Abstract
Objectives
We aimed to determine the effect of surgical approach on the histology of the femoral head following resurfacing of the hip.
Methods
We performed a histological assessment of the bone under the femoral component taken from retrieval specimens of patients having revision surgery following resurfacing of the hip. We compared the number of empty lacunae in specimens from patients who had originally had a posterior surgical approach with the number in patients having alternative surgical approaches.
Results
We found a statistically significant increase in the percentage of empty lacunae in retrieval specimens from patients who had the posterior approach compared with other surgical approaches (p < 0.001).
Conclusions
This indicates that the vascular compromise that occurs during the posterior surgical approach does have long-term effects on the bone of the femoral head, even if it does not cause overt avascular necrosis.
Cite this article: Bone Joint Res 2013;2:200–5.
Article focus
We studied the long-term effects of surgical approach on vascularity of femoral head using retrieval specimens from failed hip resurfacings
We used a nuclei-counting method to compare the vascularity of femoral heads between posterior and anterior type approaches, such as the trochanteric flip and anterolateral approaches
The research question we aimed to answer by this study is: “Does the posterior surgical approach have any long-term effects on vascularity of the femoral head compared with other anterior-type approaches?”
Key messages
There is a statistically significant increase in the percentage of empty lacunae in retrieval specimens from patients who had the posterior approach compared with other surgical approaches
The posterior surgical approach does have long-term effects on the bone of the femoral head, even if it does not cause overt avascular necrosis
Strengths and limitations
The main strength of this study was the systematic analysis of 47 histology slides from 12 retrieval specimens
Limitations include the relatively small number of specimens and the potential effect of thermal necrosis in cemented specimens
Introduction
Modern hip resurfacing arthroplasty has been a popular option for treating young active patients with arthritis of the hip for the last decade.1 Its potential advantages over conventional total hip replacement (THR) include relatively less bone resection, lower dislocation rates and the ability to convert to a THR in a subsequent operation if the need arises.2,3
There have been encouraging reports of medium-term survival of metal-on-metal (MoM) resurfacing of the hip,4 but complications such as avascular necrosis, persistent groin pain, neck reabsorption, pseudotumours and metallosis have been noted as reasons for failure.5,6 Several studies have reported these complications and attempted to understand the underlying causes.7-9 When resurfacing was first introduced, the role of blood flow to the femoral head and the surgical approach was considered to play a key role in deciding the final outcome of surgery.10-12 In a previous study,13 we reported that the fall in the intra-operative blood flow to the femoral head is higher with the posterior approach compared with the trochanteric flip approaches during resurfacing of the hip.13 However, a follow-up study revealed that at the end of one year, the vascularity had returned to similar levels in both groups.14
In this study our aim is to assess the vascular compromise between posterior surgical approach and alternative approaches in 12 retrieval specimens taken from failed hip resurfacing arthroplasties.
Materials and Methods
The regional Research and Development department and the local ethics committee approved the study.
We retrieved femoral heads from consecutive patients who underwent revision for failed resurfacing arthroplasty at a single institution (University Hospitals of Coventry and Warwickshire NHS Trust) between August 2007 and August 2009. The exclusion criteria were badly damaged femoral heads that were unsuitable for the retrieval analysis and patients with multiple surgeries of the resurfaced hip before revision. None of the initial surgery was performed for avascular necrosis (AVN) as it is considered a contraindication for hip resurfacing in our institution.
A total of 19 implants were retrieved. Seven were excluded: five femoral heads were badly damaged and unsuitable for retrieval analysis and two patients had undergone multiple surgeries on the hip. The 12 remaining femoral heads were divided into two groups based on the surgical approach used during the initial resurfacing operation. The patient details are given in Table I.
Table I
Patient details of the two groups
| Characteristics | Other approaches*(n = 6) | Posterior approach (n = 6) | ||
|---|---|---|---|---|
| Mean age at revision (yrs) (range) | 52 (37 to 66) | 54 (40 to 57) | ||
| Male (n, %) | 3 (50) | 2 (33) | ||
| Fixation (n, %) | ||||
| Cemented | 4 (67) | 4 (67) | ||
| Uncemented | 2 (33) | 2 (33) | ||
| Mean time since initial surgery to revision (mths) (range) | 43 (11 to 70) | 40 (8 to 120) | ||
-
* comprising four trochanteric flip and two anterolateral approaches
Preparation of specimens
At the time of revision surgery, the specimens were transported in 0.9% normal saline containers and then stored in 10% neutral buffered formalin solution. All specimens were handled according to national guidelines on human tissue use.15
The specimens were transported to the retrieval analysis laboratory (Orthopaedic Hospital, University of California Los Angeles). At the laboratory, each specimen was orientated and fixed in a supportive cement block. Two sections 2.5 mm thick were cut using an implant cutting saw (Exakt Technologies, Gottingen, Germany); one from the anterior and the other from the posterior half of each specimen. In order to maintain uniformity and accuracy of the cuts, a cutting diagram (Fig. 1) was used as a template to mark each specimen.

Fig. 1
Cutting diagram showing the coronal sections of the femoral head.
Preparation of histological slides
The metal implant and adjacent bone cement was then removed from each section. Each anterior and each posterior section was further cut into a superior and an inferior half. The sections were then decalcified using decalcifying solution (Surgipath Decalcifier II solution; Surgipath Medical Industries, Inc., Richmond, Illinois).
Once decalcification was complete, the sections were embedded in paraffin, and sectioned at 5 µm. The sections were mounted on slides and stained with haematoxylin & eosin. A total of 47 slides were analysed; 24 in the posterior approach group and 23 in the non-posterior group.
Each slide was scanned using a slide scanner (3DHistech Ltd, Budapest, Hungary)and viewed using the corresponding software on a computer.The degree of bone necrosis was assessed by counting the percentage of empty lacunae within a single strip of bone in the middle of each section.8,16 A rectangular area 0.5 mm wide extending vertically from the proximal to the distal end at the mid-point of the slide was selected as the counting area (Fig. 2).
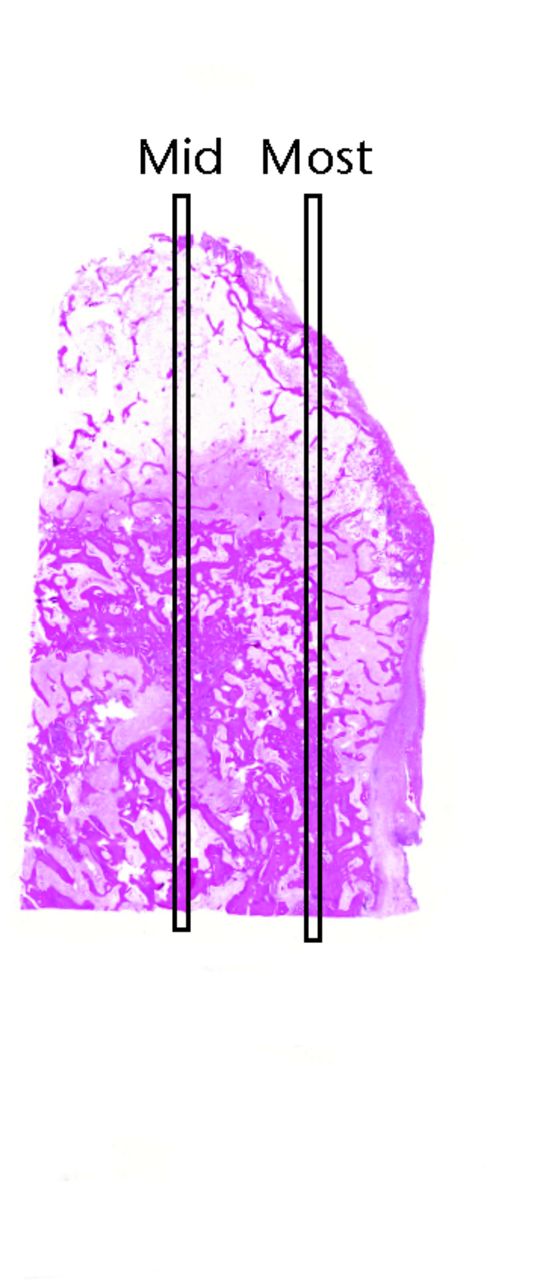
Fig. 2
Sample slide showing where the ‘mid’ and ‘most’ strips are marked for counting.
The mid strip was selected as it gave the widest cross section from margin to the centre of the bone. However, to ensure that the ‘mid’ strip was representative of the bone within the slide, we performed a comparison study in 13 slides: seven from the non-posterior and six from the posterior group (Table II). The number of nuclei in the ‘mid’ strip was compared with an identical strip from another area identified as the ‘most’ strip (Fig. 2).
Table II
Comparison study results
| Slide | Empty lacunae in ‘mid’ strip (%) | Empty lacunae in ‘most’ strip (%) | p-value (chi-squared) | |||
|---|---|---|---|---|---|---|
| Non-posterior | ||||||
| 1 | 23.80 | 26.88 | 0.745 | |||
| 2 | 21.42 | 22.61 | 0.896 | |||
| 3 | 15.45 | 19.28 | 0.356 | |||
| 4 | 16.38 | 14.06 | 0.665 | |||
| 5 | 19.56 | 18.09 | 0.989 | |||
| 6 | 16.88 | 15.51 | 0.958 | |||
| 7 | 8.22 | 7.65 | 0.975 | |||
| Posterior | ||||||
| 1 | 18.37 | 18.37 | 0.910 | |||
| 2 | 18.93 | 20.84 | 0.601 | |||
| 3 | 23.36 | 24.85 | 0.708 | |||
| 4 | 26.50 | 27.71 | 0.765 | |||
| 5 | 24.95 | 36.47 | < 0.001 | |||
| 6 | 54.44 | 51.16 | 0.525 |
Data and statistical analysis
Any lacunae that did not have a nucleus were defined as ‘empty’. Any nuclei that were seen within a lacuna or within the counting area were included in the count. All lacunae that were on the borders were considered as inside and counted.
We tested the intra- and inter-observer variability in nuclei count (Table III). A selected area of six slides were assessed, the lacunae were counted and classified as either nucleated or empty by two authors (HWA, JM) and by the same individual (HWA) at two separate occasions two days apart. The proportions of empty cells were compared using a two-sample test for equality of proportions (R statistical software; University of Vienna, Vienna, Austria).17
Table III
Total cell counts and empty cell rates by group (non-posterior and posterior) and section (anterior and posterior)
| Group/Section | Cell count (n) | Nucleated (n) | Empty (n, %) |
|---|---|---|---|
| Non-posterior | |||
| Anterior | 2307 | 1758 | 549 (23.8) |
| Posterior | 4329 | 3251 | 1078 (24.9) |
| Posterior | |||
| Anterior | 3993 | 2559 | 1434 (35.9) |
| Posterior | 4117 | 2659 | 1458 (35.4) |
| Total | 14 746 | 10 227 | 4519 (30.6) |
Once the comparison study and the agreement study were completed, we compared the proportion of empty lacunae in the posterior approach group and non-posterior groups using a chi-squared test (Table III). We looked for any interaction between the surgical approach and the position (anterior of posterior) of the section using logistic regression analysis.
Finally, as a control test, the results were compared with the histology of a femoral head taken from a patient who had a hip replacement for established Ficat and Arlet stage 418,19 avascular necrosis (AVN). In this AVN slide, we counted three identical strips of bone similar to the ‘mid’ strips used in the posterior approach and non-posterior groups; the strips were identified as ‘mid’, ‘left’ and ‘right’.
Results
Table II shows the percentage of empty lacunae in the ‘mid’ strip compared with the ‘most’ strip in the 13 comparison slides taken from the two groups. These results show that there was no statistically significant difference between the ‘mid’ and ‘most’ strips in either group (except one in the posterior group: p < 0.001), indicating that the ‘mid’ strips were representative of the bone in each specimen.
The inter- and intra-observer agreement was good. The first observer counted a total 379 cells (124 empty) and the second observer counted 343 (99 empty). Estimated rates of empty cells were 32.7% and 28.9% respectively (p-value = 0.299; two-sample test for equality of proportions) providing no evidence that rates differed between individuals. The first observer repeated the assessment and counted a total of 372 cells (112 empty), giving a rate of 30.1%. A further test of equality of proportions provided no evidence to indicate that this was different from the first count (p = 0.489).
Table III and Figure 3 show the comparison between the posterior approach group and non-posterior group.
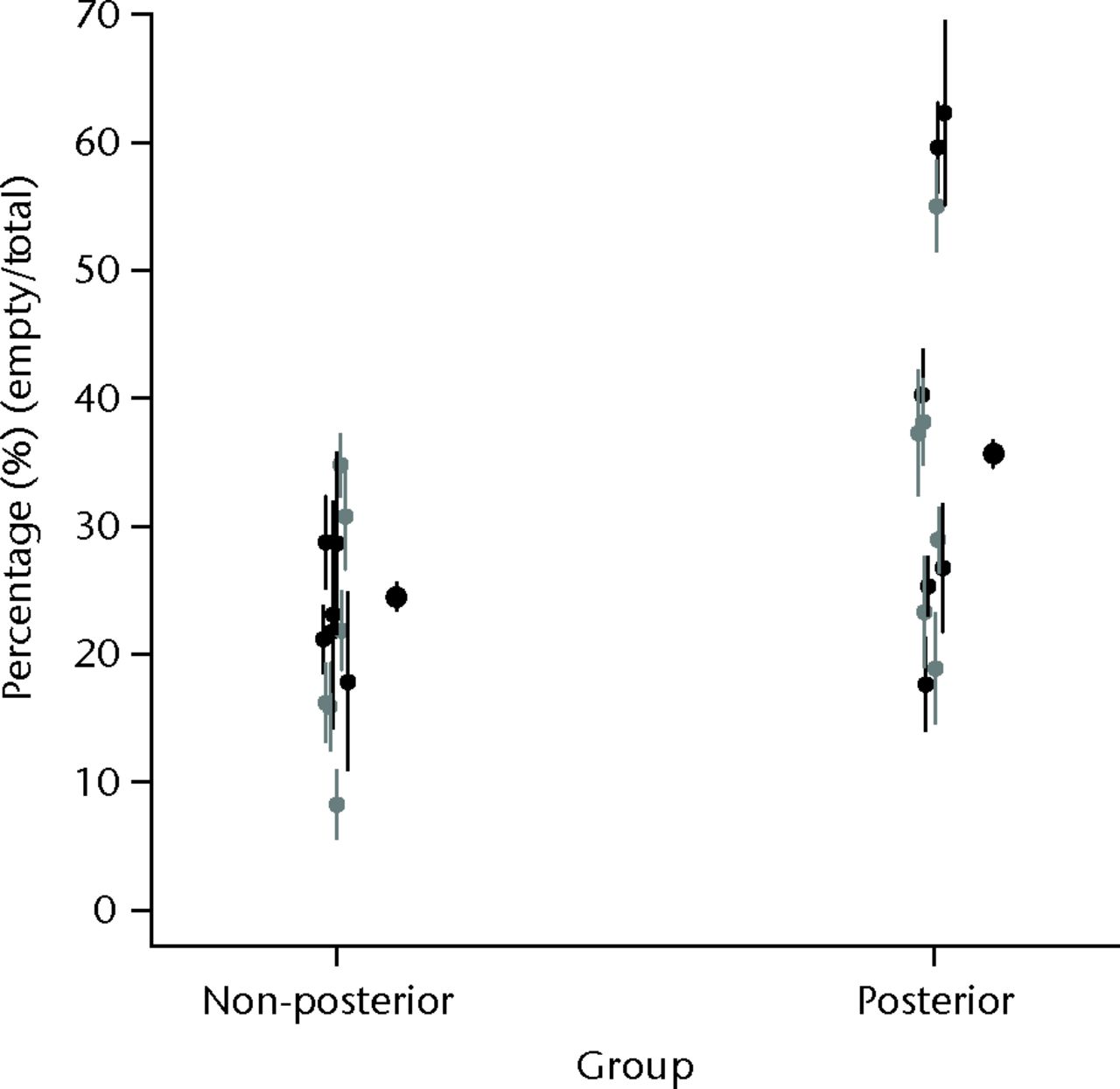
Fig. 3
Strip plot of the mean percentage empty nuclei (bars represent 95% confidence intervals), by group (non-posterior and posterior), with shading to indicate section (anterior, black; posterior, grey) within groups; the x-axis values have been jittered by adding a small random component to aid presentation.
Logistic regression analysis for empty cell numbers showed a significant effect due to surgical approach (p < 0.001, for model regression coefficient), but no evidence of statistical significance for anterior or posterior location within the head (p = 0.319) or for an interaction between position and group (p = 0.282). Parameter estimates from the regression model suggest that the odds ratio for empty nuclei was 1.71 (95% confidence interval (CI) 1.59 to 1.83) for the posterior approach group versus the non-posterior group; i.e. the data indicates that there were significantly more empty cells in the posterior approach group than the non-posterior group.
Control study
When comparing the AVN slide with both posterior and non-posterior groups we found that the AVN slide to have a mean percentage of empty lacunae of 88%, compared with 35% in the non-posterior group was 35% and 24% in the posterior group (Table IV, Fig. 4).
Table IV
Proportion of empty lacunae counted from the three strips in the control (avascular necrosis)
| Strip | Total (n) | Empty (n, %) |
|---|---|---|
| Mid | 41 | 38 (92.7) |
| Right | 81 | 68 (84.0) |
| Left | 39 | 35 (89.7) |
| Mean | 161 | 141 (87.6) |


Figs. 4a - 4b
Slide from a) the non-posterior group showing nucleated lacunae (magnification ×20) and b) the hip with avascular necrosis, showing mostly empty lacuna (magnification ×40, both haematoxylin and eosin).
Discussion
The relationship between surgical approach and blood flow to the femoral head following resurfacing of the hip has been a subject of interest to surgeons for several years.12,20-22 Most intra-operative studies have confirmed a drop in blood flow during the posterior approach compared with other surgical approaches.13,23 However, the question still remains as to whether this reduction in blood flow during surgery leads to permanent changes in the bone of the femoral head?
We found a statistically significant difference in the percentage of empty lacunae in retrieval specimens from patients who underwent the posterior approach compared with other surgical approaches. This indicates that the vascular compromise that occurs during the posterior surgical approach does have long-term effects on the bone of the femoral head.
Steffen et al8 reported only 9% empty lacunae in retrieval specimens from hip replacements done for osteoarthritis, compared with 85% in revision hip replacement done for failed resurfacings due to femoral neck fractures where avascular necrosis would be expected. Other studies of histological retrieval specimens have also shown high rates of avascular necrosis after hip resurfacing.24,25 In contrast, our study demonstrated only 35% empty lacunae in the posterior surgical approach group and 23% empty lacunae in the non-posterior group. These results suggests that although the posterior approach seems to cause more vascular compromise than other surgical approaches, it may not be enough to cause avascular necrosis, at least in the majority of cases.
In our study, the average percentage of empty lacunae in the femoral head of a patient with well-established avascular necrosis was 89% (Table IV), which is in keeping with other studies.8 The advantage of our study is that we took pre-defined 2.5 mm thick coronal sections from the mid-point of the anterior and posterior half of the femoral head (Fig. 1), such that comparisons between groups were made from the same areas of the femoral head. This also gives a larger representative sample of bone extending from the outer margin of the epiphysis to inner margin of the head-neck junction.
One limitation of this study was the fact there was a mix of cemented and uncemented implants. In cemented specimens, the outer margin (cement-bone interphase) may be exposed to thermal necrosis,26,27 which may affect the number of empty lacunae. However, both groups of patients had the same number of cemented specimens (Table I). The total number of specimens was also relatively small. However, each specimen was analysed in great depth, including 24 cut sections and 47 histological slides. Finally, as the study involved an exhaustive manual counting process, error due to observer eccentricity or (unintentional) bias was a possibility. We addressed this by conducting an agreement study.
We analysed both anterior and posterior sections from each femoral head. The posterior part of the head is mainly supplied by retinacular branches of the medial circumflex femoral artery while the anterior part is supplied by retinacular branches from both the medial and lateral circumflex femoral arteries.28,29 In theory, we might therefore expect the posterior sections to show a significant difference in empty lacunae compared with the anterior sections. However, we did not find any statistically significant difference between the anterior and posterior sections (p = 0.319) (Fig. 3). This may suggest that the difference between the two groups is not directly associated with a reduction in the posterior blood supply caused by damage to medial circumflex femoral artery and there may be considerable overlap between areas of bone supplied by medial and lateral circumflex femoral artery.
In conclusion, we found a statistically significant difference in the percentage of empty lacunae in retrieval specimens from patients who had the posterior approach compared with other surgical approaches during resurfacing of the femoral head. This indicates that the vascular compromise that occurs during the posterior surgical approach does have long-term effects on the bone of the femoral head, even if it does not cause overt avascular necrosis.
1 McMinn D , DanielJ. History and modern concepts in surface replacement. Proc Inst Mech Eng H2006;220:239–251.CrossrefPubMed Google Scholar
2 Quesada MJ , MarkerDR, MontMA. Metal-on-metal hip resurfacing: advantages and disadvantages. J Arthroplasty2008;23:69–73.CrossrefPubMed Google Scholar
3 Barrack RL . Metal-metal hip resurfacing offers advantages over traditional arthroplasty in selected patients. Orthopedics2007;30:725–726.CrossrefPubMed Google Scholar
4 Ollivere B , DuckettS, AugustA, PorteousM. The Birmingham Hip Resurfacing: 5-year clinical and radiographic results from a District General Hospital. Int Orthop2010;34:631–634.CrossrefPubMed Google Scholar
5 Shimmin AJ , BareJ, BackDL. Complications associated with hip resurfacing arthroplasty. Orthop Clin North Am2005;36:187–193.CrossrefPubMed Google Scholar
6 Smith AJ , DieppeP, HowardPW, BlomAW. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales. Lancet2012;380:1759–1766.CrossrefPubMed Google Scholar
7 Morlock MM , BishopN, ZustinJ, et al.Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J Bone Joint Surg [Am]2008;90-A(Suppl 3):89–95.CrossrefPubMed Google Scholar
8 Steffen RT , AthanasouNA, GillHS, MurrayDW. Avascular necrosis associated with fracture of the femoral neck after hip resurfacing: histological assessment of femoral bone from retrieval specimens. J Bone Joint Surg [Br]2010;92-B:787–793.CrossrefPubMed Google Scholar
9 Campbell P , TakamuraK, LunderganW, EspositoC, AmstutzHC. Cement technique changes improved hip resurfacing longevity: implant retrieval findings. Bull NYU Hosp Jt Dis2009;67:146–153. Google Scholar
10 Griffiths DE , ActonK, HullJB. Hip resurfacing with preservation of femoral head blood supply. Surgeon2008;6:115–119.CrossrefPubMed Google Scholar
11 Campbell P , MirraJ, AmstutzHC. Viability of femoral heads treated with resurfacing arthroplasty. J Arthroplasty2000;15:120–122.CrossrefPubMed Google Scholar
12 Forrest N , WelchA, MurrayAD, et al.Femoral head viability after Birmingham resurfacing hip arthroplasty: assessment with use of [18F] fluoride positron emission tomography. J Bone Joint Surg [Am]2006;88-A(Suppl 3):84–89.CrossrefPubMed Google Scholar
13 Amarasekera HW , CostaML, FoguetP, et al.The blood flow to the femoral head/neck junction during resurfacing arthroplasty: a comparison of two approaches using laser doppler flowmetry. J Bone Joint Surg [Br]2008;90-B:442–445.CrossrefPubMed Google Scholar
14 Amarasekera H , RobertsP, CostaM, et al.Scintigraphic assessment of bone status at one year following hip resurfacing: comparison of two surgical approaches using SPECT-CT scan. Bone Joint Res2012;1:86–92.CrossrefPubMed Google Scholar
15 No authors listed. Human Tissue Act 2004. http//www.legislation.gov.uk/ukpga/2004/30/contents (date last accessed 10 July 2013). Google Scholar
16 Catto M . A histological study of avascular necrosis of the femoral head after transcervical fracture. J Bone Joint Surg [Br]1965;47-B:749–776.PubMed Google Scholar
17 No authors listed. R Documentation: Test of Equal or Given Proportions. http://stat.ethz.ch/R-manual/R-patched/library/stats/html/prop.test.html (date last accessed 11 July 2013). Google Scholar
18 Ficat RP, Arlet J. Treatment of bone ischemia and necrosis. In: Hungerford DS, ed. Ischemia and necrosis of bone. Baltimore: Williams & Wilkins, 1980:171–182. Google Scholar
19 Arlet J, Ficat P. Non-traumatic avascular femur head necrosis: new methods of examination and new concepts. Chir Narzadow Ruchu Ortop Pol 1977;42:269–276 (in Polish). Google Scholar
20 Gerdesmeyer L, Gollwitzer H, Bader R, Rudert M. Surgical approaches in hip resurfacing. Orthopade 2008;37:650–658 (in German). Google Scholar
21 Steffen R , O’RourkeK, GillHS, MurrayDW. The anterolateral approach leads to less disruption of the femoral head-neck blood supply than the posterior approach during hip resurfacing. J Bone Joint Surg [Br]2007;89-B:1293–1298.CrossrefPubMed Google Scholar
22 Steffen RT , FernD, NortonM, MurrayDW, GillHS. Femoral oxygenation during hip resurfacing through the trochanteric flip approach. Clin Orthop Relat Res2009;467:934–939.CrossrefPubMed Google Scholar
23 Steffen RT , SmithSR, UrbanJP, et al.The effect of hip resurfacing on oxygen concentration in the femoral head. J Bone Joint Surg [Br]2005;87-B:1468–1474.CrossrefPubMed Google Scholar
24 Zustin J , SauterG, MorlockMM, RutherW, AmlingM. Association of osteonecrosis and failure of hip resurfacing arthroplasty. Clin Orthop Relat Res2010;468:756–761.CrossrefPubMed Google Scholar
25 Howie DW , CornishBL, Vernon-RobertsB. The viability of the femoral head after resurfacing hip arthroplasty in humans. Clin Orthop Relat Res1993;291:171–184.PubMed Google Scholar
26 Gill HS , CampbellPA, MurrayDW, De SmetKA. Reduction of the potential for thermal damage during hip resurfacing. J Bone Joint Surg [Br]2007;89-B:16–20.CrossrefPubMed Google Scholar
27 Little JP , GrayHA, MurrayDW, BeardDJ, GillHS. Thermal effects of cement mantle thickness for hip resurfacing. J Arthroplasty2008;23:454–458.CrossrefPubMed Google Scholar
28 Tucker FR . Arterial supply to the femoral head and its clinical importance. J Bone Joint Surg [Br]1949;31-B:82–93.PubMed Google Scholar
29 Trueta J , HarrisonMH. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg [Br]1953;35-B:442–461.PubMed Google Scholar
Funding statement:
The costs of analysis of specimens were met by a research grant from Corin Group PLC, The Corinium Centre, Cirencester, United Kingdom.
Author contributions:
H. W. Amarasekera: Project design, Collecting, preparing and analysis of specimens, Data collection and analysis, Manuscript writing
P. C. Campbell: Project design and supervision, Data analysis, Manuscript assistance
N. Parsons: Data analysis, Writing manuscript
J. Achten: Project design, Manuscript writing
J. Masters: Data collection, Cell count analysis
D. R. Griffin: Supervision, Project design, Collection of specimens, Manuscript writing
M. L. Costa: Project design, Collection of specimens, Analysis, Manuscript writing
ICMJE Conflict of Interest:
None declared
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









