scand J Rheumatol2012;41:66–72.CrossrefPubMed Google Scholar
11 Shung KK . High frequency Ultrasonic Imaging. J Med Ultrasound2009;17:25–30. Google Scholar
12 Shung K , CannataJ, Qifa ZhouM, LeeJ. High frequency ultrasound: a new frontier for ultrasound. Conf Proc IEEE Eng Med Biol Soc2009;2009:1953–1955.CrossrefPubMed Google Scholar
13 Grégoire JM , SerrièreS, GeorgescoG, et al.Techniques and applications of noninvasive high-resolution ultrasound imaging. J Radiol2006;87:1920-1936 (in French). Google Scholar
14 Jimenez PA , GlassonSS, TrubetskoyOV, HaimesHB. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci1997;47:598–601.PubMed Google Scholar
15 Dindyal S , KyriakidesC. Ultrasound microbubble contrast and current clinical applications. Recent Pat Cardiovasc Drug Discov2011;6:27–41.CrossrefPubMed Google Scholar
Funding statement:
This article was funded by the Purdue Research Foundation Trask Innovation Fund. A. Panitch reports that monies have been received by the NIH and Symic Biomedical to Purdue University which is not related to this article.
Author contributions:
N. Vázquez-Portalatín: Data collection, Data analysis, Injection assistance, Writing the paper.
G. J. Breur: Data analysis, Injection assistance, Editing the paper.
A. Panitch: Data analysis, Editing the paper, Providing financial support.
C. J. Goergen: Data collection, Data analysis, Injection assistance, Writing and editing the paper.
ICMJE Conflict of Interest:
None declared
©2015 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









