Abstract
Objectives
The most concerning infection of allografts and operative procedures is methicillin resistant Staphylococcus aureus (MRSA) and no current iontophoresed antibiotics effectively combat this microbe. It was initially hypothesised that iontophoresis of vancomycin through bone would not be effective due to its large molecular size and lack of charge. The aim of this study was to determine whether this was a viable procedure and to find the optimum conditions for its use.
Methods
An iontophoresis cell was set up with varying concentrations of Vancomycin within the medulla of a section of sheep tibia, sealed from an external saline solution. The cell was run for varying times, Vancomycin concentrations and voltages, to gain information on optimisation of conditions for impregnating the graft. Each graft was then sectioned and dust ground from the exposed surface. The dust was serially washed to extract the Vancomycin and concentrations measured and plotted for all variables tested.
Results
Vancomycin was successfully delivered and impregnated to the graft using the iontophoresis technique. The first order fit to the whole data set gave a significant result (p = 0.0233), with a significant concentration (p = 0.02774) component. The time component was the next most significant (p = 0.0597), but did not exceed the 95% confidence level.
Conclusions
Iontophoresis is an effective method for delivering Vancomycin to allograft bone. The concentrations of the vancomycin solution affected the bone concentration, but results were highly variable. Further study should be done on the effectiveness of delivering different antibiotics using this method.
Cite this article: Bone Joint Res 2014;3:101–7.
Article focus
To determine whether iontophoresis of vancomycin through bone was a viable procedure.
To find the optimum conditions for iontophoresis of vancomycin.
Key messages
Iontophoresis of allograft bone with vancomycin is a viable procedure
The optimum conditions for iontophoresis are 5% vancomycin in sterile water at 110 v for 30 minutes.
Iontophoresis of allograft bone with vancomycin delivers doses of vancomycin to the allograft, exceeding the minimum inhibitory concentration (MIC) necessary for effective antimicrobial activity.
Strengths and limitations
Strengths: we have effectively demonstrated both that iontophoresis is a viable procedure to deliver therapeutic doses of vancomycin to allograft bone, and the optimum conditions (which are easily reproducible) to do this.
Limitations: we did not perform Micro CT to ascertain the effect of porosity of the graft on the loading of the bone and how this affects the conditions required.
Introduction
The use of allograft bone is well established in limb salvage after tumour surgery1-6 and in revision arthroplasty7-11 as morcellised11,12 and site-specific structural grafts.13-15 Infection is a common complication. The outcome in patients who develop infection is poor and often requires either two-stage revision3,5,7,15,16 or amputation.2,5,15
Allograft infections generally present early, with approximately 75% occurring within four months.13,15 It is well known that peri-operative infection is the most likely cause. The most common organisms isolated are Gram-positive (54%), followed by Gram-negative (36%) and mixed (10%).13
One solution to combat infection is the use of antibiotic impregnated bone graft. There are several techniques suggested in the literature, but most do not directly augment the allograft (such as mixing with antibiotic impregnated cement beads). Soaking has been suggested17 as has iontophoresis and pressurisation.18
Iontophoresis is a method of transporting antibiotics through bone using an electrical current and can get very high levels of antibiotic to the allograft bone that elute for up to two weeks.18,19 All previous published work has described iontopheresis of gentamicin and flucloxacillin.18 Given that the most common causes of orthopaedic infections are Gram positive microbes13 and in particular MRSA, vancomycin is becoming a desirable antibiotic for orthopaedic allograft implantation. The larger size and different shape of the vancomycin molecule (Fig. 1) and the molecule’s neutral charge at neutral pH, led to the belief that iontophoresis was not very effective for vancomycin. If it did work, the optimal conditions for iontophoresis were expected to be very different from gentamicin.
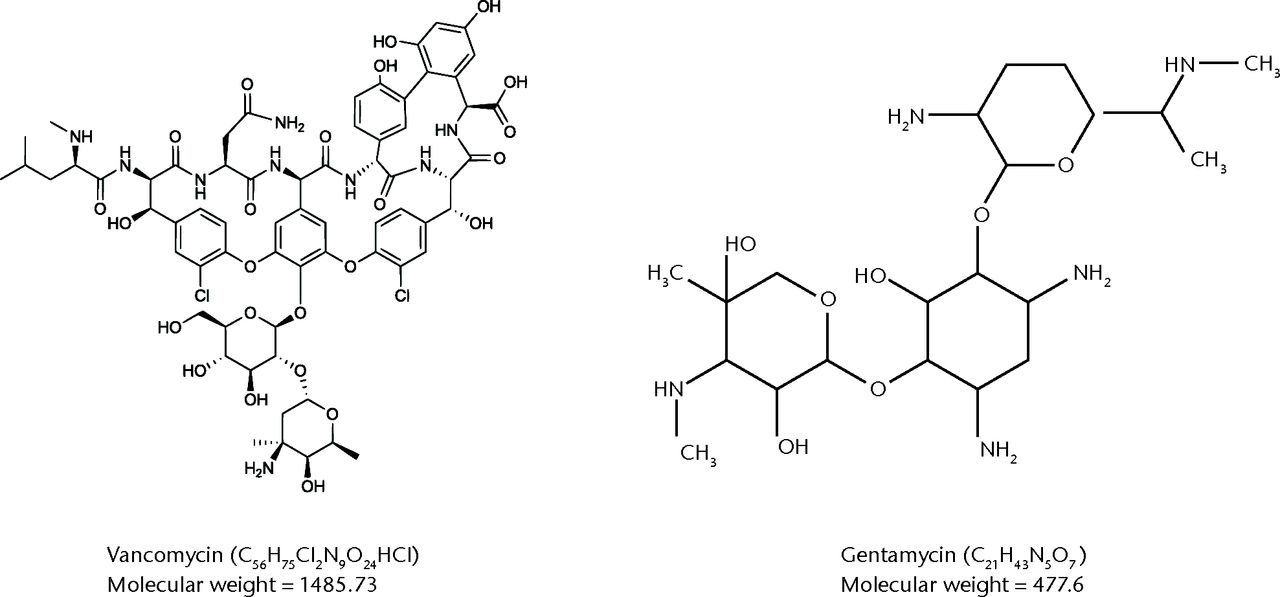
Fig. 1
Structural diagrammatic comparison between vancomycin and gentamycin molecules.
There is a maximum useful time for iontophoresis18 as if there is a finite reservoir of antibiotic, it is possible to drive all of the available ions through the bone and out into the external solution, reducing the amount retained in the bone. The exact relationship between bone geometry, current and time is unclear, but preliminary results indicate that gentamicin iontophoresis for up to 30 minutes can be sustained before depletion occurs with this apparatus.18 Accordingly, a conservative approach suggests that iontophoresis for about 20 minutes will give maximum antibiotic loading, while ensuring that depletion does not occur.18
In this study, we aimed to determine whether iontophoresis of vancomycin through bone was a viable procedure and to find its optimum conditions. We did this by evaluating the vancomycin loading produced via differing concentrations of drug, voltages and time taken for iontophoresis. A central-composite design was chosen to allow a response surface to be fitted and optimal conditions estimated.20 The design was chosen to be rotatable (to allow uniform variance) and to cover the whole reasonable range of conditions that could be achieved with the apparatus.
Materials and Methods
For the model, we used sections of sheep tibial diaphysis 20 mm long with the marrow removed, which were sealed at one end with an acrylic disc and extended at the other end with a 30 mm acrylic tube. Both were attached to the bone with cyanoacrylate glue. This effectively extended the medullary canal, increasing the internal volume and allowing the experimental segment to be completely submerged in an electrolyte solution, while restricting any ion exchange to the experimental segment of bone. This solution aids in the movement of charged antibiotic particles through the bone, will not impair the function or survival of bone cells, and has been used in those experiments testing gentamycin.18 The prepared bone was placed in a beaker surrounded by a metal mesh to form the anode. The beaker was then filled with Ringer’s solution for irrigation (Baxter, Sydney, Australia) to just below the open end of the acrylic tube. We then placed 3.4 ml of varying concentrations of vancomycin into the medullary canal (enough to fill completely) and placed a metal rod electrode (cathode) centrally into the canal to form an iontophoresis cell with radial geometry and with the bone as a membrane between the two half cells18 (Fig. 2).
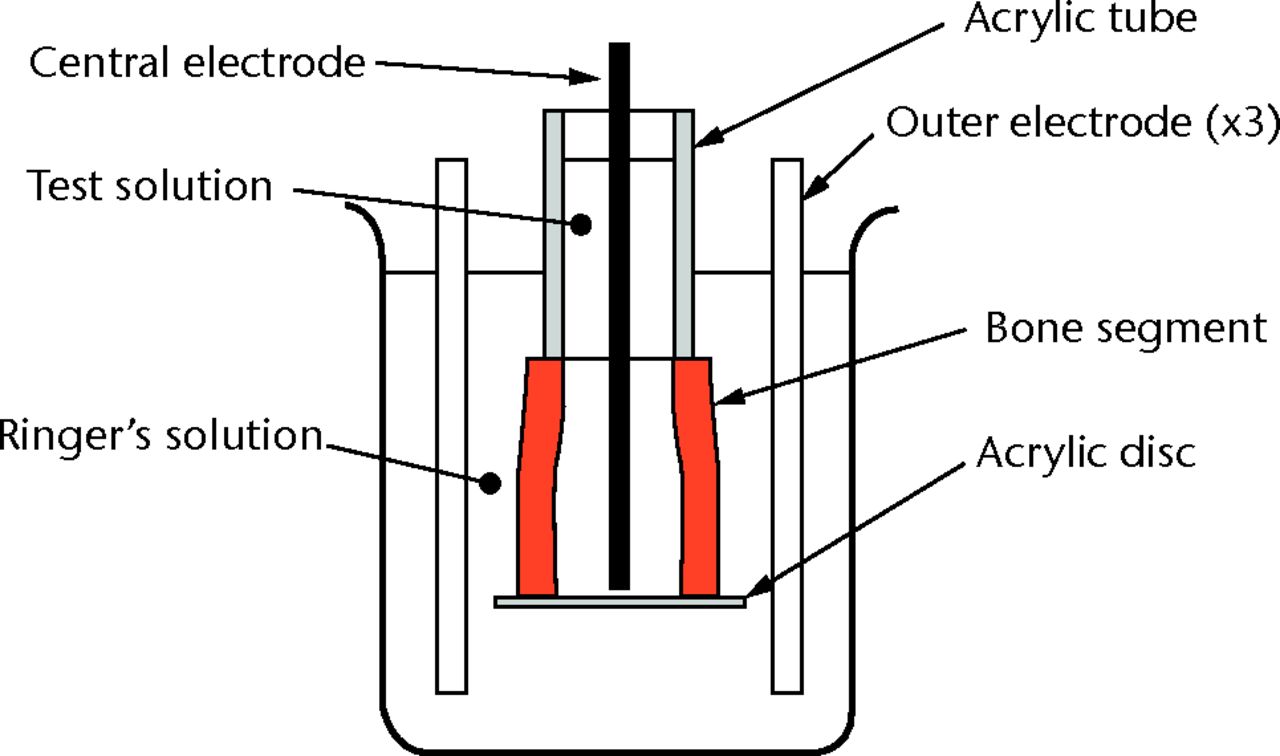
Fig. 2
Diagram showing the iontophoresis cell.
The range of experimental conditions was chosen to cover the whole practical range (Table I). Within these limits, the combination of conditions were chosen to implement a rotatable central composite design, as shown in Table II.
Table I
Limits on conditions
| Time (mins) | Voltage (v) | Concentration (%) | |
|---|---|---|---|
| Min | 10 | 10 | 1 |
| Max | 60 | 110 | 5 |
Table II
Experimental parameters
| Group | Sample | Mass (grams) | Time (mins) | Voltage (v) | Concentration (%) | Bone | End* |
|---|---|---|---|---|---|---|---|
| 1 | C1.1 | 0.105 | 14 | 30.3 | 1.8 | 1 | prox |
| 1 | C1.2 | 0.1058 | 26 | 30.3 | 1.8 | 2 | prox |
| 1 | C1.3 | 0.102 | 14 | 89.7 | 1.8 | 3 | prox |
| 1 | C1.4 | 0.1024 | 26 | 89.7 | 1.8 | 4 | prox |
| 1 | C1.5 | 0.1021 | 14 | 30.3 | 4.2 | 5 | prox |
| 1 | C1.6 | 0.108 | 26 | 30.3 | 4.2 | 6 | prox |
| 1 | C1.7 | 0.102 | 14 | 89.7 | 4.2 | 7 | prox |
| 1 | C1.8 | 0.1028 | 26 | 89.7 | 4.2 | 8 | prox |
| 1 | C1.9 | 0.1029 | 20 | 60 | 3 | 9 | prox |
| 1 | C1.10 | 0.1026 | 20 | 60 | 3 | 10 | prox |
| 1 | C1.11 | 0.1026 | 20 | 60 | 3 | 11 | prox |
| 2 | S2.1 | 0.103 | 10 | 60 | 3 | 2 | dist |
| 2 | S2.2 | 0.101 | 30 | 60 | 3 | 1 | dist |
| 2 | S2.3 | 0.101 | 20 | 10 | 3 | 3 | dist |
| 2 | S2.4 | 0.102 | 20 | 110 | 3 | 7 | dist |
| 2 | S2.5 | 0.103 | 20 | 60 | 1 | 9 | dist |
| 2 | S2.6 | 0.103 | 20 | 60 | 5 | 8 | dist |
| 2 | S2.7 | 0.101 | 20 | 60 | 3 | 5 | dist |
| 2 | S2.8 | 0.103 | 20 | 60 | 3 | 4 | dist |
| 2 | S2.9 | 0.101 | 20 | 60 | 3 | 6 | dist |
| 3 | C1.1 | 0.0999 | 14 | 30.3 | 1.8 | 1 | prox |
| 3 | C1.2 | 0.1024 | 26 | 30.3 | 1.8 | 2 | prox |
| 3 | C1.3 | 0.1037 | 14 | 89.7 | 1.8 | 3 | prox |
| 3 | C1.4 | 0.102 | 26 | 89.7 | 1.8 | 4 | prox |
| 3 | C1.5 | 0.1005 | 14 | 30.3 | 4.2 | 5 | prox |
| 3 | C1.6 | 0.0999 | 26 | 30.3 | 4.2 | 6 | prox |
| 3 | C1.7 | 0.105 | 14 | 89.7 | 4.2 | 7 | prox |
| 3 | C1.8 | 0.1006 | 26 | 89.7 | 4.2 | 8 | prox |
| 3 | C1.9 | 0.1 | 20 | 60 | 3 | 9 | prox |
| 3 | C1.10 | 0.1015 | 20 | 60 | 3 | 10 | prox |
| 3 | C1.11 | 0.102 | 20 | 60 | 3 | 11 | prox |
| 4 | MVC1.1 | 0.1 | 60 | 110 | 5 | 12 | dist |
| 4 | MVC1.2 | 0.1 | 60 | 110 | 5 | 12 | prox |
| 4 | MVC1.3 | 0.1 | 60 | 110 | 5 | 13 | prox |
-
*Prox, proximal; Dist, distal
Quantitative analysis
Following iontophoresis, the bone segments were washed in distilled water and dried before a central ring of 2 mm was cut from the iontophoresed bone using a slow speed diamond saw (Buehler IsoMet; Buehler Ltd., Lake Bluff, Illinois). This was then placed on an agar plate flooded with a heavy suspension of sensitive Staphylococcus aureus (S. aureus) for qualitative analysis (Fig. 3). A small amount of bone dust was then ground from the two cut ends of the tibia. A magnet was passed over the powder (to remove any impurities from the filing) and 2 ml of distilled water was added to a measured mass (0.01g) of the powdered sample. This was then agitated using ultrasound for 30 minutes. After centrifuging, the supernatant fluid was removed. A further 2 ml of fluid was added and the agitation and centrifuging process was repeated twice more, the last (4th) was left to soak for 12 hours before centrifuging. The specimens were refrigerated to 4°C during this period to minimise degradation. Pilot studies established that no further vancomycin could be extracted after this process.
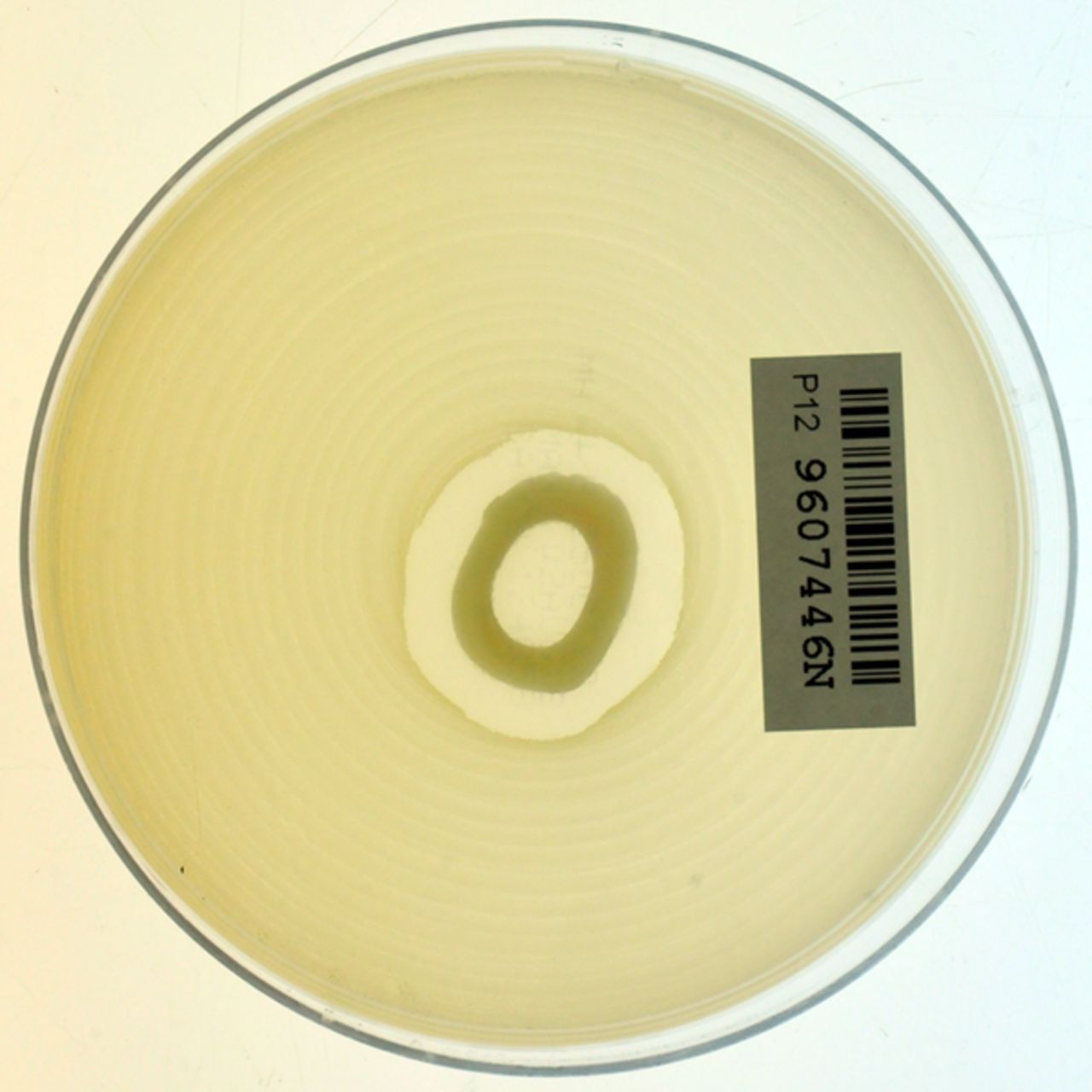
Fig. 3
Agar showing antibiotic activity (against MRSA) of iontophoresed allograft.
The supernatant solutions were analysed for vancomycin using fluorescence polarisation immunoassay (Abbott Axsym Analyser; Abbott Laboratories, Abbott Park, Illinois).
Statistical analysis
The results of the vancomycin assay were assessed using the rsm package version 2.0 (IBM, Armonk, United States)21 and R version 2.15.3 (Bell laboratories, Murray Hill, New Jersey).22 The experiment was designed as a balanced, rotatable central-composite design to allow for a full second order analysis.20 This was done as it was expected that there would be a peak for vancomycin loading as a result of some combination of conditions, based on previous experience with gentamicin iontophoresis.18
A first order (linear) model was initially fit to the results. If the lack of fit was significant then a second order (quadratic) model was used. Significance was set at p < 0.05 (95% confidence interval (CI)).
Results
Results of the iontophoresis with vancomycin at different concentrations, voltages and times are recorded in Table III. The minimum concentration of vancomycin recorded post iontophoresis was 309 µg/g bone, and the maximum was 2204 µg/g bone (Table III). Even at the minimum, we achieved concentrations at least comparable with gentamycin iontophoresis (180 µg/g).
Table III
Results
| Sample | Group | Bone | Time (min) | Voltage (v) | Concentration (%) | Dust mass (g) | Vancomycin amount (µg) | Vancomycin concentration (µg vanc/g bone) |
|---|---|---|---|---|---|---|---|---|
| S2.2 | 2 | 1 | 30 | 60 | 3 | 0.101 | 87.05 | 861.8811881 |
| S2.1 | 2 | 2 | 10 | 60 | 3 | 0.103 | 84.825 | 823.5436893 |
| S2.3 | 2 | 3 | 20 | 10 | 3 | 0.101 | 71.05 | 703.4653465 |
| S2.8 | 2 | 4 | 20 | 60 | 3 | 0.103 | 67.95 | 659.7087379 |
| S2.7 | 2 | 5 | 20 | 60 | 3 | 0.101 | 209.475 | 2074.009901 |
| S2.9 | 2 | 6 | 20 | 60 | 3 | 0.101 | 145.7 | 1442.574257 |
| S2.4 | 2 | 7 | 20 | 110 | 3 | 0.102 | 159.725 | 1565.931373 |
| S2.6 | 2 | 8 | 20 | 60 | 5 | 0.103 | 50.575 | 491.0194175 |
| S2.5 | 2 | 9 | 20 | 60 | 1 | 0.103 | 31.575 | 306.5533981 |
| MVC1.1 | 4 | 12 | 60 | 110 | 5 | 0.1 | 41.625 | 416.25 |
| C1.1 | 1 | 1 | 14 | 30.3 | 1.8 | 0.105 | 29.7 | 282.8571429 |
| C1.1 | 3 | 1 | 14 | 30.3 | 1.8 | 0.0999 | 35.825 | 358.6086086 |
| C1.2 | 1 | 2 | 26 | 30.3 | 1.8 | 0.1058 | 86.7 | 819.4706994 |
| C1.2 | 3 | 2 | 26 | 30.3 | 1.8 | 0.1024 | 83.15 | 812.0117188 |
| C1.3 | 1 | 3 | 14 | 89.7 | 1.8 | 0.102 | 75.7 | 742.1568627 |
| C1.3 | 3 | 3 | 14 | 89.7 | 1.8 | 0.1037 | 61.675 | 594.7444552 |
| C1.4 | 1 | 4 | 26 | 89.7 | 1.8 | 0.1024 | 83.725 | 817.6269531 |
| C1.4 | 3 | 4 | 26 | 89.7 | 1.8 | 0.102 | 57.975 | 568.3823529 |
| C1.5 | 1 | 5 | 14 | 30.3 | 4.2 | 0.1021 | 113.275 | 1109.451518 |
| C1.5 | 3 | 5 | 14 | 30.3 | 4.2 | 0.1005 | 35.325 | 351.4925373 |
| C1.6 | 1 | 6 | 26 | 30.3 | 4.2 | 0.108 | 174.2 | 1612.962963 |
| C1.6 | 3 | 6 | 26 | 30.3 | 4.2 | 0.0999 | 80.625 | 807.0570571 |
| C1.7 | 1 | 7 | 14 | 89.7 | 4.2 | 0.102 | 143.65 | 1408.333333 |
| C1.7 | 3 | 7 | 14 | 89.7 | 4.2 | 0.105 | 69.675 | 663.5714286 |
| C1.8 | 1 | 8 | 26 | 89.7 | 4.2 | 0.1028 | 351.475 | 3419.01751 |
| C1.8 | 3 | 8 | 26 | 89.7 | 4.2 | 0.1006 | 204.975 | 2037.524851 |
| C1.9 | 1 | 9 | 20 | 60 | 3 | 0.1029 | 29.45 | 286.2001944 |
| C1.9 | 3 | 9 | 20 | 60 | 3 | 0.1 | 30.975 | 309.75 |
| C1.10 | 1 | 10 | 20 | 60 | 3 | 0.1026 | 216.05 | 2105.750487 |
| C1.10 | 3 | 10 | 20 | 60 | 3 | 0.1015 | 227.375 | 2240.147783 |
| C1.11 | 1 | 11 | 20 | 60 | 3 | 0.1026 | 170.875 | 1665.448343 |
| C1.11 | 3 | 11 | 20 | 60 | 3 | 0.102 | 102.375 | 1003.676471 |
| MVC1.2 | 4 | 12 | 60 | 110 | 5 | 0.1 | 64.45 | 644.5 |
| MVC1.3 | 4 | 13 | 60 | 110 | 5 | 0.1 | 114.375 | 1143.75 |
The minimum Inhibitory concentration (MIC) for vancomycin is > 1 µg/ml. We have clearly exceeded this in our experiments, thus we can be sure that the loaded bone would be effective in combatting local MRSA infections.
The first order fit of vancomycin to the whole data set gave a statistically significant result (p = 0.0233), with a significant concentration (p = 0.02774) component (Fig. 4). The time component was the next most significant (p = 0.0597) but did not exceed the 95% CI level (Fig. 5). Increasing voltage showed a tendency to improve loading of bone but this was not statistically significant (Fig. 6). The overall lack of fit was not significant (p = 0.3984), so no second order fit was attempted.
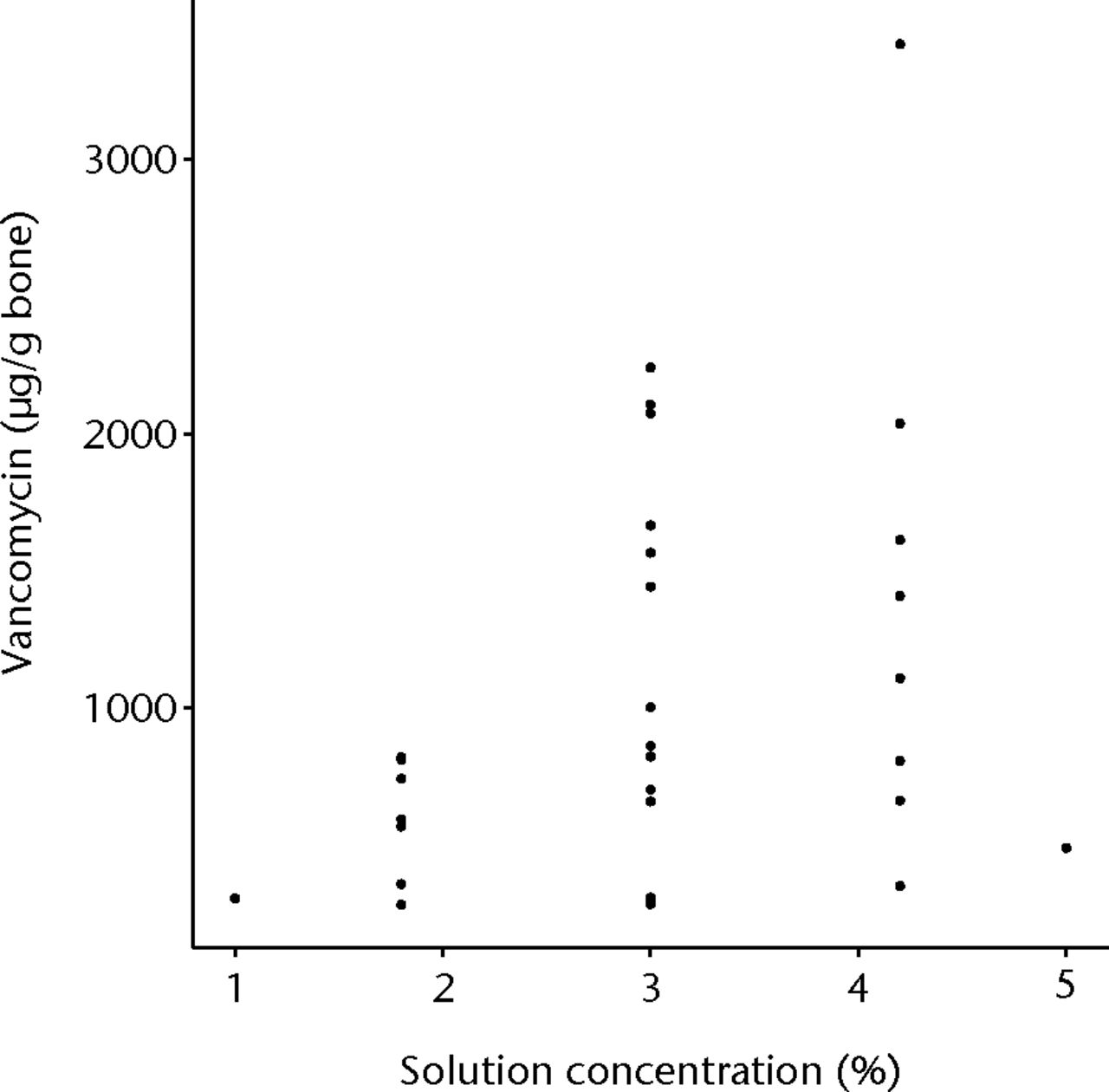
Fig. 4
Graph showing vancomycin in bone, with increasing concentration of vancomycin solution
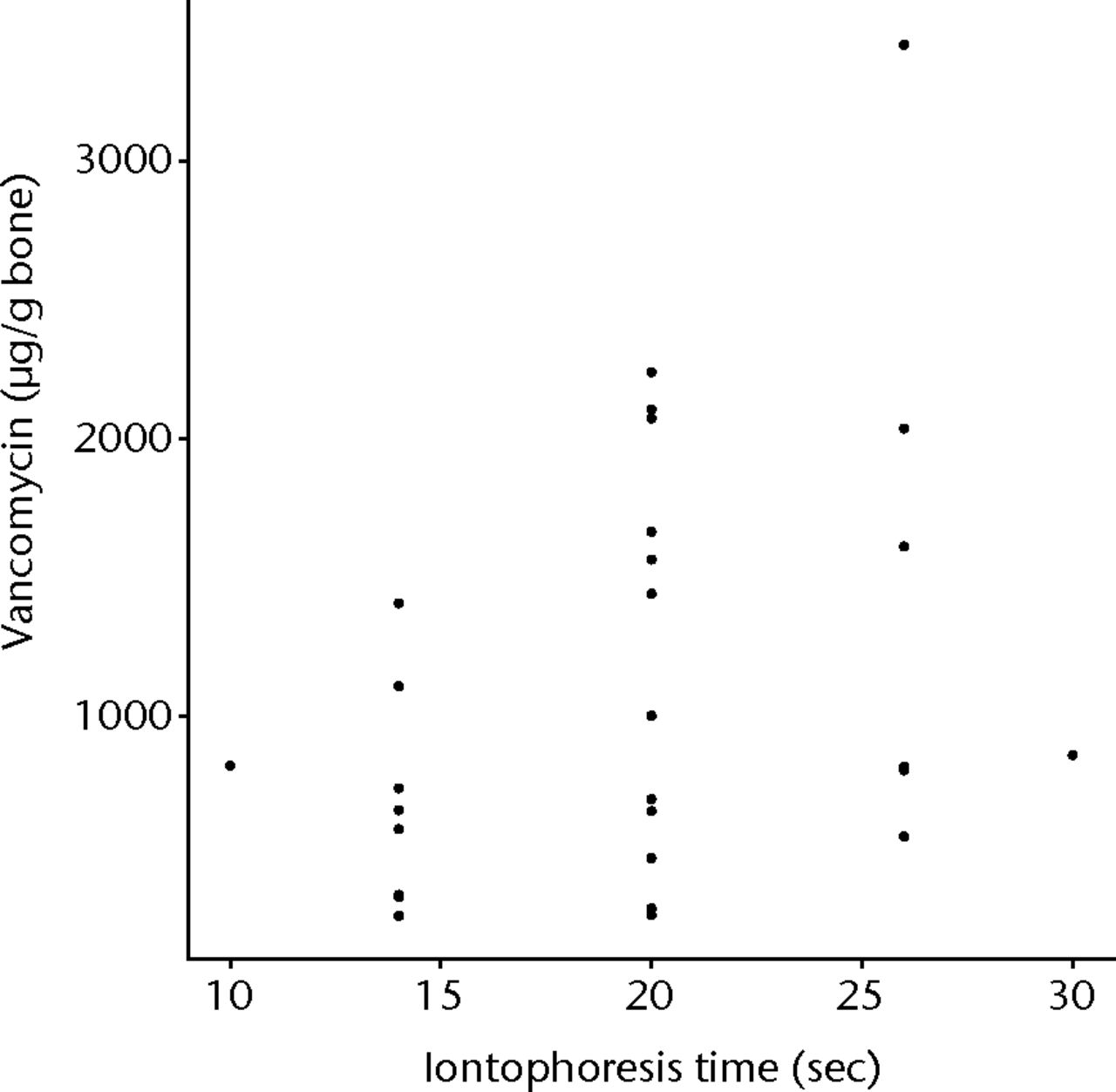
Fig. 5
Graph showing vancomycin found within allograft when iontophoresed for increasing lengths of time
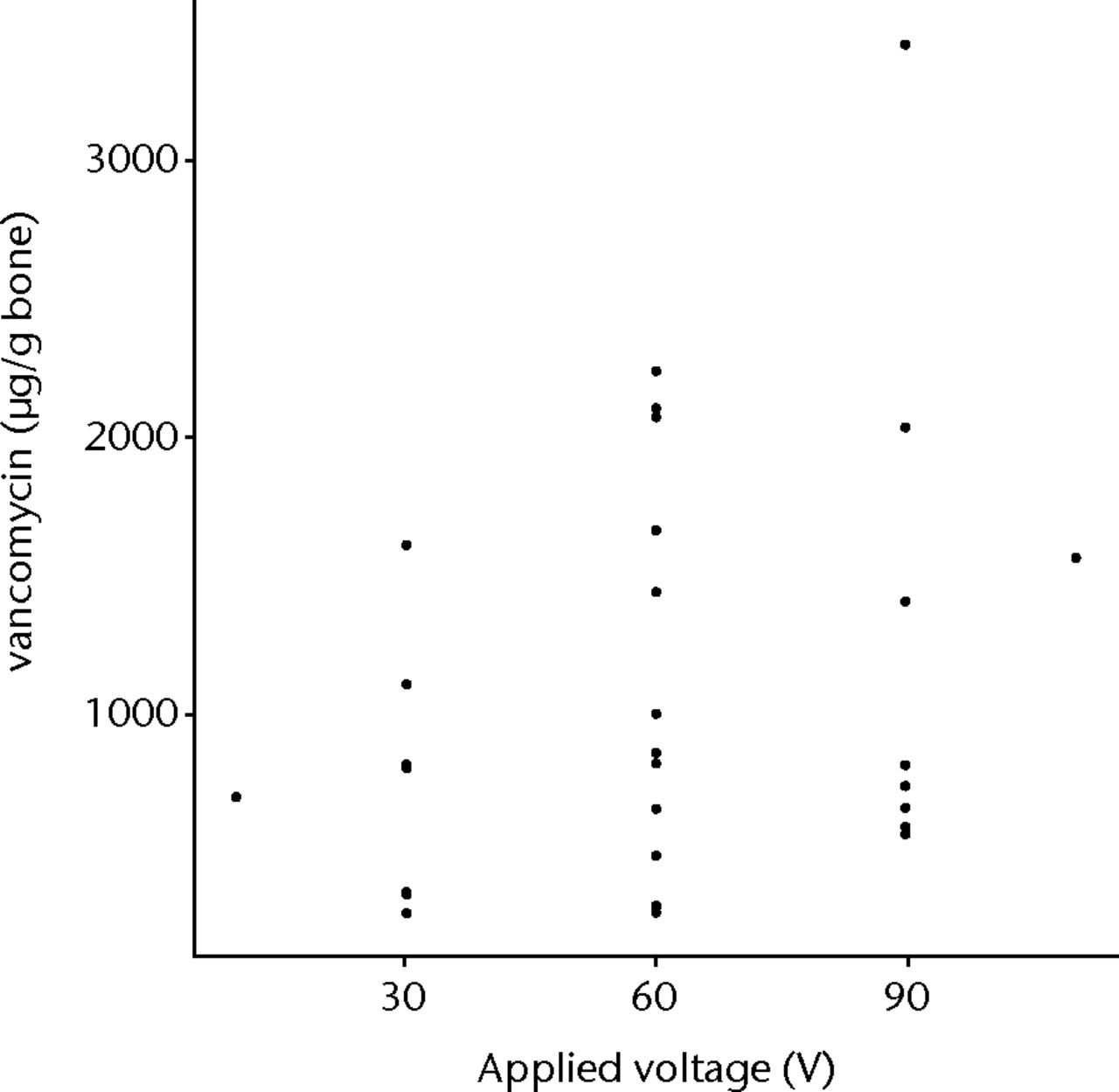
Fig. 6
Graph showing vancomycin found within allograft when iontophoresed with increasing voltage
Examination of the plot of the response surface showed that the highest predicted vancomycin load would be with all conditions at the maximum levels. As the voltage and concentration could not be increased and to determine if there was a falling off of loading with time, (as is the case with gentamicin) a further three segments were loaded for 60 minutes using 110 v and a 5% concentration. The mean value for this experiment was 734.83 µg/g bone (645.21 to 864.86), well below the model predicted 4433.7 µg bone. We believe that this is because the vancomycin is driven into, through and out of the bone as time goes on.
Discussion
The use of local antibiotic therapy for prophylaxis and treatment of infection in orthopaedic practice is well established.23,24 Much work has been done on the use of polymethylmethacrylate beads and antibiotic loaded cement in the clinical setting.23 The aim of local treatment is to achieve high concentrations of the therapeutic agent at the site of the pathology, while avoiding the possible complications of high systemic levels. Allograft associated infections are problematic and treatment often involves resection arthroplasty.25,26 Iontophoretically-loaded allograft bone can achieve this desirable situation, while able to incorporate and load the host bone effectively. Furthermore, because the antibiotics diffuse out from all surfaces of the bone, it is surmised that the concentration of antibiotics will be highest at the surface of the bone, even inside small holes and cracks. This is in contrast with supplementary antibiotics introduced into the wound, whether from the bloodstream or by antibiotic-loaded cement, since the concentration gradient is in the opposite direction. By supplementing the bone directly, iontophoresis should ensure antibiotic cover even in the smallest crevices and cracks on the surface, where bacteria would normally be protected from the body’s defences.
We know from in vitro studies that the majority of antibiotic elution from the allograft occurs over the first two weeks18 and that this is effective in preventing early post-operative infections, which are the significant concern in these cases. Furthermore, in Khoo et al’s study27 iontophoresed segmental allografts (proximal and distal femoral) were implanted for infected arthroplasty salvage. They studied the elution of antibiotics from the grafts and found that clinically significant (above MIC) levels of antibiotic were found locally for up to 48 hours, post iontophoresis. They noted a 100% graft retention with no infective complications at a mean of 47 months.27 As our study with vancomycin iontophoresis was as effective as the in vitro gentamicin iontophoresis, we can assume that the elution of antibiotic is similar.
Previously, it has been demonstrated that iontophoresis can produce concentrations of 180 µg bone of gentamycin, and 34 µg/g of flucloxacillin within treated bone, and that elution of the antibiotic from bone continues for up to two weeks.18 The minimum concentration of vancomycin recorded post iontophoresis was 309 µg/g bone, and the maximum was 2204 µg/g bone. Even in the worst case, these concentrations were comparable with gentamicin iontophoresis (which we know to be effective against early allograft infections).27 As the MIC against S. aureus for vancomycin is > 1 µg/g and > 2 for gentamicin, we can be confident that both drugs can be successfully loaded to segmental graft using this method and deliver clinically effective concentrations locally. As we had far exceeded the effective MIC for vancomycin (1 µg/ml) for it to be active, even in the worst case scenario by 300%, we felt that no further testing was necessary to prove this an effective method for impregnating segmental allograft with vancomycin. Admittedly further optimisation testing may have shown statistical significance with increasing voltage and time parameters. However, this would be clinically unimportant as with easily manageable parameters (of 110 mV for 30 minutes with a concentration of 5% vancomycin) we could clearly load segmental allograft reproducibly at levels far exceeding the MIC required.
There is significant variation in the concentrations of vancomycin found within the individual bone segments. This may be due to variations in the cortical thickness, density or porosity of the individual bone segments, all of which would be expected to affect vancomycin loading. Theoretically, we could have performed micro CT to determine the porosity and microstructures of the grafts prior to iontophoresis in order to have standardised this variable; this is not routinely performed prior to implanting allograft bone and thus seemed unimportant in our study. However, notwithstanding the variation, we determined that there was a statistically significant linear increase in the amount of vancomycin that can be iontophoresed into segmental allograft with increasing concentration (p = 0.02774). There were also increases with larger voltage and with time, although these were not statistically significant in this study. Given the large variation between segments, many more results would be required to determine if there is a significant effect of these variables. It is unlikely that such information would change the clinical application of this technique.
We wanted to see whether, as with gentamicin, increasing the time would lead to depletion of vancomycin within the graft as it is driven through, and out of, the graft. In fact, we found that up to an hour of iontophoresis increased the amount of vancomycin in the bone compared with the mean 30 minute value, but this was much less than the predicted amount from the linear model. This is likely due to the fact that the antibiotic is driven into and then out of the other side of the bone at longer periods than 30 minutes. This would tend to indicate that the linear model is not a good fit in this region of the process, but no experiments were carried out to verify this, as the information we have gathered from this study is enough to use this design in a clinical capacity.
Conclusion
We have demonstrated that vancomycin can be successfully delivered to segmental allograft bone using iontophoresis and that increasing the concentration, voltage and time can improve delivery. Optimal conditions reproducible in theatre, based on our current practice for gentamycin iontophoresis, are to use 5% vancomycin in sterile water at 110 v for 30 minutes. Longer times are not detrimental, but only give a small increase in the amount of vancomycin delivered and are therefore, we feel, unnecessary.
Acknowledgements: We wish to thank S. Y. Lim for preparing the analysis samples, and the Perth Bone and Tissue Bank (AKA Plusfile) for their support with the study.
1 Clohisy DR , MankinHJ. Osteoarticular allografts for reconstruction after resection of a musculoskeletal tumor in the proximal end of the tibia. J Bone Joint Surg [Am]1994;76-A:549–554.CrossrefPubMed Google Scholar
2 Delloye C , De NayerP, AllingtonN, et al.Massive bone allografts in large skeletal defects after tumor surgery: a clinical and microradiographic evaluationArch. Orthop Trauma Surg1988;107:31–41. Google Scholar
3 Jofe MH , GebhardtMC, TomfordWW, MakinHJ. Reconstruction for defects of the proximal part of the femur using allograft arthroplasty. J Bone Joint Surg [Am]1988;70-A:507–516.PubMed Google Scholar
4 Mankin HJ , GebhardtMC, JenningsLC, SpringfieldDS, TomfordWW. Long term results of allograft replacement in the management of bone tumorsClin Orthop. Relat Res1996;324:86–97. Google Scholar
5 Musculo DL , AyerzaMA, CalabreseME, GruenbergM. The use of a bone allograft for reconstruction after resection of giant-cell tumor close to the knee. J Bone Joint Surg [Am]1993;75-A:1656–1662.CrossrefPubMed Google Scholar
6 Dion N , SimFH. The use of allografts in orthopaedic surgery. Part I: the use of allografts in musculoskeletal oncology. J Bone Joint Surg [Am]2002;84-A:644–654. Google Scholar
7 Alexeef M , MahomedN, MorsiE, GarbuzD, GrossA. Structural allograft in two stage revisions for failed septic hip arthroplasty. J Bone Joint Surg [Br]1996;78-B:213–216. Google Scholar
8 Berrey BH Jr , LordCF, GebhardtMC, MankinHJ. Fractures of allografts: frequency, treatment and end-results. J Bone Joint Surg [Am]1990;72-A:825–833. Google Scholar
9 Edwards SA , PanditHG, GroverML, ClarkeHJ. Impaction bone grafting in revision hip surgery. J Arthroplasty2003;18:852–859.CrossrefPubMed Google Scholar
10 Haddad FS , GarbuzDS, MasriBA, DuncanCP. Structural proximal femoral allografts for failed total hip replacements: a minimum review of five yearsJ Bone. Joint Surg [Br]2000;82-B:830–836. Google Scholar
11 Toms AD , BarkerRL, JonesRS, KuiperJH. Impaction bone-grafting in revision joint replacement surgery. J Bone Joint Surg [Am]2004;86-A:2050–2060.CrossrefPubMed Google Scholar
12 Chan Y- S , UengSWN, WangCJ, et al.Management of small infected tibial defects with antibiotic-impregnated autogenic cancellous bone grafting. J Trauma1998;45:758–764.CrossrefPubMed Google Scholar
13 Lord CF , GebhardtMC, TomfordWW, MankinHJ. Infection in bone allografts: incidence, nature and treatment. J Bone Joint Surg [Am]1988;70-A:369–376. Google Scholar
14 Cara JA , LaclérigaA, CañadellJ. Intercalary bone allografts: 23 tumour cases followed for 3 years. Acta Orthop Scand1994;65:42–46. Google Scholar
15 Dick HM , StrauchRJ. Infection of massive bone allografts. Clin Orthop Relat Res1994;306:46–53.PubMed Google Scholar
16 McGann W , MankinHJ, HarrisWH. Massive allografting for severe failed total hip replacement. J Bone Joint Surg [Am]1986;68-A:4–12.PubMed Google Scholar
17 Witso E , PersenL, LosethK, BenumP, BerghK. Cancellous bone as an antibiotic carrier. Acta Orthop Scand2000;71:80–84.CrossrefPubMed Google Scholar
18 Day RE , MegsonS, and WoodD. Iontophoresis as a means of delivering antibiotics into allograft bone. J Bone Joint Surg [Br]2005;87-B:1568–1574.CrossrefPubMed Google Scholar
19 Michalak KA , KhooPP, YatesPJ, DayRE, WoodDJ. Iontophoresed segmental allografts in revision arthroplasty for infection. J Bone Joint Surg [Br]2006;88-B:1430–1437.CrossrefPubMed Google Scholar
20 No authors listed. NIST/SEMATECH e-Handbook of Statistical Methods http://www.itl.nist.gov/div898/handbook/pri/section3/pri33.htm (date last accessed 13 February 2014). Google Scholar
21 Lenth RV . Response-surface methods in r, using rsm. J Stat Soft2009;7:1-17. Google Scholar
22 No authors listed. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ (date last accessed 13 February 2014). Google Scholar
23 Leigh D , MarrinerJ, NisbetD, et al.Bone concentrations of cefuroxime and cegamandole in the femoral head in 96 patients undergoing total hip replacement surgery. J Antimicrob Chemother1982;9:303–311. Google Scholar
24 Henry SL , GallowayKP. Local antibacterial therapy for the management of orthopaedic infections: pharmocokinetic considerations. Clin Pharmacokinet1995;29:36–45. Google Scholar
25 Ammon P , StockleyI. Allograft bone in two-stage revision of the hip for infection: is it safe?J Bone Joint Surg [Br]2004;86-B:962–965.CrossrefPubMed Google Scholar
26 Berry DJ , ChandlerHP, ReillyDT. The use of bone allografts in two-stage reconstruc- tion after failure of hip replacements due to infection. J Bone Joint Surg [Am]1991;73-A:1460–1468. Google Scholar
27 Khoo PP , MichalakKA, YatesPJ, et al.Iontophoresis of antibiotics into segmental allografts. J Bone Joint Surg [Br]2006;88-B:1149–1157.CrossrefPubMed Google Scholar
Funding statement:
This work was partially supported by NH& MRC grant 513865
Author contributions:
M. C. Edmondson: Performed experiments, Data collection, Data analysis, Writing the paper
R. Day: Assisted with planning experiments, Data analysis, Assisted with writing the paper
D. Wood: Iontophoresis concept
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









