Abstract
Objectives
An experimental piglet model induces avascular necrosis (AVN) and deformation of the femoral head but its secondary effects on the developing acetabulum have not been studied. The aim of this study was to assess the development of secondary acetabular deformation following femoral head ischemia.
Methods
Intracapsular circumferential ligation at the base of the femoral neck and sectioning of the ligamentum teres were performed in three week old piglets. MRI was then used for qualitative and quantitative studies of the acetabula in operated and non-operated hips in eight piglets from 48 hours to eight weeks post-surgery. Specimen photographs and histological sections of the acetabula were done at the end of the study.
Results
The operated-side acetabula were wider, shallower and misshapen, with flattened labral edges. At eight weeks, increased acetabular cartilage thickness characterised the operated sides compared with non-operated sides (p < 0.001, ANOVA). The mean acetabular width on the operated side was increased (p = 0.015) while acetabular depth was decreased anteriorly (p = 0.007) and posteriorly (p = 0.44). The cartilage was thicker, with delayed acetabular bone formation, and showed increased vascularisation with fibrosis laterally and focal degenerative changes involving chondrocyte hypocellularity, chondrocyte cloning, peripheral pannus formation and surface fibrillation.
Conclusions
We demonstrate that femoral head AVN in the young growing piglet also induced, and was coupled with, secondary malformation in acetabular shape affecting both articular and adjacent pelvic cartilage structure, and acetabular bone. The femoral head model inducing AVN can also be applied to studies of acetabular maldevelopment, which is less well understood in terms of developing hip malformation.
Cite this article: Bone Joint Res 2014;3:130–8.
Article focus
Assessing development of secondary acetabular deformation in piglets following induction of femoral head ischemia, avascular necrosis, and deformation.
Key messages
Secondary acetabular malformation is induced by, and coupled with, femoral head deformation in a piglet avascular necrosis (AVN) model.
Operated side acetabula are progressively wider, shallower and misshapen, with flattened labral edges.
Acetabular cartilage is thicker, with delayed acetabular bone formation and shows increased vascularisation with fibrosis laterally and early focal degenerative surface changes.
Strengths and limitations
This study is the first to demonstrate secondary acetabular malformation in a piglet femoral head ischemia/AVN model – and it can be a valuable and clinically relevant experimental model for studying the development of acetabular malformation.
This study only reports initial observations, and more investigation will be needed for definitive correlations.
Introduction
Intracapsular surgical ligation at the base of the femoral neck and sectioning of the ligamentum teres is an excellent model for inducing ischemia, necrosis, and deformation in the immature piglet femoral head.1-4 A piglet model creating avascular necrosis (AVN) of the femoral head by surgical intervention at the base of the neck also causes secondary changes to develop in the acetabulum, even though no surgical interventions on the acetabulum are undertaken. We have assessed these acetabular changes by multiple methods to show the close relationship between developing deformity on both sides of the joint. MRI with gadolinium enhancement, demonstrates age-related vascular changes in the developing epiphysis and metaphysis of normal piglet femurs5 and the repair/deformation response in the necrosis model.2-4 It also outlines normal acetabular development and acetabular changes following femoral head deformation. The development of the acetabulum (with involvement of three bones: ilium, ischium, and pubis) from the pelvic cartilage and the physeal tri-radiate cartilage is known from anatomical studies6-15 but is assessed infrequently in relation to the pathogenesis of hip deformity. In a recent study with the femoral neck ligation–ligamentum teres sectioning model, we applied three dimensional CT scanning to show the close coupling of acetabular deformation to femoral head deformation.16 In this study, we use a model in three-week-old piglets and assess the secondary development of acetabular deformity by inspection of the intact acetabula, their histological appearance, and qualitative sequential MR imaging.
Materials and Methods
Acetabula were assessed from eight Yorkshire piglets. Surgical procedures on the femur were undertaken at three weeks of age and all piglets were killed by eight weeks post-surgery (eleven weeks of age). Normal piglet acetabular development was assessed from the non-operated contralateral side. The piglets began walking as soon as the anesthesia wore off. They limped for two or three days on the operated side, but resumed full weight-bearing on the operated lower extremity, which continued throughout the time of the experiment. The femoral findings have been described in detail in our previous article.4
Operative technique
Anesthesia was induced with an intramuscular (IM) injection of midazolam hydrochloride (Baxter, Deerfield, Illinois) 40 mg/kg, and ketamine hydrochloride (Ketalar; Parke-Davis, Morris Plains, New Jersey) 20 mg/kg. Ketamine hydrochloride 20 mg/kg and xylazine 5 mg/kg (Rompun; Mobay, Shawnee, Kansas) were given 30 minutes later. A continuous intravenous (IV) infusion of diprivan 1% (Propofol; AstraZeneca, Wilmington, Delaware) in 5% dextrose was used at 0.002 mg/kg/minute for maintenance. An oral airway was placed and blow-by oxygen was given at 4 L/minute.
The right hip was approached by lateral incision, with the piglet lying on its left side. A linear 6 cm incision, parallel to the femur, was centred over the tip of the greater trochanter. Dissection to the trochanter and anterior joint capsule was done between muscles, leaving them intact. Anterior capsulotomy and longitudinal traction on the lower extremity allowed for subluxation of the femoral head from the acetabulum. Long-curved scissors were used to cut the ligamentum teres. Femoral head ischemia was induced using a doubled #2 silk ligature placed circumferentially and tied tightly around the base of the femoral neck within the hip joint capsule. The acetabulum was not interfered with surgically. The capsule, musculotendinous structures, subcutaneous tissues and skin were closed with absorbable sutures. Skin dressings and post-surgical splinting were not used. The non-operated left hip served as a control.
MR Imaging technique
MRI at 1.5 Tesla (GE Medical Systems, Milwaukee, Wisconsin) used a pair of 3 inch receive-only surface coils. Each study was performed under general anesthesia using a single IM injection of ketamine hydrochloride 20 mg/kg and xylazine 5 mg/kg, followed by a continuous IV infusion of diprivan 1% in 5% dextrose at 0.002 mg/kg/min. Studies were performed with the piglets lying in the lateral decubitus position, with the non-operated hip down. In all animals, conventional MRI including T1, T2 and spoiled gradient recalled echo images, were obtained with 2.5 mm section thickness, 0.625 mm in-plane resolution, and 20 cm field of view. T1-weighted images were acquired, with repetition time (msec)/echo time (msec) of 500/9 and one signal acquired. T2-weighted images were acquired with 2000/60 and two signals acquired. Depending on the age and size of the piglet, there were usually six to nine coronal plane images per femoral head and acetabulum. Gadopentetate dimeglumine (Magnevist; Berlex, Wayne, New Jersey) an IV contrast agent was also used at 0.2 mmol/kg, injected in a rapid bolus into an ear vein 10 seconds after beginning dynamic gadolinium-enhanced MRI. Enhancement was evaluated using a spoiled gradient echo sequence (200/2; flip angle, 60°; section thickness, 3 mm; in-plane resolution, 0.625 mm; field of view, 20 cm). Five images were acquired per section. MRI was performed post-surgery under general anesthesia at 48 hours and one, two, four and eight weeks. One piglet was killed at two weeks to document early changes. One developed limping, discomfort and delayed infection on the operated side and was killed at four weeks, with analyses used only from 48 hours and one and two week time periods, when clinical and MRI were unremarkable. The remaining piglets were assessed until eight weeks post-surgery.
Statistical analysis
Measurements were made of surface cartilage thickness (articular cartilage and adjacent acetabular cartilage) at each time period on operated and non-operated sides at anterior, middle, and posterior coronal plane segments. For each segment, measurements were made medially, in the middle, and laterally, and the mean was determined to provide a single value. Differences between the two sides (ligated versus non-operated control hips) at each time point were evaluated using repeated-measures analysis of variance (ANOVA), with Bonferroni-adjusted comparisons preceded by the Greenhouse-Geisser F-test to determine significance. Two-tailed p-values < 0.05 were considered statistically significant. Data analysis was performed using SPSS software (version 19.0, SPSS Inc., IBM, Chicago, Illinois). Additional measurements were made on the MR images of the operated side and non-operated side acetabula at eight weeks post-surgery in five piglets to document acetabular width and depth. The width of the acetabulum was measured in the same planes on both sides from the tips of the acetabular labrum, from anterior to posterior. The acetabular depths were then measured from this transverse line passing radially at right angles to anterior and posterior segments to the acetabular articular cartilage surface.
Structural photographic and histologic studies
After the final MRI, each piglet remained anesthetised and was killed with an intracardiac injection of Fatal-Plus (pentobarbital sodium; Vortech Pharmaceuticals, Dearborn, Michigan). The acetabula on both sides from four piglets were removed intact by sectioning the pubic, iliac, and ischial bones to include the complete tri-radiate cartilage. Photographs were taken of operated and non-operated acetabula and proximal femurs after removal. Multiple histological sections in multiple planes from the four operated acetabula and the four non-operated contralateral control acetabula, were made. Acetabular specimens were fixed in 10% neutral buffered formalin for two to four weeks, followed by decalcification in 25% formic acid until soft. Some specimens were cut in the coronal plane, but most were cut to include the complete cross-sectional diameter of the acetabulum from the outer labrum to the tri-radiate cartilage posterior to the socket. Tissues were placed in increasing concentrations of alcohol, infiltrated and embedded in paraffin, cut at 7 µm thickness, and stained with 1% toluidine blue or hematoxylin and eosin. The study was approved by the institution’s Animal Care and Use Committee.
Results
All piglets recovered well from surgery and at 48 hours, all demonstrated completely ischemic and fully located femoral heads. Photographs of coronal plane decalcified sections of operated and non-operated side acetabula eight weeks following proximal femoral surgery showed considerable secondary deformation of the acetabulum on the operated side (Fig. 1). The markedly deformed shape of the AVN femoral head compared with the normal opposite side non-operated head could have been expected to cause abnormal modelling of the actively growing adjacent acetabulum (Figs 2 and 3). With specific inspection, we found the acetabula on the operated side to be wider with flattened labral rims, shallower and misshapen in relation to the abnormally shaped femoral head. The three-dimensional nature of the acetabular deformities on the operated side could be appreciated in the intact acetabular photographs. A posterior view of the tri-radiate cartilages showed them to be present and open on the operated side, even in the presence of marked acetabular deformation. They were relatively wider in some regions, but not of uniform thickness.
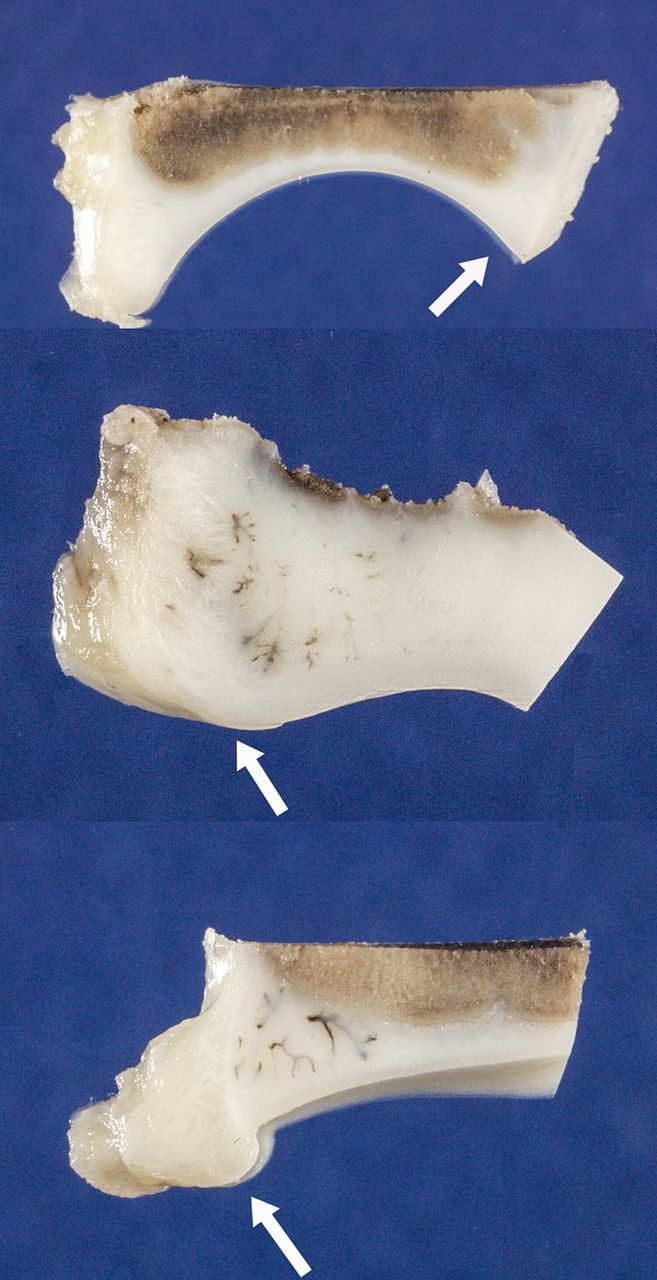
Fig. 1
Photographs showing acetabula from mid-coronal plane decalcified sections. Arrows point to the lateral acetabular region. Top: normal acetabulum from non-operated side of piglet at 11 weeks of age. Middle: outer portion of acetabulum from operated side at 11 weeks of age illustrates shallower arc of the articular surface, widened and blunted outer rim of acetabulum and reactive vessel accumulation (brown linear densities). Bottom: outer portion of acetabulum from operated hip at 11 weeks of age illustrates diminished arc of articular surface and asymmetric overgrowth of cartilage instead of the normal triangular outer rim.

Fig. 2
Top: photographs show operated side acetabulum on the left and non-operated side on the right. Note well defined outer rim on normal side and flattened rim with wider opening on operated side. Below, the corresponding femoral heads are shown. The femoral head and neck with avascular necrosis (AVN) shows flatter, wider head with shorter neck and more prominent greater trochanter.

Fig. 3
Top. Photographs show deformed acetabulum from operated side with flattened outer rim, shallow socket and flattened outer cartilage surface (left). Normal non-operated acetabulum (right) shows differences from operated side. Below, femoral heads are seen in superior views. Asymmetric shape of operated femoral head (left) is seen.
Qualitative inspection of the MRI scans from both sides showed the associated acetabular under-development on the operated side primarily at the anterior and supero-lateral region affecting the shape of the articular socket (sloping or more oblique), with diminished subchondral bone formation (Fig. 4). By eight weeks, MRI measurements demonstrated statistically significant acetabular cartilage thickness changes, characterising the operated sides in comparison with normal non-operated sides (p < 0.001); the operated side cartilage was thicker (Table I). Differences in anterior, middle, and posterior regions in operated versus non-operated segments were 2.78 mm (sd 1.37) vs 1.76 mm (sd 0.36); 2.48 mm (sd 0.88) vs 1.60 mm (sd 0.33); and 2.45 mm (sd 0.91) vs 1.62 mm (sd 0.38). Significant differences were not seen at 48 hours, and one and two weeks. By four weeks, significant differences were noted in each of the segments with p-values < 0.001. Reactions to surgery of the tri-radiate cartilage were slow to develop and reached statistical significance only at eight weeks post-surgery. The ligated side remained thicker than the non-operated side as the latter narrowed (Table I). MRI changes between 48 hours and eight weeks are highlighted in Table II.

Fig. 4
MRI (left) from operated hip eight weeks post-surgery shows irregularly shaped femoral head (white arrow) and abnormal acetabulum with bony under-development anteriorly. Tri-radiate cartilage is abnormal and widened in parts. The ilium is shown above with iliac crest apophysis. MRI (right) from the normal hip shows the sphericity of the femoral head (white arrow) and adjacent acetabulum. Tri-radiate cartilage and ilium are normal.
Table I
Acetabulum MRI measurements for ligated and control hips after surgery (n = 8). Data represent the mean (sd). (ANOVA, analysis of variance)
| Cartilage (mm) | Ligated Side | Control Side | Repeated-Measures ANOVA (p-value) |
|---|---|---|---|
| Tri-radiate | |||
| 48 hours | 1.41 (sd 0.26) | 1.69 (sd 0.31) | 0.050* |
| One week | 1.48 (sd 0.34) | 1.63 (sd 0.40) | 0.279 |
| Two weeks | 1.54 (sd 0.28) | 1.61 (sd 0.40) | 0.661 |
| Four weeks | 1.58 (sd 0.16) | 1.60 (sd 0.23) | 0.909 |
| Eight weeks | 1.50 (sd 0.36) | 1.16 (sd 0.15) | 0.049* |
| Anterior | |||
| 48 hours | 1.65 (sd 0.24) | 1.69 (sd 0.16) | 0.849 |
| One week | 1.83 (sd 0.41) | 1.70 (sd 0.24) | 0.527 |
| Two weeks | 1.73 (sd 0.22) | 1.63 (sd 0.18) | 0.558 |
| Four weeks | 2.68 (sd 0.76) | 1.84 (sd 0.18) | < 0.001* |
| Eight weeks | 2.78 (sd 1.37) | 1.76 (sd 0.36) | < 0.001* |
| Middle | |||
| 48 hours | 1.53 (sd 0.24) | 1.48 (sd 0.14) | 0.799 |
| One week | 1.59 (sd 0.40) | 1.58 (sd 0.29) | 0.949 |
| Two weeks | 1.61 (sd 0.38) | 1.40 (sd 0.29) | 0.242 |
| Four weeks | 2.80 (sd 1.11) | 1.66 (sd 0.15) | < 0.001* |
| Eight weeks | 2.48 (sd 0.88) | 1.60 (sd 0.33) | < 0.001* |
| Posterior | |||
| 48 hours | 1.25 (sd 0.54) | 1.36 (sd 0.18) | 0.564 |
| One week | 1.48 (sd 0.38) | 1.41 (sd 0.21) | 0.748 |
| Two weeks | 1.69 (sd 0.55) | 1.29 (sd 0.20) | 0.039* |
| Four weeks | 2.56 (sd 0.89) | 1.62 (sd 0.26) | < 0.001* |
| Eight weeks | 2.45 (sd 0.91) | 1.62 (sd 0.38) | 0.002* |
-
* Statistically significant.
Table II
Acetabulum MRI changes between 48 hours and eight weeks*
| Control side | Tri-radiate: p = 0.003 (significant decrease at eight weeks, (31%)) | |
| Anterior: p = 0.82 (no change) | ||
| Middle: p = 0.64 (no change) | ||
| Posterior: p = 0.25 (no change) | ||
| Ligated side | Tri-radiate p = 0.38 (no change) | |
| Anterior p < 0.001 (significant increase at eight weeks (68%)) | ||
| Middle p < 0.001 (significant increase at eight weeks, (62%)) | ||
| Posterior p < 0.0001 (significant increase at eight weeks, (96%)) | ||
-
*p values calculated based on the repeated-measures ANOVA for each of the four measurements.
The acetabular width was increased on the operated side compared with the non-operated side at eight weeks in each of the five piglets measured. The mean width on the operated side was 33.2 mm (sd 2.3) (30.3 to 36.5) and on the non-operated side it was 29.2 mm (sd 1.4) (p = 0.015) (27.8 to 31.1). Acetabular depth was less on the operated side but was more abnormal anteriorly than posteriorly. The anterior depth was a mean of 4.4 mm (sd 1.2) (3.2 to 5.8) on the operated side compared with 6.5 mm (sd 0.7) (p = 0.007) (6.0 to 7.8) on the non-operated side and the posterior depth was decreased less to a mean of 6.3 mm (sd 1.3) (4.7 to 7.6) compared with the normal 6.7 mm (sd 0.6) (p = 0.44) (6.1 to 7.7). Acetabular width and anterior depth values were statistically significant, but posterior depth value was not.
Each of the three bones comprising the acetabulum (ilium, pubis, and ischium) forms similar to an epiphyseal secondary ossification centre (Fig. 5). On the non-operated side (Fig. 5), the articular surface was spherical with well-ordered bone growth of iliac, ischial, and pubic components, while the operated side (Fig. 6) lacked sphericity, and showed individual bone underdevelopment and a misshapen articular surface. At eight weeks post-surgery, histological sections of the operated-side acetabula showed asymmetric shaping of these boney centres and of the arc of the surface cartilage (Figs 1 and 6), thicker cartilage (especially at the outer periphery), reactive cartilage nodules at the peripheral labral area, increased vascularisation (cartilage canals) at the peripheral cartilage accumulations, and early degenerative cartilage changes involving empty chondrocyte lacunae, areas of hypocellular cartilage, chondrocyte cloning, and some surface fibrillation (Figs 1, 6, 7 and 8). The changes were focal and superficial and were concentrated at the lateral periphery of the acetabular cartilage surfaces. On occasion, peripheral fibrovascular pannus expanded onto the cartilage surface (Figs 7 and 8). Chondrocyte death (empty lacunae), hypocellular to acellular areas, and extensive chondrocyte circular cloning with excess glycosaminoglycan synthesis surrounding each clone (Figs 7 and 8) are characteristic of early osteoarthritic change. Clefts were sometimes seen passing at right angles, or obliquely from the surface of the cartilage and then transversely within the involved hypocellular/acellular areas. Deeper changes involved pathologic vascular invasion, leading to fibrosis with pelvic cartilage disruption (Fig. 8).

Fig. 5
Left: Histological cross-section of acetabulum of non-operated side at 11 weeks of age at time of death. Note sphericity of articular cartilage surface and well-ordered endochondral bone development of each of ischial, iliac, and pubic bones. At right, photomicrograph of bipolar physeal region is shown between two adjacent bones in the normal non-operated side (hematoxylin and eosin stain).

Fig. 6
Photomicrograph of histologic cross-section of the acetabulum from the operated side eight weeks post-surgery shows markedly abnormal articular cartilage surface shape, under-developed and asymmetric bones and relatively more extensive persisting cartilage (hematoxylin and eosin stain).

Fig. 7
Histologic sections from the lateral region of the acetabular articular cartilage showing (top figure) multiple abnormal intracartilaginous vessels and fibrovascular invasion (lower right); (middle figure) peripheral fibrocartilaginous reactive tissue, with pannus fibrovascular overgrowth and circular cartilaginous clones in otherwise hypocellular area; and (lower figure) hypocellular cartilage surface with fibrillation and transverse cleft.

Fig. 8
Histology sections show normal sequence from the articular surface to the bone of the ilium (left side). On the right, abnormal articular cartilage from the operated-side acetabula shows surface abnormalities with acellular regions, superficial transverse tears (top); pannus, acellular cartilage with empty chondrocyte lacunae, chondrocyte clones (middle) and superficial transverse fissures through acellular cartilage clone region (lower).
Discussion
The piglet femoral head AVN model also induces secondary changes in acetabular structure as the growing acetabulum relates to the progressively misshapen femoral head. The piglet femoral head always becomes misshapen in this model, due to the induced necrosis, continued weight-bearing, and asymmetric repair with revascularisation.1,3,4 The femoral head deformities previously described,4 to which the acetabula in this study were relating, involved a shortened head and neck with normal trochanteric growth (coxa vara), with the head widened and oval to resemble a partially flattened shape. The articular surface of the heads was intact but uneven, with localised flattening and depression most prominent laterally and centrally; the normal spherical shape of the medial portion of the femoral head was better preserved and often close to normal (Fig. 2).
The acetabular changes were significantly developed in all piglet hips and in all segments of the cartilage by four weeks post-surgery, persisting and worsening at eight weeks (Table I). The abnormalities included shape changes (asymmetric circumferences, wider and shallower acetabula), persistence of cartilage with delayed iliac supra-acetabular bone formation, cartilage labral flattening with cartilaginous lipping, and early signs of articular cartilage degeneration. This report and our recent CT imaging study16 are the first to demonstrate the development and characteristics of acetabular maldevelopment in the piglet femoral head ischemia model. In this model, the head and neck deformities are analogous to those in childhood Legg–Perthes disease. MRI and specimen measurements begin to quantify the observations of acetabular changes in a valuable way. We have expanded our work with the AVN model to further study the development of acetabular deformation coupled with femoral head deformation, by use of serial CT imaging, to which we apply a shape analysis computational algorithm technique that captures the temporal shape changes of the femurs and acetabula, along with their co-dependencies.16 This method concentrates on the quantitative tracking of the temporal progression of the disease and the deformation process.
With early development of the piglet hip in utero, the tri-radiate cartilage forms and acetabular development receives contributions from iliac, ischial, and pubic segments.6-15 While not seen on single two-dimensional histological sections or on plain radiographs, there is continuity of acetabular articular and adjacent pelvic cartilage (acetabular cartilage hemisphere) and the tri-radiate cartilages. The intervening pelvic cartilage (between the physeal tri-radiate and the acetabular cartilage) is essentially epiphyseal cartilage (within which the iliac, ischial, and pubic bone centres appear) homologous with the epiphyseal cartilages at the ends of long bones.9 Bucholz et al11 referred to the acetabular articular cartilage and the adjacent pelvic cartilage as the acetabular hemisphere. Ponseti8 referred to yet another region of acetabular cartilage at its periphery as the ring apophysis in continuity with the growth plates of ilium, ischium, and pubis and with the three flanges of the tri-radiate cartilage. Harrison9,15 and others10,11 refer to this area as a natural continuation of more internal tissue, calling it “the articular cartilage at the acetabular rim”15 rather than an implied specific structure. Some studies have demonstrated these cartilage continuities.9,10,11
In children with Legg–Perthes, the acetabulum reacts with altered growth to abnormalities in both the shape and position of the femoral head17-23 in a similar way to that shown in the piglet AVN findings. Acetabular and femoral head overgrowth in parallel fashion have been noted,17 along with premature fusion of the tri-radiate cartilage, hypertrophy of articular cartilage, changes in acetabular dimensions, and increased acetabular uptake in bone scans indicative of increased vascular supply.18,19 Decreases in depth and irregularity in the shape of acetabula with Perthes have been reported, with secondary acetabular changes developing in proportion to femoral head involvement.20 Multiple measurements on affected and non-involved acetabula of 34 patients with Perthes had findings similar to the piglet model.21 Irregularity of the acetabulum was directly proportional to the extent of femoral head involvement, with increase in size, decrease in depth, an irregular contour on the affected side and sloping and shallowness of the superolateral cartilaginous part of the acetabulum. The malformed femoral head also seemed to affect the peripheral lateral superior labrum, an observation also made in the model. CT and MRI scans have documented acetabular changes in children with Perthes’ disease22 and studies of 62 acetabula in Perthes disease found the opening angle and diameter of the acetabulum increased on the involved side.23
Acetabular maldevelopment similar to developmental dysplasia of the hip has been induced experimentally in skeletally immature rat and rabbit modelling by removing or displacing the femoral head15,24 or by immobilising the knee in extension with a Kirschner (K-)-wire.25 Acetabular growth becomes narrower, shallower, and smaller primarily at lateral acetabular cartilage, with the tri-radiate cartilage relatively unaffected. Studies specifically damaging the tri-radiate cartilage (rabbit) show the ilio-ischial segment to be the most extensive component for normal growth of the acetabulum.26,27 In the rabbit acetabular dysplasia model referred to above,25 the abnormalities were reversed by selective epiphysiodesis of the ilio-ischial limb of the tri-radiate cartilage. AVN can also be produced in the lamb by ligating the pericapsular vessels of the neck and sectioning the ligamentum teres.28
While the deforming effects of asymmetric growth at long bone epiphyses are well understood clinically, negative effects on acetabular and tri-radiate cartilages are not as well understood in relation to clinical disorders of the hip. Figures 4 (right side) and 5 show the well-ordered developing ischial, iliac, and pubic bones surrounded by the endochondral sequence physis and hypertrophic cartilage zones, while those bones undergo asymmetric development relating to deformed femoral heads (Figs 4 (left side) and 6) with AVN. In this model, the growth deformity of the acetabulum occurs primarily at the more laterally and anteriorly positioned acetabular articular and adjacent pelvic cartilage (acetabular hemisphere) but each of the three bones is also affected.
While far from definitive, measurements of the tri-radiate cartilage are generally the same for several weeks in operated and non-operated sides (Table I) until the later and final measurements, when the operated side is statistically wider than the non-operated side (Table II). This decrease on the control side may represent the beginning of slowdown of normal growth, while the operated side shows an abnormal pattern. This will be investigated in future studies. The acetabular articular cartilage undergoes focal changes superficially, while the non-operated side remains normal. These changes are concentrated laterally and are similar to the well-known histological patterns of early osteoarthritic change. The deeper adjacent pelvic cartilage suffers premature vascular and fibrovascular invasion, damaging the normal sequence of cartilage and pelvic bone formation. This study in the immature piglet hip shows the close coupling of abnormal acetabular development to deformity of the femoral head induced by AVN, with the most prominent changes in the peripheral acetabular cartilage. It allows for more detailed studies of the pathogenesis of acetabular deformation and of possible surgical intervention, leading to biologic corrections.
1 Kim HK , SuPH, QiuYS. Histopathologic changes in growth-plate cartilage following ischemic necrosis of the capital femoral epiphysis: an experimental investigation in immature pigs. J Bone Joint Surg [Am]2001;83-A:688–697. Google Scholar
2 Jaramillo D , ConnollySA, VajapeyamS, et al.Normal and ischemic epiphysis of the femur: diffusion MR imaging study in piglets. Radiology2003;227:825–832.CrossrefPubMed Google Scholar
3 Menezes NM , ConnollySA, ShapiroF, et al.Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology2007;242:129–136.CrossrefPubMed Google Scholar
4 Shapiro F , ConnollyS, ZurakowskiD, et al.Femoral head deformation and repair following induction of ischemic necrosis: a histologic and magnetic resonance imaging study in the piglet. J Bone Joint Surg [Am]2009;91-A:2903–2914.CrossrefPubMed Google Scholar
5 Jaramillo D , Villegas-MedinaOL, DotyDK, et al.Age-related vascular changes in the epiphysis, physis, and metaphysis: normal findings on gadolinium-enhanced MRI of piglets. AJR Am J Roentgenol2004;182:353–360.CrossrefPubMed Google Scholar
6 Breathnach AS Frazer’s Anatomy of the Human Skeleton. 6th ed. London: J & A Churchill Ltd, 1965. Google Scholar
7 Payton CG . The Growth of the Pelvis in the Madder-fed Pig. J Anat1935;69:326–343.PubMed Google Scholar
8 Ponseti IV . Growth and development of the acetabulum in the normal child. Anatomical, histological, and roentgenographic studies. J Bone Joint Surg [Am]1978;60-A:575–585.PubMed Google Scholar
9 Harrison TJ . The growth of the pelvis in the rat; a mensural and morphological study. J Anat1958;92:236–260.PubMed Google Scholar
10 Portinaro NM , MurrayDW, BensonMK. Microanatomy of the acetabular cavity and its relation to growth. J Bone Joint Surg [Br]2001;83-B:377–383.CrossrefPubMed Google Scholar
11 Bucholz RW , EzakiM, OgdenJA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg [Am]1982;64-A:600–609.PubMed Google Scholar
12 Siffert RS , FeldmanDJ. The growing hip: the dynamic development of the normal adult hip and the deformed hip of Legg-Calvé-Perthes' disease. Acta Orthop Belg1980;46:443–476.PubMed Google Scholar
13 Laurenson RD . Development of the acetabular roof in the fetal hip; an arthrographic and Histological Study. J Bone Joint Surg [Am]1965;47-A:975–983.PubMed Google Scholar
14 Gardner E , GrayDJ. Prenatal development of the human hip joint. Am J Anat1950;87:163–211.CrossrefPubMed Google Scholar
15 Harrison TJ . The influence of the femoral head on pelvic growth and acetabular form in the rat. J Anat1961;95:12–24.PubMed Google Scholar
16 Tsai A , ConnollyS, NedderA, ShapiroF. Visualization and analysis of the deforming piglet femur and hip following experimentally induced avascular necrosis of the femoral head. IEEE Trans Biomed Eng2013;60:1742–1750.CrossrefPubMed Google Scholar
17 Yngve DA , RobertsJM. Acetabular hypertrophy in Legg-Calvé-Perthes disease. J Pediatr Orthop1985;5:416–421.CrossrefPubMed Google Scholar
18 Joseph B . Morphological changes in the acetabulum in Perthes' disease. J Bone Joint Surg [Br]1989;71-B:756–763.CrossrefPubMed Google Scholar
19 Grzegorzewski A , SynderM, KozłowskiP, SzymczakW, BowenRJ. The role of the acetabulum in Perthes disease. J Pediatr Orthop2006;26:316–321.CrossrefPubMed Google Scholar
20 Meurer A, Böhm B, Decking J, Heine J. Analysis of acetabular changes in Morbus Perthes disease with radiomorphometry. Z Orthop Ihre Grenzgeb 2005;143:100-5. [article in German]. Google Scholar
21 Madan S , FernandesJ, TaylorJF. Radiological remodelling of the acetabulum in Perthes' disease. Acta Orthop Belg2003;69:412–420.PubMed Google Scholar
22 Cho TJ , ChoiIH, ChungCY, YooWJ, LeeKS. The bicompartmental acetabulum in Perthes' disease: 3D-CT and MRI study. J Bone Joint Surg [Br]2005;87-B:1127–1133.CrossrefPubMed Google Scholar
23 Cahuzac JP , de GauzyJS, VidalH, GaubertJ. The acetabular opening angle in Perthes' disease. Radiographic study of 62 unilateral cases. Acta Orthop Scand1992;63:278–281.CrossrefPubMed Google Scholar
24 Langenskiold A , SarpioO, MichelssonJE. Experimental dislocation of the hip in the rabbit. J Bone Joint Surg [Br]1962;44-B:209–215. Google Scholar
25 Moraleda L , AlbiñanaJ, ForriolF. Selective epiphysiodesis of the triradiate cartilage for treatment of residual experimental acetabular dysplasia. J Pediatr Orthop2013;33:821–828.CrossrefPubMed Google Scholar
26 Hallel T , SalvatiEA. Premature closure of the triradiate cartilage. A case report and animal experiment. Clin Orthop Relat Res1977;:278–281.PubMed Google Scholar
27 Gepstein R , WeissRE, HallelT. Acetabular dysplasia and hip dislocation after selective premature fusion of the triradiate cartilage. An experimental study in rabbits. J Bone Joint Surg [Br]1984;66-B:334–336.CrossrefPubMed Google Scholar
28 Martinez-Álvarez S, Azorin D, Epeldegui T, Forriol F. Avascular necrosis of the femoral head: experimental study in lambs. Trauma Fund Mapfre 2011;22:188-96 [article in Spanish]. Google Scholar
Funding statement:
This study was supported by the National Institutes of Health grant 2 RO1 AR042396-09 and the Peabody Foundation Inc.
Author contributions:
F. Shapiro: Data collection, Data analysis, Performed surgeries, Writing the paper, Initial concept
S. Connolly: Data collection, MR imaging, Data analysis, Writing the paper, Initial concept
D. Zurakowski: Data analysis, Writing the paper
E. Flynn: Histological studies
D. Jaramillo: Data collection, MR imaging, Data analysis, Writing the paper, Initial concept
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
Supplementary material. Figures showing acetabula on the operated side to be wider with flattened labral rims, shallower and misshapen in relation to the abnormally shaped femoral head, are available with the online version of this article www.bjr.boneandjoint.org.uk









