Abstract
Introduction
Negative pressure wound therapy (NPWT) and vessel loop assisted closure are two common methods used to assist with the closure of fasciotomy wounds. This retrospective review compares these two methods using a primary outcome measurement of skin graft requirement.
Methods
A retrospective search was performed to identify patients who underwent fasciotomy at our institution. Patient demographics, location of the fasciotomy, type of assisted closure, injury characteristics, need for skin graft, length of stay and evidence of infection within 90 days were recorded.
Results
A total of 56 patients met the inclusion criteria. Of these, 49 underwent vessel loop closure and seven underwent NPWT assisted closure. Patients who underwent NPWT assisted closure were at higher risk for requiring skin grafting than patients who underwent vessel loop closure, with an odds ratio of 5.9 (95% confidence interval 1.11 to 31.24). There was no difference in the rate of infection or length of stay between the two groups. Demographic factors such as age, gender, fracture mechanism, location of fasciotomy and presence of open fracture were not predictive of the need for skin grafting.
Conclusion
This retrospective descriptive case series demonstrates an increased risk of skin grafting in patients who underwent fasciotomy and were treated with NPWT assisted wound closure. In our series, vessel loop closure was protective against the need for skin grafting. Due to the small sample size in the NPWT group, caution should be taken when generalising these results. Further research is needed to determine if NPWT assisted closure of fasciotomy wounds truly leads to an increased requirement for skin grafting, or if the vascular injury is the main risk factor.
Article focus
Is the type of assisted closure technique for fasciotomy wounds predictive of the need for skin grafting to achieve definitive coverage?
Is there a difference in the rate of infection or length of stay when comparing vessel loop assisted closure and negative pressure wound therapy (NPWT) assisted closure for treatment of fasciotomy wounds?
Article summary
Patients who underwent NPWT assisted closure were at higher risk for requiring skin grafting than patients who underwent vessel loop closure, with an odds ratio of 5.9 (95% confidence interval 1.11 to 31.24)
There was no difference in the rate of infection or length of stay between the two groups
Demographic factors such as age, gender, fracture mechanism, location of fasciotomy, and presence of open fracture were not predictive of the need for skin grafting
Strengths and limitations
A major limitation of our study is the relatively small number of patients that underwent NPWT treatment and the large difference in numbers between groups
The retrospective nature of this study introduces selection bias, although statistical analysis did not demonstrate any differences between the NPWT and vessel loop groups with regard to demographic, mechanism of injury or presence of open fracture. It is possible that patients with more severe injuries were selected to undergo NPWT and therefore this group had worse outcomes
Continuous pressure monitoring was not employed, which several authors have suggested increases the false positive rate for the diagnosis of compartment syndrome
Introduction
The use of decompressive fasciotomy, in the setting of an acute compartment syndrome, is potentially a limb--saving procedure. Compartment syndrome, a situation where the compartment pressure is higher than perfusion pressure, occurs in both the upper and lower extremities and can result from insults such as fracture, ischemia, reperfusion, crush injury, burns and over-exertion.1,2 The sequelae from an untreated compartment syndrome are devastating. Therefore, early recognition and treatment via surgical decompression are paramount. However, the fasciotomy incisions can lead to large, unsightly, chronic wounds after surgical intervention.
A popular method of assisted closure of fasciotomy wounds uses a negative pressure environment created by negative pressure wound therapy (NPWT).3 NPWT dressings are a closed system whereby a vacuum applies subatmospheric pressure to a wound through a porous foam dressing, reducing extravascular pressure and oedema within the compartment, leading to improved circulation, granulation and approximation of wound edges, as well as less bacterial colonisation.3 Orthopaedic indications for NPWT include closure of fasciotomy wounds, degloving injuries and infectious wounds after debridement.4,5 It has been suggested that the use of the NPWT dressing leads to a higher rate of primary wound closure, less hospitalisation time and earlier rehabilitation than traditional wet-to-dry dressing changes.6 Other authors have shown that definitive closure of fasciotomy wounds, either primary or with split-thickness skin graft, is accelerated by the use of the NPWT7,8 and that it may help avoid the need for flap coverage.9
Dynamic wound closure using the vessel loop or shoelace technique has also been described as a viable management option (Fig. 1).10-16 This method entails approximation of wound edges using vessel loops anchored by skin staples and gradually tensioning them across the wound margins. Early versions of this technique used materials such as heavy nylon sutures and were claimed to lead to gradual closure of fasciotomy wounds without further need for surgery.16 As the technique has evolved, it has been suggested that vessel loop closure may avoid the need for split-thickness skin grafts, resulting in better cosmesis and avoidance of donor site morbidity.10-16 Vessel loop closure has been praised as cost-effective and simple due to the readily available materials needed for its execution.17 It may even have other applications such as the treatment of complex wounds associated with open fractures.18 Other commercially available devices for dermatotraction are available.19,20 Examples of these devices include the Dermaclose RC (Chanhassen, Minnesota) and the Sure-Closure skin-stretching system (Zimmer, Warsaw, Indiana). Despite this, the simplified vessel loop technique may be the cheapest and most readily available. Several modifications to the technique have been described, including the use of drains placed in the wound in order to prevent myonecrosis.21
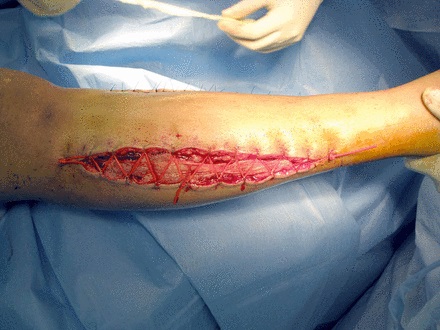
Fig. 1
Photograph showing an example of vessel loop closure in a lower leg fasciotomy.
The purpose of this study is to describe two commonly employed methods of fasciotomy closure and report on the need for skin graft, length of hospital stay and incidence of infection.
Patients and Methods
After obtaining Institutional Review Board approval, a retro-spective search was performed of our orthopaedic database from January 2004 to July 2010 to identify patients who underwent fasciotomy at our level I trauma centre. Patients were included if the following criteria were met: 1) age between 18 and 89 years at the time of fasciotomy; 2) either NPWT or vessel loops were applied at the time of initial fasciotomy and at each subsequent debridement; 3) outpatient records were available for review. Patients were excluded if any of the following -criteria were met: 1) neither NPWT nor vessel loops were used at the initial fasciotomy or not used at one of the subsequent debridements; 2) outpatient charts were not available for review; 3) the method of assisted closure was unable to be determined from records; 4) fasciotomy was performed due to exertional compartment syndrome or burns. The Vacuum Assisted Closure device (KCI, San Antonio, Texas) was the NPWT device used in all cases.
Patient demographics including age, gender, race, body mass index (BMI), history of diabetes and history of smoking were recorded. The location of the fasciotomy, type of assisted closure, mechanism of injury, presence of fracture, bone(s) fractured, presence of vascular injury, number of debridements before final closure/graft, need for skin graft, length of stay, evidence of infection within 90 days and time to infection were recorded. Compartment syndrome was diagnosed based on clinical findings and confirmed intra-operatively using a handheld compartment pressure measurement device (Stryker, -Kalamazoo, Michigan), with a threshold of a compartment pressure within 30 mm Hg of the diastolic blood pressure declared positive for compartment syndrome. Patients were indicated for skin grafting if the attending surgeon determined that their fasciotomy wound could not be closed primarily. This decision was most commonly made after three debridements and/or NPWT changes. Time to infection was defined as the time from injury to initiation of antibiotics and/or -operative debridement.
Statistical analysis
Baseline, demographic and clinical variables were tested for group differences using t-tests for continuous variables and Fisher’s exact test for categorical variables. The analysis of binary outcomes (skin graft, infection) was carried out using univariate logistic regression. The odds ratios with 95% confidence intervals (CI) were calculated for each predictor. Analysis of -variance (ANOVA) was used to determine if there was a significant difference when three or more groups were present. Time to event data (time to infection) was -analysed using univariate and multivariate Cox proportional hazards regression. Hazard ratios with 95% CI were calculated for each predictor. Differences between groups (rejection of the null hypothesis) and regression effects were considered significant if the probability of chance occurrence was ≤ 0.05 using two-tailed tests.
Results
The retrospective medical record search identified 72 patients who underwent fasciotomy over the study period. A total of 16 patients were excluded; two of whom received both NPWT and vessel loops during their course and 14 who did not have treatment with NPWT or vessel loops to assist with closure. Of the remaining 56 patients, 49 underwent vessel loop closure and seven underwent NPWT assisted closure. The two groups (NPWT and vessel loop) were statistically similar in regards to age, race, gender, mechanism of injury, -presence of vascular injury, open fracture, infection and length of stay (Table I). Patients who underwent NPWT assisted closure were at higher risk for requiring skin grafting than patients who underwent vessel loop closure, with an odds ratio of 5.9 (95% CI 1.11 to 31.24). Patients treated with vessel loop assisted closure were found to be protected from requiring a skin graft in this series, with an odds ratio of 0.17 (95% CI 0.03 to 0.89). Patients treated with vessel loop assisted closure had a mean length of stay of 19.2 days (3 to 113) while patients treated with NPWT assisted closure had a mean length of stay of 23.7 days (6 to 75), which was not statistically significant (p = 0.61). There was no difference in the frequency of infection between the patients treated with NPWT and those treated with vessel loop closure (p = 0.11). Location and mechanism of injury were not predictive of skin graft requirement (Table II). Vascular injury demonstrated a trend for increased risk of skin graft, odds ratio 4.33 (95% CI 0.9 to 20.7), but the confidence interval crossed unity and was therefore found to be non-significant. ANOVA was used to determine that there was no difference in rate of skin graft between groups (forearm, lower leg, thigh) (p = 0.12).
Table I
Patient demographics and characteristics (NPWT, negative pressure wound therapy)
| Vessel loop (n = 49) | NPWT (n = 7) | p-value* | |
|---|---|---|---|
| Mean age (yrs) | 37.1 (18 to 82) | 41.7 (19 to 72) | 0.4† |
| Male (n, %) | 40 (81.6) | 6 (85.7) | 1.00 |
| Mean body mass index (kg/m2) | 27.9 (18.3 to 52.4) | 28.9 (23.9 to 35.8) | 0.6† |
| Diabetes (n, %) | 4 (8.2) | 1 (14.3) | 0.50 |
| Smoker (n, %) | 11 (22.4) | 1 (14.3) | 1.00 |
| White/Caucasian (n, %) | 7 (14.3) | 2 (28.6) | 0.31 |
| Mechanism (n, %) | |||
| Gunshot wound | 12 (24.5) | 1 (14.3) | 1.00 |
| Blunt trauma | 20 (40.8) | 2 (28.6) | 0.69 |
| Automobile vs pedestrian | 13 (26.5) | 3 (42.9) | 0.39 |
| Other | 4 (8.2) | 1 (14.3) | - |
| Vascular injury (n, %) | 6 (12.2) | 2 (28.6) | 0.26 |
| Fracture (n, %) | 44 (89.8) | 7 (100) | 1.00 |
| Open fractures | 18 (36.7) | 1 (14.3) | 0.40 |
| Required skin graft (n, %) | 9 (18.4) | 4 (57.1) | 0.04 |
| Infection within 90 days (n, %) | 3 (6.1) | 2 (28.6) | 0.11 |
| Mean length of stay (days) | 19.2 (3 to 113) | 23.7 (6 to 75) | 0.61† |
-
* Fisher’s exact test, unless otherwise stated † t-test
Table II
Comparison of patients who had a skin graft versus those who did not
| No skin graft (n = 42) | Skin graft (n = 14) | Odds ratio (95% CI) | |
|---|---|---|---|
| Mean age (yrs) | 38.3 (18 to 82) | 35.6 (19 to 72) | 0.99 (0.94 to 1.03) |
| Male (n, %) | 34 (81.0) | 12 (85.7) | 3.18 (0.36 to 27.76) |
| Mean body mass index (kg/m2) | 28.2 (18.3 to 52.4) | 27.7 (23.5 to 34.4) | 0.99 (0.92 to 1.05) |
| Diabetes (n, %) | 3 (7.1) | 2 (14.3) | 2.42 (0.36 to 16.36) |
| Smoker (n, %) | 9 (21.4) | 3 (21.4) | 1.13 (0.26 to 5.0) |
| White/Caucasian (n, %) | 7 (16.7) | 2 (14.3) | 0.94 (0.17 to 5.17) |
| NPWT* (n, %) | 3 (7.1) | 4 (28.6) | 5.93 (1.11 to 31.24) |
| Vessel loop (n, %) | 40 (95.2) | 9 (64.3) | 0.17 (0.03 to 0.89) |
| Location (n, %) | |||
| Forearm | 7 (16.7) | 5 (35.7) | 3.21 (0.81 to 12.77) |
| Lower leg | 30 (71.4) | 9 (64.3) | 0.69 (0.19 to 2.53) |
| Thigh | 6 (14.3) | 0 (0) | - |
| Mechanism (n, %) | |||
| Gunshot wound | 11 (26.2) | 2 (14.3) | 0.53 (0.10 to 2.76) |
| Blunt trauma | 16 (38.1) | 6 (42.9) | 1.44 (0.41 to 5.07) |
| Automobile vs pedestrian | 12 (28.6) | 4 (28.6) | 1.15 (0.30 to 4.44) |
| Other | 3 (7.1) | 2 (14.3) | - |
| Vascular injury (n, %) | 4 (9.5) | 4 (28.6) | 4.33 (0.9 to 20.7) |
| Fracture (n, %) | 38 (90.5) | 14 (100) | - |
| Open fractures | 14 (33.3) | 5 (35.7) | 1.30 (0.36 to 4.69) |
| Infection (n, %) | 4 (9.5) | 1 (7.1) | 1.1 (0.87 to 1.24) |
| Mean length of stay (days) | 17 (3 to 87) | 28 (8 to 113) | - |
-
* NPTW, negative pressure wound therapy
Discussion
Fasciotomy is a limb saving procedure that can carry significant morbidity. Although our series clearly suffers from an imbalance in the number of patients in each group, it demonstrates that NPWT assisted closure of fasciotomy wounds may lead to an increased risk of skin grafting, while vessel loop closure may be protective against patients requiring skin graft. Demographic and patient factors such as BMI, fracture mechanism, age, gender, and presence of open fracture did not affect the rate of skin graft. Patients with vascular injury demonstrated a trend toward skin grafting after use of a NPWT. The mechanism of this association is unclear, however, the negative pressure environment created by the NPWT may lead to increased soft-tissue oedema due to the ‘leakiness’ of cell basement membranes created during ischemia. NPWT has been purported to decrease bacterial counts in soft-tissue wounds, theoretically decreasing the rate of infection.6 The potential benefit of NPWT therapy in decreasing infection rates is not reflected in the data from our study.
Zannis et al6 compared wet-dry saline dressings with NPWT assisted closure, and found that NPWT significantly increased the rate of primary closure and reduced the time to closure. While NPWT increased the rate of primary closure in their series, the rate of skin grafting was similar for upper extremity fasciotomies treated with NPWT (42.6%) and those treated with saline dressings (47.3%).6 The percentage of patients requiring skin grafting for lower extremity fasciotomy was also similar (16.5% vs 20.4% in the saline dressing group), although significance values were not provided.
Forearm fasciotomy wounds were treated exclusively with vessel loop closure in our series, resulting in no infections, but a 42% rate of skin grafting. The high rate of skin grafting may be reflective of the minimal amount of redundant skin in this location. A series of patients random-ised to either NPWT or vessel loop closure will be needed to determine the optimal closure method for upper extremity fasciotomy wounds.
A major limitation of our study is the relatively small number of patients that underwent NPWT treatment. This likely stems from anecdotal experience in our department that fasciotomy wounds treated with NPWT tended to require skin grafting, therefore surgeons were less inclined to employ NPWT for this use. The retrospective nature of this study also introduces selection bias, although statistical analysis did not demonstrate any differences between the NPWT and vessel loop groups with regard to demographic, mechanism of injury, or presence of open fracture. It is possible that patients with more severe injuries were selected to undergo NPWT and therefore this group had worse outcomes. Another potential weakness is the use of clinical findings and a single -compartment measurement for diagnosis of compartment syndrome. Several authors have found a high rate of false positives in using a single compartment measurement, as opposed to trending pressures with continuous monitoring, meaning that some of the patients in our series may have had an ‘unnecessary’ fasciotomy.22-24 Given the potential for false positives, our groups could have a different number of true positive compartment syndromes, possibly affecting the rate of skin grafting given that patients with a false positive compartment syndrome would theoretically have a higher change of delayed primary closure. It would have been better to trend compartment measurements and record absolute values to determine the severity of the compartment -syndromes and determine if pressure correlates with need for skin graft.
In conclusion, this retrospective descriptive case series demonstrates an increased risk of skin grafting in patients who underwent fasciotomy and were treated with NPWT assisted wound closure. In our series, vessel loop closure was protective against the need for skin grafting. Due to the small sample size in the NPWT group, caution should be taken when generalising these results. Further research is needed to determine if NPWT assisted closure of fasciotomy wounds truly leads to an increased requirement for skin grafting, or if the vascular injury is the main risk factor.
1 McQueen MM Gaston P Court-Brown CM . Acute compartment syndrome: who is at risk?J Bone Joint Surg [Br]2000;82-B:200–203.PubMed Google Scholar
2 McQueen MM Christie J Court-Brown CM . Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg [Br]1996;78-B:95–98.PubMed Google Scholar
3 Morykwas MJ Argenta LC Shelton-Brown EI McGuirt W . Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg1997;38:553–562.CrossrefPubMed Google Scholar
4 Webb LX . New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg2002;10:303–311.CrossrefPubMed Google Scholar
5 DeFranzo AJ Argenta LC Marks MW , et al.. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg2001;108:1184–1191.CrossrefPubMed Google Scholar
6 Zannis J Angobaldo J Marks M , et al.. Comparison of fasciotomy wound closures using traditional dressing changes and the vacuum-assisted closure device. Ann Plast Surg2009;62:407–409.CrossrefPubMed Google Scholar
7 Yang CC Chang DS Webb LX . Vacuum-assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Surg Orthop Adv2006;15:19–23.PubMed Google Scholar
8 Scherer LA Shiver S Chang M Meredith JW Owings JT . The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg2002;137:930–934.CrossrefPubMed Google Scholar
9 Molnar JA DeFranzo AJ Hadaegh A , et al.. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg2004;113:1339–1346.CrossrefPubMed Google Scholar
10 Asgari MM Spinelli HM . The vessel loop shoelace technique for closure of fasciotomy wounds. Ann Plast Surg2000;44:225–229.CrossrefPubMed Google Scholar
11 Berman SS Schilling JD McIntyre KE Hunter GC Bernhard VM . Shoelace technique for delayed primary closure of fasciotomies. Am J Surg1994;167:435–436.CrossrefPubMed Google Scholar
12 Harris I . Gradual closure of fasciotomy wounds using a vessel loop shoelace. Injury1993;24:565–566.CrossrefPubMed Google Scholar
13 Janzing HM Broos PL . Dermatotraction: an effective technique for the closure of fasciotomy wounds: a preliminary report of fifteen patients. J Orthop Trauma2001;15:438–441.CrossrefPubMed Google Scholar
14 Baum TP Strauch B . Delayed primary closure using Silastic vessel loops and skin staples: description of the technique and case reports. Ann Plast Surg1999;42:337–340.CrossrefPubMed Google Scholar
15 Zorrilla P Marin A Gomez LA Salido JA . Shoelace technique for gradual closure of fasciotomy wounds. J Trauma2005;59:1515–1517.CrossrefPubMed Google Scholar
16 Almekinders LC . Tips of the trade #32: gradual closure of fasciotomy wounds. Orthop Rev1991;20:82–84. Google Scholar
17 Marek DJ Copeland GE Zlowodzki M Cole PA . The application of dermatotraction for primary skin closure. Am J Surg2005;190:123–126.CrossrefPubMed Google Scholar
18 Schnirring-Judge MA Anderson EC . Vessel loop closure technique in open fractures and other complex wounds in the foot and ankle. J Foot Ankle Surg2009;48:692–699.CrossrefPubMed Google Scholar
19 Barnea Y Gur E Amir A , et al.. Delayed primary closure of fasciotomy wounds with Wisebands, a skin- and soft tissue-stretch device. Injury2006;37:561–566.CrossrefPubMed Google Scholar
20 Medina C Spears J Mitra A . The use of an innovative device for wound closure after upper extremity fasciotomy. Hand (N Y)2008;3:146–151.CrossrefPubMed Google Scholar
21 Galois L Pauchot J Pfeffer F , et al.. Modified shoelace technique for delayed primary closure of the thigh after acute compartment syndrome. Acta Orthop Belg2002;68:63–67.PubMed Google Scholar
22 McQueen MM Court-Brown CM . Compartment monitoring in tibial fractures: the pressure threshold for decompression. J Bone Joint Surg [Br]1996;78-B:99–104. Google Scholar
23 Williams PR Russell ID Mintowt-Czyz WJ . Compartment pressure monitoring: current UK orthopaedic practice. Injury1998;29:229–232. Google Scholar
24 Harris IA Kadir A Donald G . Continuous compartment pressure monitoring for tibia fractures: does it influence outcome?J Trauma2006;60:1330–1335.CrossrefPubMed Google Scholar
None declared
J. R. Fowler: Study design, Data collection, Writing and proofreading the paper
M. T. Kleiner: Data collection, Writing and proofreading the paper
R. Das: Data collection, Writing and proofreading the paper
J. P. Gaughan: Data analysis, Writing and proofreading the paper
S. Rehman: Data analysis, Writing and proofreading the paper
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









