Abstract
Objectives
The purpose of this study was to evaluate chronological changes in the collagen-type composition at tendon–bone interface during tendon–bone healing and to clarify the continuity between Sharpey-like fibres and inner fibres of the tendon.
Methods
Male white rabbits were used to create an extra-articular bone–tendon graft model by grafting the extensor digitorum longus into a bone tunnel. Three rabbits were killed at two, four, eight, 12 and 26 weeks post-operatively. Elastica van Gieson staining was used to colour 5 µm coronal sections, which were examined under optical and polarised light microscopy. Immunostaining for type I, II and III collagen was also performed.
Results
Sharpey-like fibres comprised of type III collagen in the early phase were gradually replaced by type I collagen from 12 weeks onwards, until continuity between the Sharpey-like fibres and inner fibres of the tendon was achieved by 26 weeks.
Conclusions
Even in rabbits, which heal faster than humans, an observation period of at least 12 to 26 weeks is required, because the collagen-type composition of the Sharpey-like fibre bone–tendon connection may have insufficient pullout strength during this period. These results suggest that caution is necessary when permitting post-operative activity in humans who have undergone intra-bone tunnel grafts.
Article focus
When and how do the chronological changes in the collagen-type composition of the Sharpey-like fibres at the tendon–bone interface occur?
Key messages
Sharpey-like fibres comprised of type III collagen in the early phase were gradually replaced by type I collagen from 12 weeks onwards until continuity between the Sharpey-like fibres and inner fibres of the tendon was achieved by 26 weeks
Strengths and limitations of this study
The period of this study was long enough to observe chronological changes in the collagen type composition of Sharpey-like fibres
The present histological evaluation was qualitative, so both quantitative and statistical conclusions could not be reached
Introduction
In current procedures for knee cruciate ligament or collateral ligament reconstruction using the hamstring tendon, tunnels are drilled into the femur and tibia, and a free tendon graft is transplanted into each bone tunnel.1 The tendon graft is anchored to the inside wall of the bone tunnel by means of fibrous tissues. The normal bone–ligament junction consists of four distinct layers (bone, calcified cartilage, uncalcified cartilage and ligament).2 In this method, however, the tendon graft is anchored to the inside wall of the bone tunnel through the fibrous tissues, and the normal bone–ligament junction does not appear.2-5
In the early healing phase, fibrovascular tissue is formed at the interface between the tendon graft and the inside wall of the bone tunnel.6,7 Collagen fibres are subsequently deposited at the tendon–bone interface, and the tendon and bone become directly joined by characteristic anchoring fibres, which resemble Sharpey’s fibres and firmly connect the periosteum to the bone.6,8,9 These fibres have come to be called ‘Sharpey-like fibres’; they are important structures for inhibition of pullout failures of tendon grafts from bone tunnels.6 Liu et al10 observed that anchoring fibres resembling Sharpey-like fibres were comprised of type III collagen fibres at six weeks after surgery in a rabbit model. Similarly, Kanazawa et al8 observed Sharpey-like fibres up to eight weeks after surgery and found that they were solely comprised of type III collagen in a rabbit model. Type III collagen is generally only a fibrous collagen that acts as a framework for cells or other structures by forming slender reticulate structures known as reticular fibres. It increases in the early phase of the wound-healing process and, in many cases, is eventually either replaced by type I collagen or coexists in tissue in which type I collagen is the main component.11,12 This inevitably leads to the question of whether anchoring fibres comprised of type III collagen really offer sufficient mechanical strength.
However, irrespective of how firmly the Sharpey-like fibres are attached to the surface of the tendon, sufficient pullout strength is unlikely to be achieved unless they firmly continue to the inner fibres of the tendon. In fact, Tomita et al13 indicated that the pullout failure of the tendon graft at six weeks after surgery in a canine anterior cruciate ligament reconstruction model occurred in a deeper area within the tendon midsubstance rather than in the thin anchoring zone on the superficial portion of the tendon graft; they also showed that the superficial portion remained attached to the bone tunnel wall. However, studies so far on changes inside the tendon graft have mostly been concerned with the intra-articular portion,14-16 and very few studies have examined changes inside the tendon graft within the bone tunnel. There are no studies that have looked at the continuity of fibres between the Sharpey-like fibre and inner fibres of the tendon.
The two aims of this study were to evaluate the chronological changes in the collagen-type composition (type I, II or III) at the tendon–bone interface and to clarify the continuity between the Sharpey-like fibre and inner fibres of the tendon. Our hypotheses were that: 1) the Sharpey-like fibres comprised of type III collagen in the early phase will gradually be replaced by type I collagen; and 2) the Sharpey-like fibres will eventually extend continuously to the inner fibres within the tendon graft.
Materials and Methods
The present study was approved by the Ethics Committee of our institution. A total of 15 skeletally mature male Japanese white rabbits (body weight: 2400 g to 3500 g) who received intravascular administration of pentobarbital sodium (0.025 mg/kg) were used to create an extra-articular bone tendon graft model by grafting the extensor digitorum longus (EDL) into a bone tunnel made in the proximal metaphysis of the tibia according to the procedure of Rodeo et al.6 Arthrotomy using the medial parapatellar approach was performed under anaesthesia, then the EDL was detached from its insertion site on the lateral femoral condyle and folded approximately 8 mm from the tip. A drill 2 mm in diameter was used to make the tibial bone tunnel perpendicular to the axis of the tibial bone toward the medial cortex from the lateral cortex, 5 mm distal from the joint. A No. 4-0 Tevdek suture (Teleflex, Tokyo, Japan) was used to pull the folded EDL tendon into the lateral aperture of the bone tunnel. The Tevdek suture was tied onto a small button placed on the medial cortex (Fig. 1). Following irrigation, the articular capsule and the skin were sutured. Post-operatively, the rabbits were allowed free movement within their cages. Three rabbits were killed at each time point of two, four, eight, 12 and 26 weeks post-operatively. The EDL and proximal tibia complexes were then harvested and immediately fixed in neutral buffered 10% formalin for 48 hours. The bone was decalcified in formic acid (29 g citric acid, 18 g trisodium citrate dihydrate and 100 ml formic acid, with distilled water added to yield a total volume of 1000 ml), dehydrated and embedded in paraffin. Coronal 5 μm sections of the bone tunnel were made. Haematoxylin and eosin (HE) and elastica van Gieson were used to stain the sections, which were examined under optical and polarised light microscopy.

Fig. 1
Schematic drawing of the surgical procedure used in the study.
Immunostaining using anticollagen type I, type II and type III monoclonal antibodies (Daiichi, Toyama, Japan) was performed. Normal mouse immunoglobulin G1 at the same concentration as the primary antibody was used as a negative control. Antigens were activated in 1% trypsin solution (0.2 g trypsin, 0.2 g calcium chloride, 200 ml 0.05 M Tris–HCl buffer) reacted at 37°C for 30 minutes. Endogenous peroxidase was then blocked by reacting with 0.3% H2O2 in methanol for 30 min at room temperature. The primary antibodies were diluted with antibody diluent (Dako S 3022). Each diluent solution was reacted overnight at 4°C. Secondary antibodies were reacted with antimouse antibodies on dextran polymer (Dako K 4000) for 30 minutes at room temperature. Diaminobenzidine (DAB; Dako) was used for staining. The washing solution in each process was 0.1% Tween 20 in 0.05 M Tris–HCl buffer with 0.3 M NaCl. Finally, haematoxylin was used to perform counterstaining, and the observations were made under optical microscopy.
Results
Two weeks post-operatively
The bone–tendon interface was filled by irregularly arranged fibrous scar tissue, which included blood vessels and numerous spindle-shaped cells and cells with round nuclei. Bone-lining cells were arranged along the surface of the bone (Fig. 2a). Immunostaining revealed almost no type I collagen (Fig. 2b), whereas a dense homogeneous deposit of irregularly arranged type III collagen was evident (Fig. 2c). Type II collagen was not observed in the interface. In tendon midsubstance, type I collagen was still densely stained (Fig. 2b), while almost no staining for type III collagen was evident (Fig. 2c).
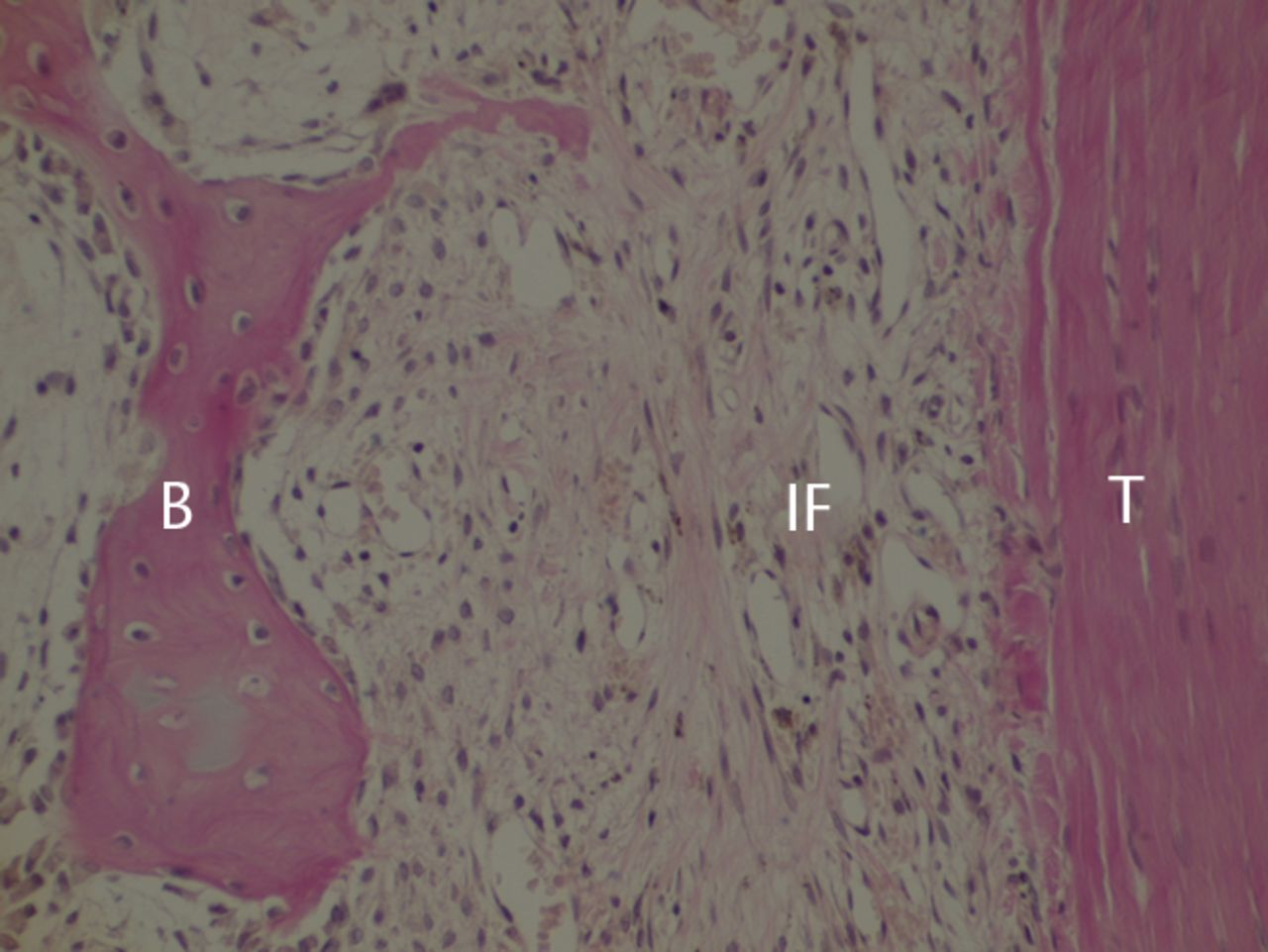
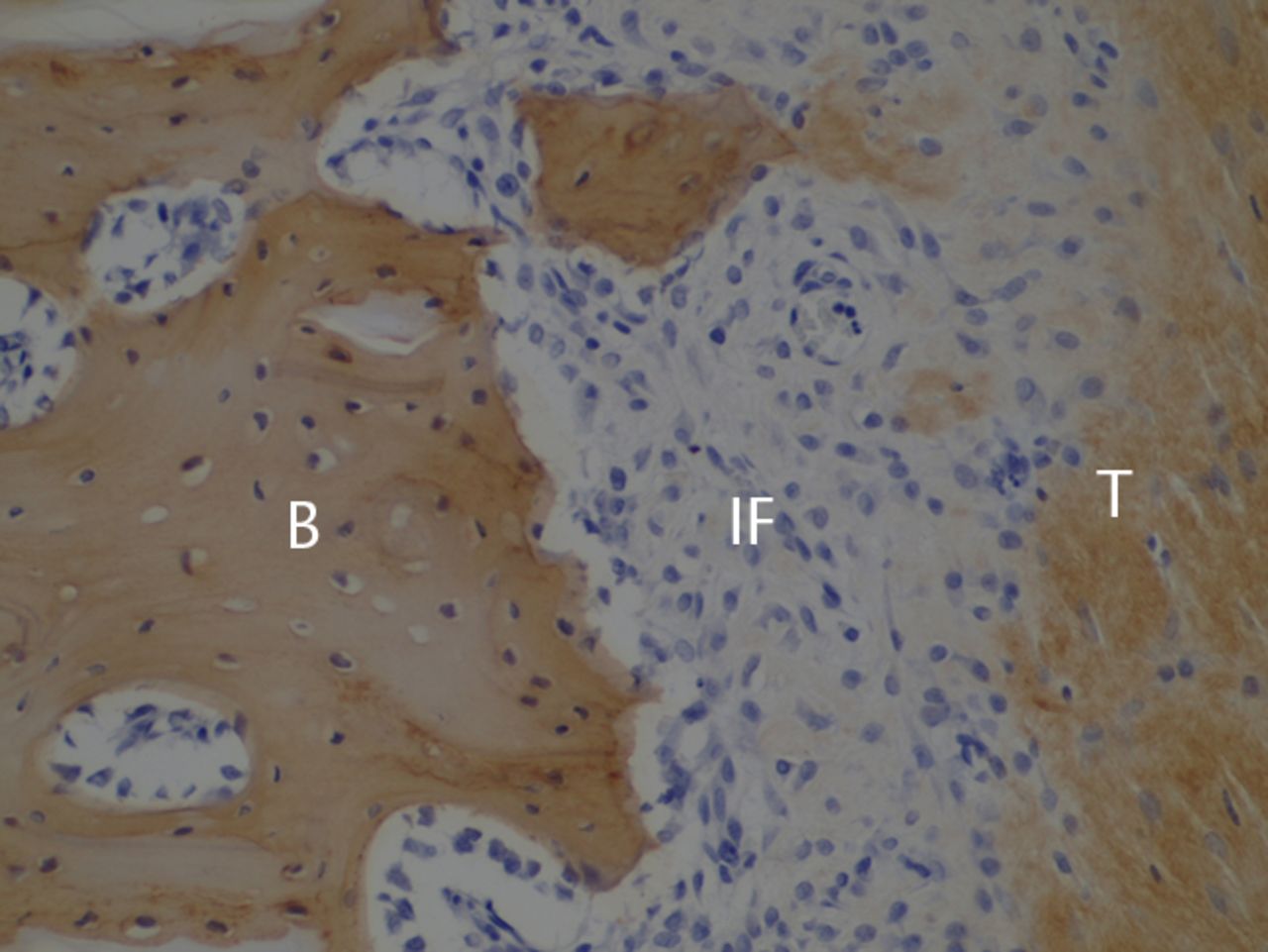
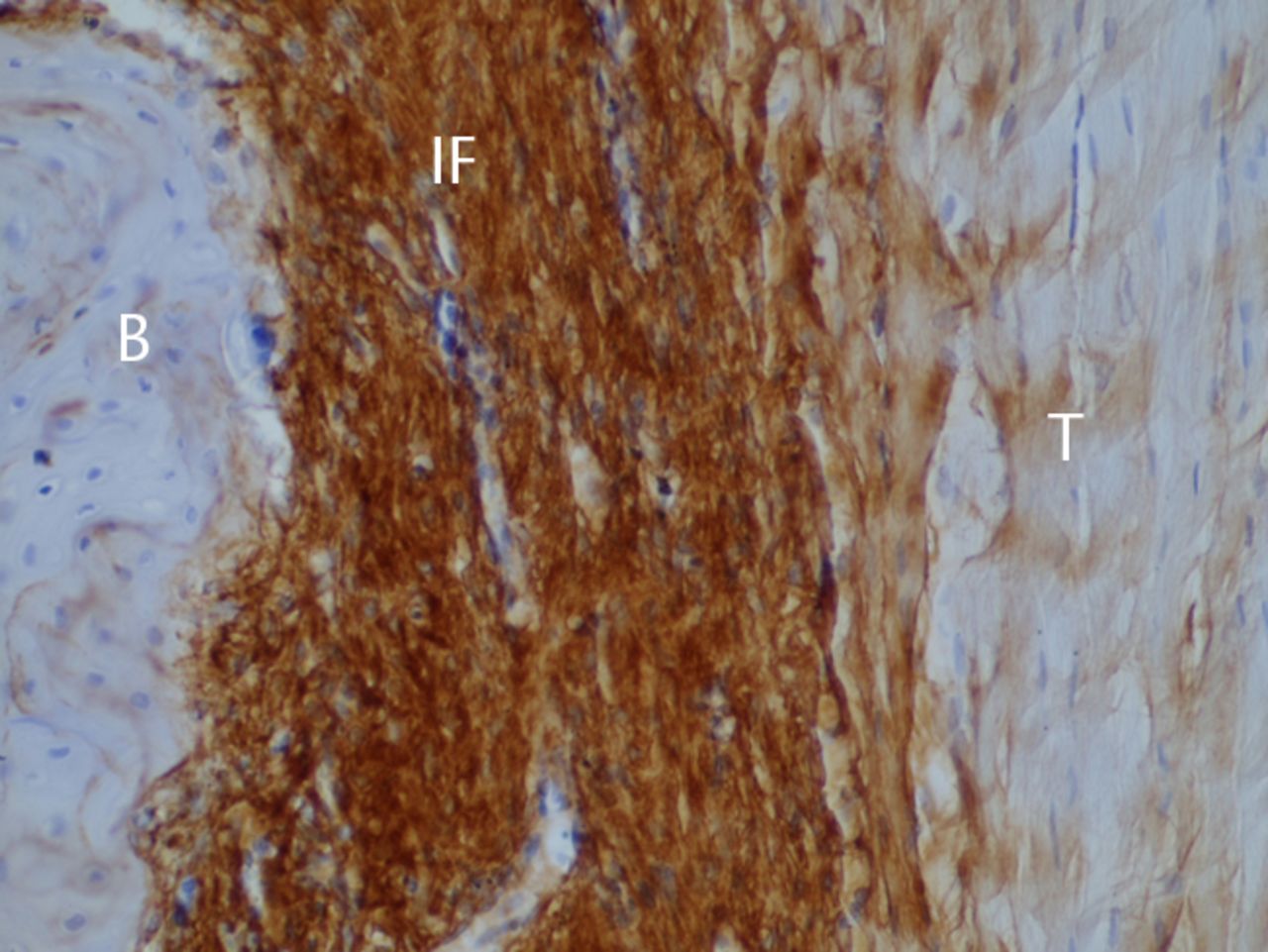
Figs. 2a - 2c
Histological images at two weeks post-operatively with a) elastic van Gieson staining, b) immunostaining for type I collagen, and c) immunostaining for type III collagen (B, bone; IF, interface; T, tendon graft; all original magnifications × 200).
Four weeks post-operatively
At four weeks, many blood vessels and cells were present in the scar tissue. Fibres that emerged perpendicular from the bone and ran into the bone–tendon interface scar tissue (Sharpey-like fibres) were observed under polarised light microscopy (Fig. 3). These fibres showed positive staining for type III collagen.
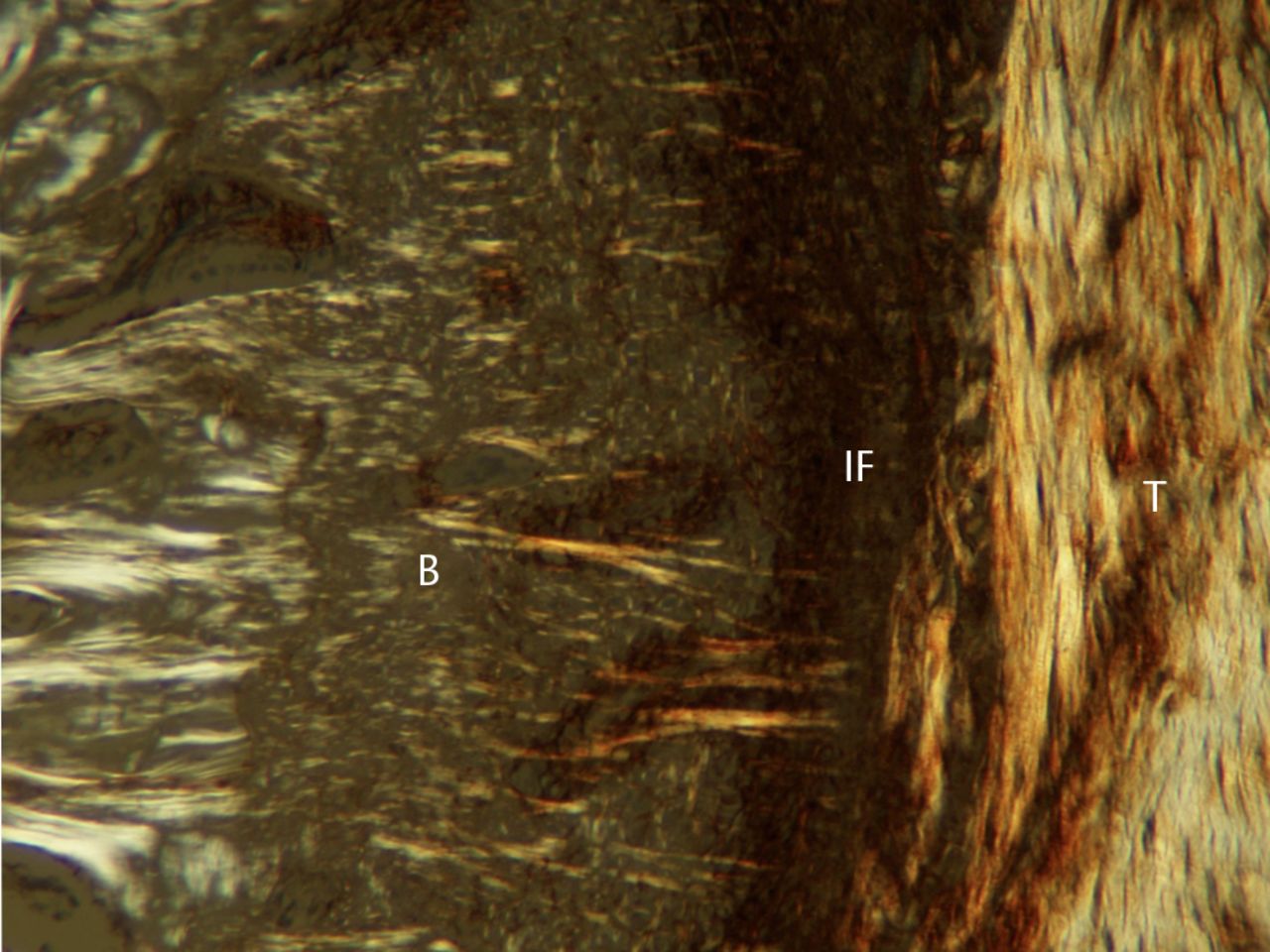
Fig. 3
Photograph of polarised light microscopy at four weeks post-operatively (T, tendon graft; IF, interface; B, bone; original magnification × 100).
Eight weeks post-operatively
Anchoring fibres observed in the bone–tendon interface at four weeks had developed further. These fibres did not show positive staining for type I collagen, but were positive for type III collagen (Fig. 4). Although irregularly arranged type I collagen fibres had also been deposited, and the inside of the scar tissue was still primarily type III collagen. The tendon midsubstance showed densely positive staining for type III collagen (Fig. 4).
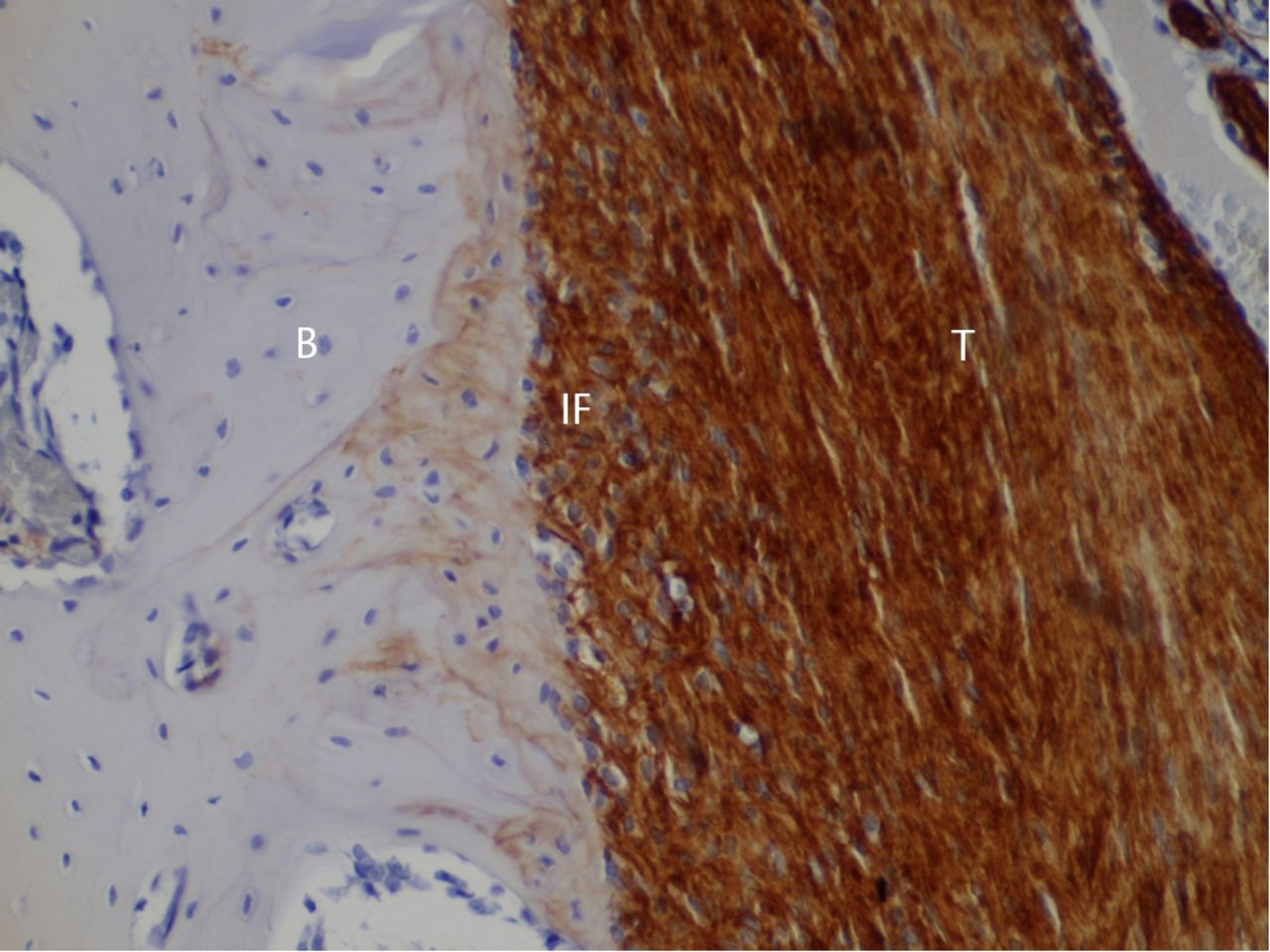
Fig. 4
Histological images at eight weeks post-operatively with immunostaining for type III collagen (T, tendon graft; IF, interface; B, bone; original magnification ×200).
12 weeks post-operatively
Many anchoring fibres extended from the bone toward the interface and passed between the bone-lining cells. Immunostaining showed type I collagen growing from the bone to the interface tissue (Fig. 5). Type III collagen fibres running in the same direction as type I were observed. The tendon midsubstance showed roughly equal staining of type I collagen and type III collagen. Type II collagen staining was not observed in the interface.

Fig. 5
Histological images at 12 weeks post-operatively with immunostaining for type I collagen (T; tendon graft; IF, interface; B, bone; original magnification × 200).
26 weeks post-operatively
The bone and tendon grafts were anchored extensively by fibres growing perpendicularly or obliquely from the bone (Figs 6 and 7). Immunostaining showed that type I collagen fibres in the bone–tendon graft interface extensively anchored the bone and tendon graft (Fig. 6b). Type III collagen fibres running in the same direction were also observed. These fibres did not stain for type II collagen. The tendon midsubstance still stained for both type I and type III collagen, but staining for type I collagen had become dense.
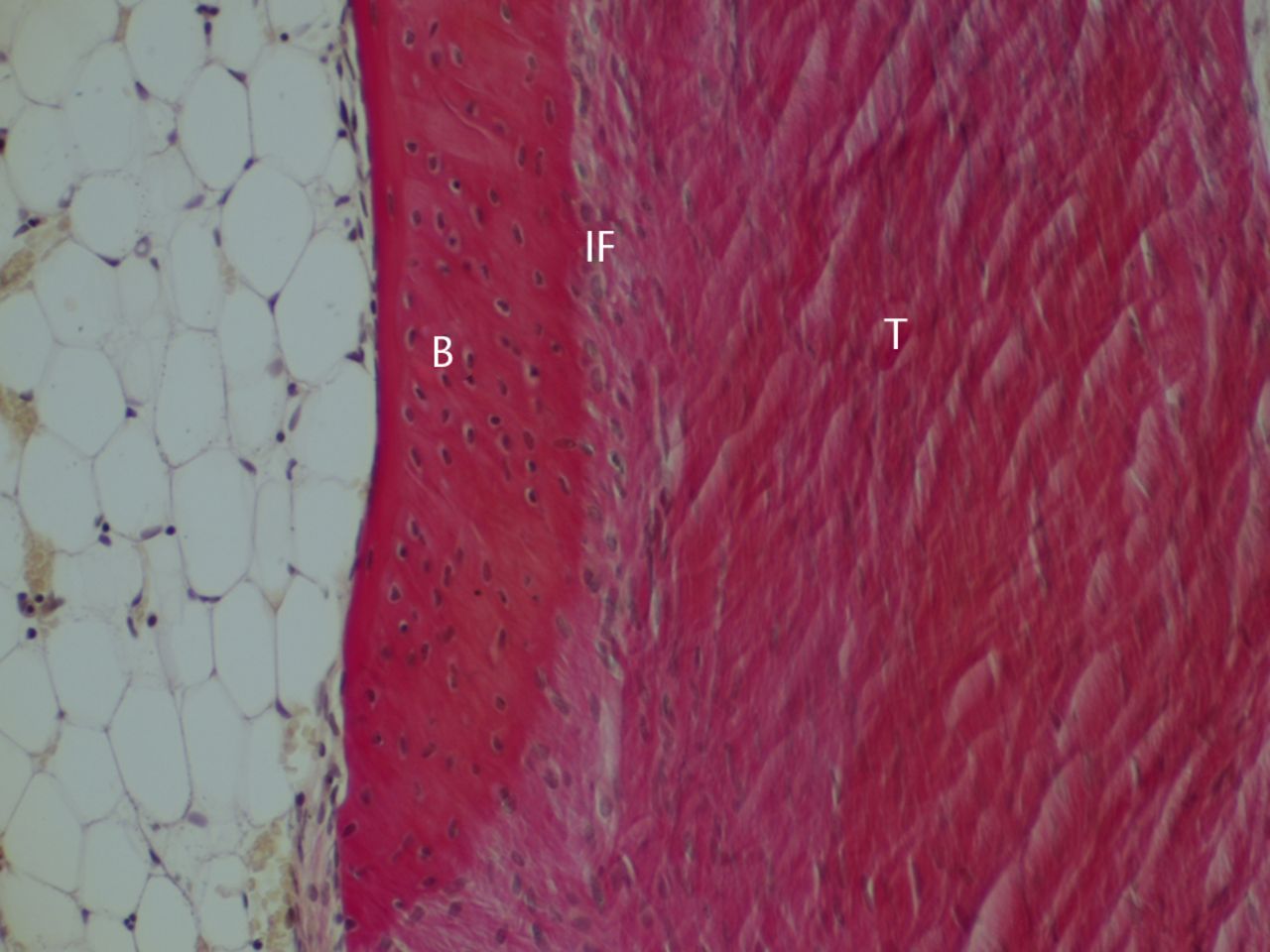

Figs. 6a - 6b
Histological images at 26 weeks post-operatively with a) elastic van Gieson staining and b) immunostaining for type I collagen (B, bone; IF, interface; T, tendon graft; all original magnifications × 200).

Fig. 7
Photograph of polarised light microscopy at 26 weeks post-operatively (T, tendon graft; IF, interface; B, bone; original magnification × 100).
Discussion
In the present study, we used a rabbit model to observe the development process and chronological changes in the collagen type composition at tendon–bone interface and intra-bone tunnel graft. Polarised light microscopy showed Sharpey-like fibres connecting the bone to the scar tissue of the bone–tendon interface at four weeks, but the fibres had not reached the tendon yet. Immunostaining showed that these fibres were composed of only type III collagen, but Sharpey-like fibres composed of type I collagen appeared at 12 weeks. At 26 weeks, the type I collagen fibres became denser and created continuity between the bone and the tendon graft. In other words, the Sharpey-like fibres anchoring obliquely from the wall of the tunnel to the tendon graft were composed of type III collagen in the early stage but gradually changed to type I collagen. This confirmed our first hypothesis. Continuity with the type I collagen Sharpey-like fibres in the bone–tendon interface began at 26 weeks. This supported our second hypothesis. These processes should result in strong adhesion of the tendon graft to the wall of the bone tunnel from the perspective of standard theories of wound healing.11,12
Previously, many reported studies have examined the integration of tendon–bone healing. While differences exist in the animals and experimental methods used in various experiments, Sharpey-like fibres are generally observed through HE staining and polarised light microscopy at between three and four weeks post-operatively. Sharpey-like fibres increase gradually from six to 12 weeks, and during this period, mechanical testing showed that tendon midsubstance ruptures occur more frequently than pullout failures. Thus, Sharpey-like fibres have been considered to be an important structure from the viewpoint of mechanics.6,13,17 However, some recent reports have pointed out that the tensile test cannot necessarily show that the strength of the tendon–bone interface increased sufficiently just because pullout failure instead of midsubstance failure occurred.13,18,19 Specifically, a change in the failure mode observed when the Sharpey-like fibres appear simply indicates that the most mechanically weak point shifted from the tendon–bone interface to the midsubstance of the tendon graft. Therefore, whether the tendon–bone interface has actually gained sufficient strength is unclear. Tsukada et al18 evaluated the strength of the interface with their original model in which the tendon graft was reinforced with suture material to prevent tendon midsubstance rupture. In their report, all reinforced tendon grafts showed pullout failure until 12 weeks and midsubstance rupture after 16 weeks, while all tendon grafts with no reinforcement showed midsubstance rupture at eight weeks after surgery. In other words, the tendon–bone interface required at least 12 to 16 weeks to provide greater strength than that of the reinforcing suture. This raises the question of whether the period generally considered adequate for the tendon–bone interface to mature is sufficient.6,13,17
In the present study, mechanical experiments were not used, but we observed the timing of histological integration in this area. Although elastica van Gieson staining and polarised light microscopy began to show Sharpey-like fibres from four weeks onwards, the fibres were comprised only of type III collagen; similar to the results reported by Liu et al10 or Kanazawa et al.8 Moreover, these fibres were mainly present in large numbers between the bone and tendon–bone interface tissue, with almost no perforating fibres found between the tendon–bone interface tissue and the tendon midsubstance. Furthermore, the tendon midsubstance became mainly amorphous type III collagen during this period, so increasing deteriorations in quality were observed histologically. This finding strongly supports previous reports of mechanical experiments; Tsukada et al18 found avulsion at the tendon side of the tendon–bone interface with Sharpey-like fibres left in the bone side until 8 weeks, and Tomita et al13 found avulsion of the tendon graft with the outermost layer of the tendon left in the tunnel side at six weeks. Type III collagen Sharpey-like fibres, therefore, seem not to anchor as far as the deep part of the tendon graft. Type I collagen Sharpey-like fibres can be barely seen from 12 weeks, and at 26 weeks and later, it gradually becomes closely continuous with type I collagen in the tendon midsubstance. Finally, the tendon is connected to the bone through dense connective tissue comprised of type I and type III collagen, with no type II collagen. This is the same composition as that found in tissue obtained by biopsy from humans, as reported by Robert et al,20 Nebelung et al21 and Peterson and Laprell.22
From an the viewpoint of immunohistological findings, the period required for the tendon–bone interface to obtain sufficient strength is from at least 12 to 26 weeks after surgery. It should be noted that the period previously considered to be necessary for tendon–bone interface maturity6,13,17 was not sufficiently long, and longer periods were needed. This is extremely important when considering clinical applications in humans. Even in rabbits, which have a greater and faster healing ability than humans, at least 12 to 26 weeks seems to be required for histological continuity that provides a sufficient degree of pullout strength between the bone and tendon.
More prudent judgment than what has previously been used is probably needed to determine when post-operative activity in humans should be permitted. Recently attempts have been made to change the anchoring between tendon and bone, including the use of mechanical stimulus,23 periosteum,24,25 bone morphogenetic protein,26,27 transforming growth factor-beta,28 hepatocyte growth factor,29 recombinant human parathyroid hormone,30 stem cells31-33 and others.34 However, most have only used general stains for morphological evaluations. We believe that tissue observation using only general stains such as HE or Alcian blue is insufficient for evaluation of the present results of basic research and that collagen typing through immunostaining is essential. According to our results, 12 or more weeks should be considered for evaluation, although the duration of most studies has not exceeded this timeframe.35
Some limitations to the present study must be considered. The first is that the rabbit healing process is much quicker than that of humans, while it is histologically similar to that of dogs or humans. The second limitation is that the tunnel is totally extra-articular, which is not similar to human ACL tunnels. Articular fluid might have altered the healing process. The third limitation is that stress distribution on the tendon graft was not similar to that of the human ACL healing process. It is important to bear in mind that this is not a perfect model of intra-articular reconstruction. However, the model is suitable for extra-articular ligament reconstruction, such as collateral ligaments. The fourth limitation is that the present histological evaluation was qualitative, so both quantitative and statistical conclusions could not be reached.
In conclusion, the type III collagen comprising the Sharpey-like fibres in the early phase was gradually replaced by type I collagen from 12 weeks onwards. Furthermore, the Sharpey-like fibres comprised of type I collagen eventually provided continuity to the inner fibres of the tendon by 26 weeks. Even in rabbits, which heal faster than humans, at least 12 to 26 weeks of observation is required because the collagen-type composition of the Sharpey-like fibre bone–tendon connection may have insufficient pullout strength during this period. These results suggested that more prudent judgment is needed than that previously used for determining when the postoperative activity in humans should be permitted.
The authors would like to thank Mr K. Yoshida for his technical assistance.
1 Yasuda K , KondoE, IchiyamaH, et al.Anatomical reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy2004;20:1015–1025. Google Scholar
2 Cooper RR , MisolS. Tendon and ligament insertion: a light and electron microscopic study. J Bone Joint Surg [Am]1970;52-A:1–20. Google Scholar
3 Benjamin M , EvansEJ, CoppL. The histology of tendon attachments to bone in man. J Anat1986;149:89–100.PubMed Google Scholar
4 Amiel D , KleinerJB, RouxRD, HarwoodFL, AkesonWH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res1986;4:162–172. Google Scholar
5 Milz S , TischerT, BuettnerA, et al.Molecular composition and pathology of entheses on the medial and lateral epicondyles of the humerus: a structural basis for epicondylitis. Ann Rheum Dis2004;63:1015–1021.CrossrefPubMed Google Scholar
6 Rodeo SA , ArnoczkySP, TorzilliPA, HidakaC, WarrenRF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg [Am]1993;75-A:1795–1803. Google Scholar
7 Grana WA , EgleDM, MahnkenR, GoodhartCW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med1994;22:344–351.CrossrefPubMed Google Scholar
8 Kanazawa T , SoejimaT, MurakamiH, et al.An immunohistological study of the integration at the bone-tendon interface after reconstruction of the anterior cruciate ligament in rabbits. J Bone Joint Surg [Br]2006;88-B:682–687.CrossrefPubMed Google Scholar
9 Yamazaki S , YasudaK, TomitaF, MinamiA, TohyamaH. The effect of graft-tunnel diameter disparity on intraosseous healing of the flexor tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med2002;30:498–505.CrossrefPubMed Google Scholar
10 Liu S , PanossianV, al-ShaikhR, et al.Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res1997;339:253–260.CrossrefPubMed Google Scholar
11 Gay S , VijantoJ, RaekallioJ, PenttinenR. Collagen types in early phases of wound healing in children. Acta Chir Scand1978;144:205–211.PubMed Google Scholar
12 Li J , ChenJ, KirsnerR. Pathophysiology of acute wound healing. Clin Dermatol2007;25:9–18.CrossrefPubMed Google Scholar
13 Tomita F , YasudaK, MikamiS, et al.Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy2001;17:461–476.CrossrefPubMed Google Scholar
14 Weiler A , PetersG, MäurerJ, UnterhauserFN, SüdkampNP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. Am J Sports Med2001;29:751–761.CrossrefPubMed Google Scholar
15 Goradia VK , RochatMC, KidaM, GranaWA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med2000;28:40–46.CrossrefPubMed Google Scholar
16 Ju YJ , TohyamaH, KondoE, et al.Effects of local administration of vascular endothelial growth factor on properties of the in situ frozen-thawed anterior cruciate ligament in rabbits. Am J Sports Med2006;34:84–91.CrossrefPubMed Google Scholar
17 Goradia VK , RochatMC, GranaWA, RohrerMD, PrasadHS. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg2000;13:143–151.PubMed Google Scholar
18 Tsukada H , IshibashiY, TsudaE, et al.The actual tendon-bone interface strength in a rabbit model. Arthroscopy2010;26:366–374.CrossrefPubMed Google Scholar
19 Wen CY , QinL, LeeKM, ChanKM. Peri-graft bone mass and connectivity as predictors for the strength of tendon-to-bone attachment after anterior cruciate ligament reconstruction. Bone2009;45:545–552.CrossrefPubMed Google Scholar
20 Robert H , Es-SayehJ, HeymannD, PassutiN, EloitS, VaneenogeE. Hamstring insertion site healing after anterior cruciate ligament reconstruction in patients with symptomatic hardware or repeat rupture: a histologic study in 12 patients. Arthroscopy2003;19:948–954.CrossrefPubMed Google Scholar
21 Nebelung W , BeckerR, UrbachD, RöpkeM, RoessnerA. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg2003;123:158–163.CrossrefPubMed Google Scholar
22 Peterson W , LaprellH. Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc2000;8:26–31.CrossrefPubMed Google Scholar
23 Wang CJ , WangFS, YangKD, et al.The effect of shock wave treatment at the tendon-bone interface: an histomorphological and biomechanical study in rabbits. J Orthop Res2005;23:274–280. Google Scholar
24 Youn I , JonesDG, AndrewsPJ, CookMP, SuhJK. Periosteal augmentation of a tendon graft improves tendon healing in the bone tunnel. Clin Orthop Relat Res2004;419:223–231.CrossrefPubMed Google Scholar
25 Li H , JiangJ, WuY, ChenS. Potential mechanisms of a periosteum patch as an effective and favourable approach to enhance tendon-bone healing in the human body. Int Orthop2012;36:665–669.CrossrefPubMed Google Scholar
26 Anderson K , SeneviratneAM, IzawaK, et al.Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med2001;29:689–698.CrossrefPubMed Google Scholar
27 Coen MJ , ChenST, RundleCH, WergedalJE, LauKH. Lentiviral-based BMP4 in vivo gene transfer strategy increases pull-out tensile strength without an improvement in the osteointegration of the tendon graft in a rat model of biceps tenodesis. J Gene Med2011;13:511–521.CrossrefPubMed Google Scholar
28 Yamazaki S , YasudaK, TomitaF, TohyamaH, MinamiA. The effect of transforming growth factor-beta 1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthroscopy2005;21:1034–1041. Google Scholar
29 Nakase J , KitaokaK, MatsumotoK, TomitaK. Facilitated tendon-bone healing by local delivery of recombinent hepatocyte growth factor in rabbits. Arthroscopy2010;26:84–90. Google Scholar
30 Hettrich CM , BeamerBS, BediA, et al.The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res2012;30:769–774.CrossrefPubMed Google Scholar
31 Ouyang HW , GohJC, LeeEH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med2004;32:321–327.CrossrefPubMed Google Scholar
32 Lim JK , HuiJ, LiL, et al.Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy2004;20:899–910.CrossrefPubMed Google Scholar
33 Li YG , WeiJN, LuJ, WuXT, TengGJ. Labeling and tracing of bone marrow mesenchymal stem cells for tendon-to-bone tunnel healing. Knee Surg Sports Traumatol Arthrosc2011;19:2153–2158.CrossrefPubMed Google Scholar
34 Mutsuzaki H , SakaneM, NakajimaH, OchiaiN. Calcium phosphate-hybridised tendon graft to reduce bone-tunnel enlargement after ACL reconstruction in goats. Knee2012;19:455–460.CrossrefPubMed Google Scholar
35 Kuang GM , YauWP, LuWW, ChiuKY. Osteointegration of soft tissue grafts within the bone tunnels in anterior cruciate ligament reconstruction can be enhanced. Knee Surg Sports Traumatol Arthrosc2010;18:1038–1051.CrossrefPubMed Google Scholar
Funding statement:
This work was supported by the Grant-in Aid for Scientific Research C: KAKENHI 20591769.
Author contributions:
K. Tabuchi: Data collection, Data analysis, Writing the paper
T. Soejima: Study design, Writing the paper
T. Kanazawa: Data collection, Data analysis
K. Noguchi: Data collection, Data analysis
K. Nagata: Study design
ICMJE Conflict of Interest:
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









