Abstract
Aims
Outcomes of current operative treatments for arthrofibrosis after total knee arthroplasty (TKA) are not consistently positive or predictable. Pharmacological in vivo studies have focused mostly on prevention of arthrofibrosis. This study used a rabbit model to evaluate intra-articular (IA) effects of celecoxib in treating contracted knees alone, or in combination with capsular release.
Methods
A total of 24 rabbits underwent contracture-forming surgery with knee immobilization followed by remobilization surgery at eight weeks. At remobilization, one cohort underwent capsular release (n = 12), while the other cohort did not (n = 12). Both groups were divided into two subcohorts (n = 6 each) – one receiving IA injections of celecoxib, and the other receiving injections of vehicle solution (injections every day for two weeks after remobilization). Passive extension angle (PEA) was assessed in live rabbits at 10, 16, and 24 weeks, and disarticulated limbs were analyzed for capsular stiffness at 24 weeks.
Results
IA celecoxib resulted in greater mean PEA at ten weeks (69.6° (SD 4.6) vs 45.2° (SD 9.6), p = 0.004), 16 weeks (109.8° (SD 24.2) vs 60.9° (SD10.9), p = 0.004), and 24 weeks (101.0° (SD 8.0) vs 66.3° (SD 5.8), p = 0.004). Capsular stiffness was significantly reduced with IA celecoxib (2.72 Newton per cm (N·cm)/° (SD 1.04), p = 0.008), capsular release (2.41 N·cm/° (SD 0.80), p = 0.008), and capsular release combined with IA celecoxib (3.56 N·cm/° (SD 0.99), p = 0.018) relative to IA vehicle (6.09 N·cm/° (SD 1.64)).
Conclusion
IA injections of a celecoxib led to significant improvements in passive extension angles, with reduced capsular stiffness, when administered to rabbit knees with established experimental contracture. Celecoxib was superior to surgical release, and the combination of celecoxib and a surgical release did not provide any additional value.
Cite this article: Bone Joint Res 2022;11(1):32–39.
Article focus
-
In a rabbit model of arthrofibrosis, does intra-articular (IA) celecoxib improve motion compared to control injections?
-
Is IA celecoxib superior to a capsular release?
-
Does IA celecoxib have synergistic effects when combined with a capsular release?
Key messages
-
IA celecoxib significantly improves passive range of motion and capsular stiffness in a validated rabbit model of arthrofibrosis.
-
IA celecoxib is superior to a capsular release.
-
IA celecoxib combined with capsular release did not provide additional value when compared to each treatment alone.
Strengths and limitations
-
Use of a validated large animal model with relatively high numbers of subjects compared to other, similar studies.
-
Direct local administration of the studied drug, thus reducing confounding variables compared to studies using subcutaneous or oral drug delivery.
-
A limitation of this study is a lack of molecular and histological data to confirm tissue-specific changes.
Introduction
Arthrofibrosis is a debilitating complication affecting roughly 4% of all patients undergoing primary total knee arthroplasty (TKA).1,2 Arthrofibrosis also complicates various other orthopaedic procedures and injuries. Nonoperative treatment options for this disease are limited, and in many cases a surgical capsular release in the form of a lysis of adhesions (LOA) is performed, often combined with exchange of one or more prosthetic components.3-17 Yet, these patients’ knees are often refractory to operative treatment as arthrofibrosis can recur after capsular release.6,14,18-20 Thus, the persistently stiff knee is a clinical challenge that would benefit from new adjuvant therapies.
Currently, there are limited pharmacological treatments for the established arthrofibrotic knee. Almost all in vivo animal studies focus on the prevention of joint contractures, with only one examining the treatment of established contractures.21 Moreover, there are no studies comparing pharmacological treatment to a surgical capsular release, and no studies investigating the possible synergistic effect of combining the two.
Previous studies laid the groundwork for evaluating the effectiveness of pharmacological methods in treating established contractures and comparing these treatments to a capsular release.22-26 Several studies have shown the effectiveness of celecoxib, a selective cyclooxygenase-2 inhibitor, in preventing the formation of scar tissue in our animal model of arthrofibrosis.24,25,27 Additionally, Barlow et al28 previously developed a modified rabbit model with a limited surgical release of an established contracture. In that investigation, authors showed a significant improvement in passive extension angles (PEAs) in the surgically treated contractures.28
The current study investigated adjuvant therapies for treatment of an established contracture through three aims. The first aim was to investigate the biomechanical effects of intra-articular (IA) celecoxib injections for the treatment of established contractures in a rabbit model of arthrofibrosis. The second aim was to compare IA celecoxib injections to a surgical capsular release. The final and third aim was to investigate the potential additive effects of combining IA celecoxib and capsular release.
Methods
Study design
Animal care committee approval (IACUC) was obtained and the ARRIVE checklist was completed to perform a study on 24 skeletally mature New Zealand White (NZW) female rabbits. Each rabbit underwent a contracture-forming surgery followed by immobilization of the right knee as previously described (Figure 1).2,22-26,28-33 After eight weeks of immobilization in a 1 m3 cage, all rabbits underwent a remobilization procedure, which involved general anaesthesia and removal of the Kirschner wire (K-wire). After K-wire removal, 12 of the 24 rabbits underwent a capsular release procedure as previously described.28 Briefly, this capsular release involved a lateral incision on the right knee with blunt dissection to the lateral meta-diaphysis. An elevator was then inserted posteriorly with periosteal elevation carried to the joint. After K-wire removal and/or capsular release, each group of 12 rabbits (capsular release and non-capsular release) was again divided into two different cohorts – with six rabbits in the capsular release group and six in the non-capsular release group receiving IA celecoxib injections every day for two weeks. The other six rabbits in the capsular release and non-capsular release groups underwent 'sham' injections with the same solution used to dissolve the celecoxib (vehicle solution). By week 10 there were four groups with six rabbits each: capsular release alone, capsular release combined with IA celecoxib injections, celecoxib injections alone, and untreated control with injection of vehicle solution but no capsular release. The injections stopped at week 10 and all rabbits were allowed 14 weeks of free cage activity until sacrifice at 24 weeks.
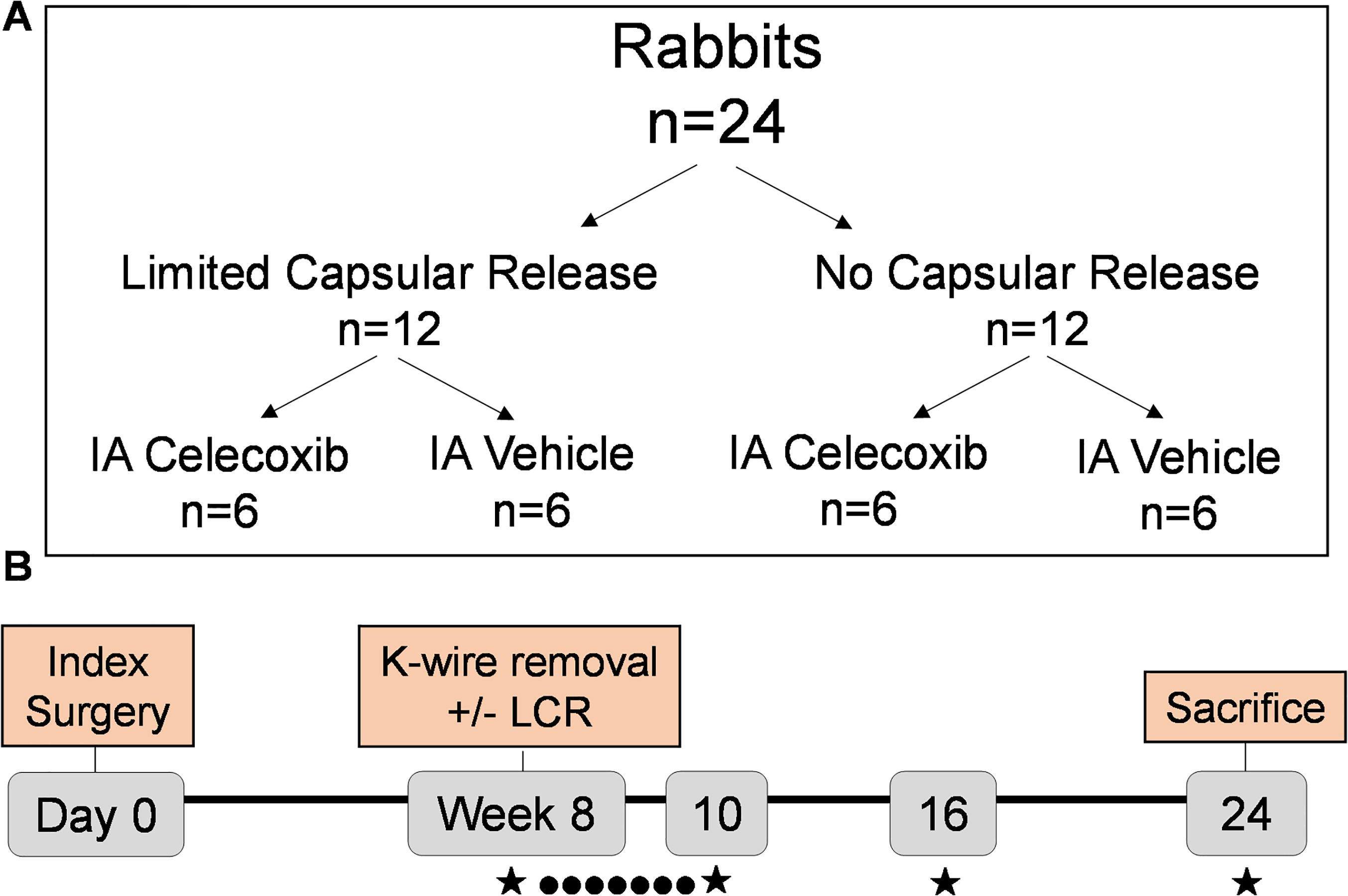
Fig. 1
Experimental design: a) 24 rabbits were divided into four groups in order to test effects of limited capsular release (LCR) and intra-articular (IA) celecoxib, both separately and together. b) Over the course of the experiment, contractures were treated every day for two weeks with IA injections of celecoxib or vehicle (●) and assessed (★) at weeks 8, 10, 16, and 24 for passive extension angle. After sacrifice (24 weeks), capsular stiffness was evaluated. K-wire, Kirschner wire.
Preparation of celecoxib and control injections
The methods to prepare the celecoxib drug solution for IA injections has been previously described.25 'Sham' injections used a solution identical to the treatment injections, but without the addition of celecoxib. These injections were administered into the joint every day for two weeks (14 days total) after the remobilization surgery at Week 8.
Static joint angle measurement
At the 8-, 10-, 16-, and 24-week timepoints, rabbits were anaesthetized and placed into a validated plunger device.32 A string tied to the experimental rabbit limb gently extended the knee at three sequential forces (20, 30, and 40 N·cm). A static lateral fluoroscopic image was taken after each force was applied, with the joint angle measured with a digital goniometer (Figure 2).
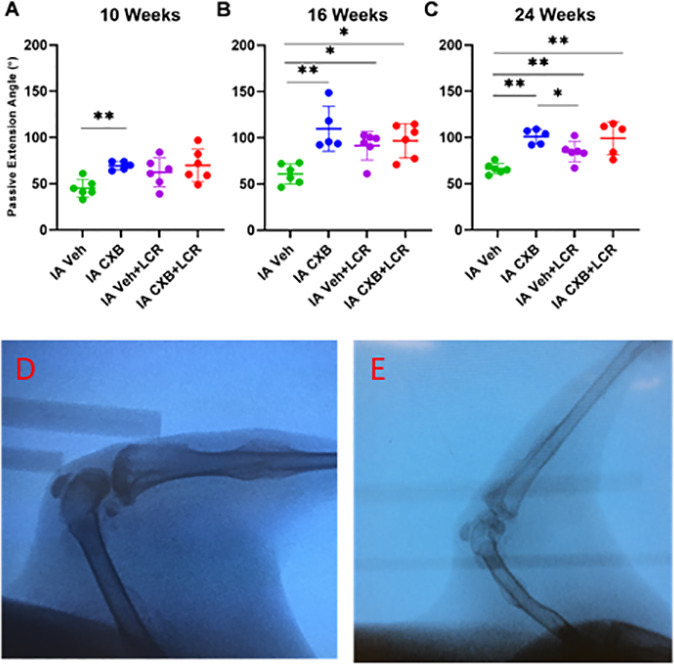
Fig. 2
Intra-articular (IA) celecoxib (CXB) injection increases the passive extension angle (PEA). PEAs were measured fluoroscopically on anaesthetized rabbits (d) and (e) at week 10 (a), week 16 (b), and week 24 (c). The graphs depict mean PEA and standard deviation (n = 5 to 6 for each group), with each data point representing one animal. When applicable, significance is noted with a standard asterisk convention (*p ≤ 0.05, **p ≤ 0.01, Mann-Whitney U test). LCR, limited capsular release. Veh, vehicle.
Dynamic stiffness measurement
At 24 weeks, and following sacrifice, the operative limbs of all animals were disarticulated at the hip and the foot was removed. Skin and other soft-tissues were removed from both the femur and tibia, while taking care to preserve the joint capsule. A 1 cm region of soft-tissue was left on both the femur and tibia nearest to the knee joint. Metal rods were then cemented into the intramedullary canals of the femur and tibia. Once dry, the limb was mounted on a dedicated dynamic load cell device (Figure 3).29,34 The maximum torque applied to the joint was 20 N·cm and the tibia was moved at a rate of 1°/second. Data were analyzed utilizing Matlab R2019a (Mathworks, USA). A previously defined 'stiffness coefficient' was used to characterize the dynamic stiffness of the joint using the slope of the steepest most linear portion of the exponential curve exported from the load cell device.26
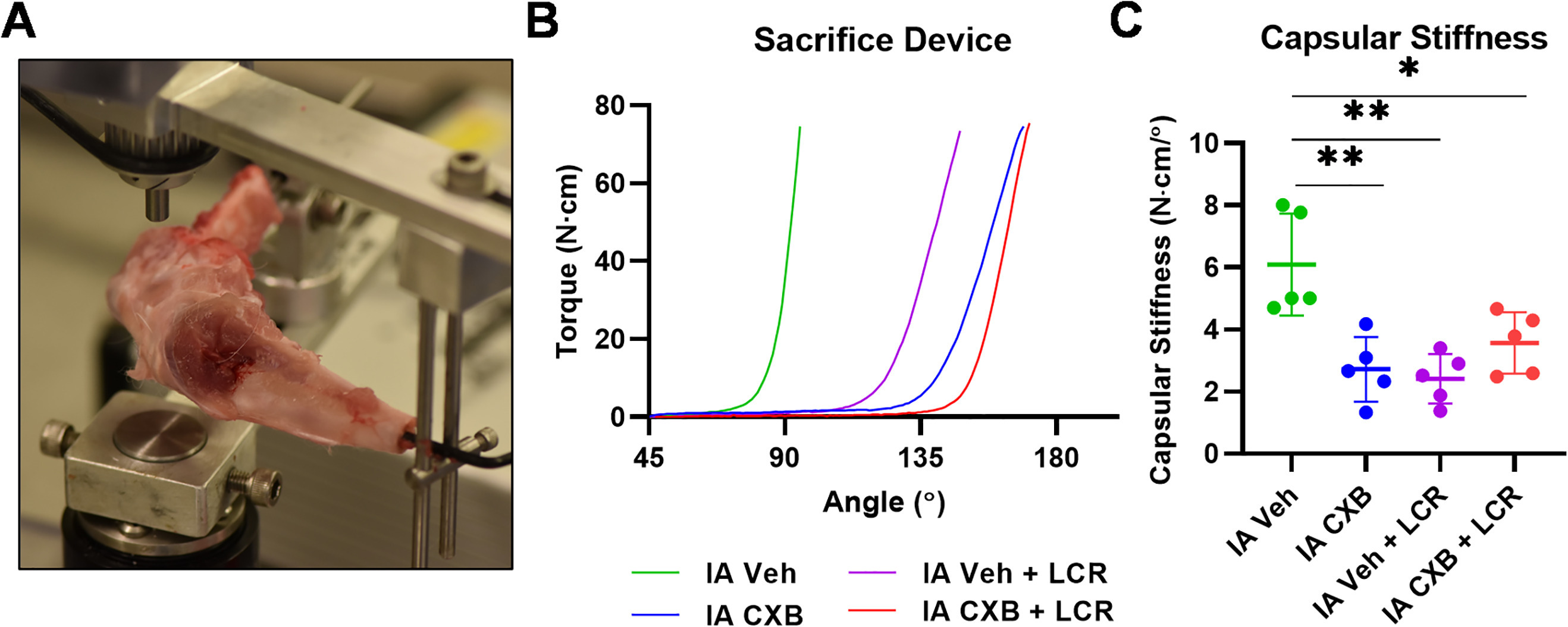
Fig. 3
Intra-articular (IA) celecoxib (CXB) injection reduces capsular stiffness. a) Capsular stiffness measurements were obtained from disarticulated rabbit limbs on a dynamic load cell device after sacrifice at 24 weeks. b) Mean curves generated by the dynamic load cell sacrifice device for each treatment group. c) Capsular stiffness data defined as the slope of a tangential line to the curves shown in panel B. The graphs depict mean capsular stiffness and standard deviation (n = 5 to 6 for each group), with each data point representing one animal. When applicable, significance is noted with a standard asterisk convention (*p ≤ 0.05, **p ≤ 0.01, Mann-Whitney U test). LCR, limited capsular release; Veh, vehicle.
Statistical analysis
Data were reported with group means and standard deviations (SDs). Kolmogorov-Smirnov normality tests were performed on all experimental data to determine if parametric (independent-samples t-test) or non-parametric tests (Mann-Whitney U test) should be used for treatment group comparisons. All statistical analyses were carried out using GraphPad Prism Version 9.0.0 for Windows (GraphPad Software, USA). Statistical significance was set at an α level of < 0.05.
Results
Static joint measurements
The experimental baseline for the effects of capsular release in vehicle control rabbits was established by showing that capsular release with vehicle injection significantly increased mean PEA at 16 weeks (91.5° (SD 15.4) vs 60.9° (SD 10.9), respectively, p = 0.015, Mann-Whitney U test) and 24 weeks (84.7° (SD 11.2) vs 66.3° (SD 5.8), respectively, p = 0.009, Mann-Whitney U test) compared to the vehicle injection group (Table I, Figure 2b to 2c). Notably, IA celecoxib animals showed statistically significant increases in mean PEA compared to the vehicle injection group at ten weeks (69.6° (SD 4.6) vs 45.2° (SD 9.6), respectively, p = 0.004, Mann-Whitney U test), 16 weeks (109.8° (SD 24.2) vs 60.9° (SD 10.9), respectively, p = 0.004, Mann-Whitney U test), and 24 weeks (101.0° (SD 8.0) vs 66.3° (SD 5.8), respectively, p = 0.004, Mann-Whitney U test (Table I, Figure 2a to 2c)). Additionally, the IA celecoxib treatment group showed statistically significant improvement in mean PEAs at 24 weeks compared to the capsular release with vehicle injections (101.0° (SD 8.0) vs 84.7° (SD 11.2), respectively, p = 0.017, Mann-Whitney U test (Figure 2c)). Importantly, no differences were observed when IA celecoxib was combined with a capsular release when compared to the IA vehicle injection with a capsular release (99.2° (SD 17.9) vs 84.7° (SD 11.2), respectively, p = 0.266, Mann-Whitney U test (Figure 2a to 2c)). Similarly, the combination of IA celecoxib and a capsular release had similar PEA when compared with rabbits treated only with IA celecoxib (99.2° (SD 17.9) vs 101° (SD 8.0), respectively, p = 0.738, Mann-Whitney U test (Figure 2a to 2c)). Thus, anti-inflammatory intervention using IA celecoxib was found to improve PEA relative to the baseline values with or without surgical release at the experimental endpoint at 24 weeks.
Table I.
Mean passive extension angles as measured by static pulley device at 40 N·cm for each treatment group. The p-values measure the statistical significance (defined as p < 0.05, measured by Mann-Whitney U test) with each treatment group compared to the intra-articular vehicle control group.
| Timepoint | IA vehicle, ° (SD) |
IA vehicle + LCR, ° (SD) |
p-value | IA CXB, ° (SD) |
p-value | IA CXB + LCR, ° (SD) |
p-value |
|---|---|---|---|---|---|---|---|
| Week 10 | 45.2 (9.6) | 62.3 (15.7) | 0.069 | 69.6 (4.6) | 0.004 | 69.8 (17.5) | 0.023 |
| Week 16 | 60.9 (10.9) | 91.5 (15.4) | 0.015 | 109.8 (24.2) | 0.004 | 96.8 (18.5) | 0.009 |
| Week 24 | 66.3 (5.8) | 84.7 (11.2) | 0.009 | 101.0 (8.0) | 0.004 | 99.2 (17.9) | 0.007 |
-
CXB, celecoxib; IA, intra-articular; LCR, limited capsular release; SD, standard deviation.
Dynamic stiffness measurement
To corroborate the PEA data, biomechanical load to failure experiments were performed to obtain stiffness coefficients. The mean stiffness coefficient evaluated at 24 weeks was significantly reduced in the capsular release cohort when compared to the vehicle-treated controls (2.41 N·cm/° (SD 0.80) vs 6.09 N·cm/° (SD 1.64); p = 0.008, Mann-Whitney U test (Table II, Figure 3a to 3c)). Similarly, the stiffness coefficient was also significantly reduced in the IA celecoxib group when compared to the vehicle treated controls (2.72 N·cm/° (SD 1.04) vs 6.09 N·cm/° (SD 1.64), respectively; p = 0.008, Mann-Whitney U test). The group with the lowest capsular stiffness was the IA vehicle with capsular release (2.41 N·cm/° (SD 0.80)). The combination of IA celecoxib with capsular release also significantly reduced capsular stiffness when compared to vehicle-treated group (3.56 N·cm/° (SD 0.99) vs 6.09 N·cm/° (SD 1.64), respectively; p = 0.018, Mann-Whitney U test). Importantly, no statistical differences in stiffness coefficients were observed when comparing IA celecoxib, capsular release, and IA celecoxib combined with capsular release (2.72 N·cm/° (SD 1.04) vs 2.41 N·cm/° (SD 0.80) vs 3.56 N·cm/° (SD 0.99), respectively (Table II)). The stiffness coefficient data largely paralleled the PEA data.
Table II.
Mean capsular stiffness (N·cm/°) for each treatment group at kill as measured by the dynamic load cell device. The p-values measure the statistical significance (defined as p < 0.05, measured by Mann-Whitney U test) with each treatment group compared to the intra-articular vehicle control group.
| Group | N·cm/° (SD) | p-value |
|---|---|---|
| IA vehicle | 6.09 (1.64) | Control |
| IA vehicle + LCR | 2.41 (0.80) | 0.008 |
| IA CXB | 2.72 (1.04) | 0.008 |
| IA CXB + LCR | 3.56 (0.99) | 0.018 |
-
CXB, celecoxib; IA, intra-articular; LCR, limited capsular release; SD, standard deviation.
Discussion
Arthrofibrosis after TKA remains a challenging complication, with poor non-surgical and surgical treatment options.7,26 Arthrofibrosis also complicates other orthopaedic conditions and procedures. For persistently stiff joints, arthroscopic or open lysis of adhesions may be indicated, although with serious limitations, as scar tissue often reaccumulates, again restricting motion.35-37 The current study demonstrated that locally administered celecoxib improved range of motion (ROM) in rabbit knees with established contractures. Furthermore, this study showed that local celecoxib treatment was superior to a surgical capsular release alone, and that these two treatment methods combined were not additive or synergistic at improving knee ROM.
The findings in this study are clinically relevant because current treatment options for established contractures are severely limited. Nonoperative treatment options focus on physical therapy and stretching.9,38 However, these options are often not successful, with physical therapy alone offering just 3° of mean improved motion in one study.39 For the persistently stiff knee, operative management includes open arthrolysis, arthroscopic lysis of adhesions, and/or a manipulation under anaesthesia (MUA), all with similar levels of improvement.39 However, 15% of patients are persistently stiff after the MUA.40,41 For these patients who fail MUA, arthroscopic or open lysis of adhesions has limited success.42,43 Furthermore, these surgical options are not without risks of periprosthetic fracture or extensor mechanism disruption.44,45 For these reasons, the discovery of a non-surgical treatment option for established contractures is important.
The findings in this study suggest that local administration of celecoxib improves motion in the stiff knee when compared to the untreated control. This finding is valuable because nearly all in vivo research on arthrofibrosis focuses on the prevention of scar formation – with only a single study looking at the treatment of established contractures.21 That single study, however, used a rat model for adhesive capsulitis of the shoulder. Interestingly, this study found that the vehicle solution with capsular release showed a less stiff capsule at 24 weeks compared to the celecoxib treatment group with capsular release. Yet, there was no statistical significance between these two groups. Ultimately, this finding reinforces the observation that celecoxib does not have an additive effect when combined with the capsular release. In fact, the celecoxib treatment group alone had statistically similar stiffness compared to both capsular release groups, suggesting that the drug itself functioned as a proxy capsular release in restoring motion in this model of arthrofibrosis.
In our study, locally delivered celecoxib was superior to a surgical capsular release in this rabbit model of arthrofibrosis. It should be noted that this superior effect was only noted at 24 weeks, and only on static measurements – with no statistical significance in the dynamic stiffness coefficients between the two groups. However, it is notable that celecoxib was at a minimum equivalent, and at best superior, to a capsular release alone. This finding is clinically relevant because for patients who fail conservative treatment for arthrofibrosis, locally delivered celecoxib might offer a less invasive and potentially more effective treatment option to improve joint arc-of-motion. Although consecutive daily IA injections for two weeks may have limited translatability into clinical practice, our findings are promising in pointing future research into the direction of more clinically relevant drug delivery – potentially a single large-dose injection, oral celecoxib, or drug-eluting scaffolds placed near or within joint at time of capsular release.46
It is important to discuss the mechanism by which celecoxib attenuated arthrofibrosis in this study. Mechanisms of fibrogenesis are widely discussed in the literature throughout various tissue types.47 More specifically, and relevant for this study, is the mechanism of fibrosis in tissues specific to the joint space. Several studies point to the activation of periarticular myofibroblasts from mesenchymal stem cells as the primary driver of arthrofibrosis.2,22-24 Salib et al25 discussed the ability of celecoxib to prevent arthrofibrosis through the disruption of post-inflammatory cytokines and subsequent interruption of myofibroblast activation. Based on this proposed mechanism, we posit that the celecoxib in our study disrupted the activation of periarticular myofibroblasts from mesenchymal stem cells and interrupted the formation of new scar tissue after the remobilization surgery. Additionally, this study found that intra-articular celecoxib was equivalent to a capsular release. This finding suggests that some reversal of well-established fibrosis occurred with celecoxib equivalent to excision of posterior capsular tissue. Importantly, any speculation on this without molecular or histological data would be conjecture, although this avenue is an important direction for future studies.
Our study has several limitations. Experimentation was constrained by low numbers of rabbits in each experimental group. For budgetary and compassionate reasons with large animal studies, the numbers of rabbits are intentionally kept at the minimum needed to adequately power the study. One rabbit was excluded from all biomechanical analyses in the IA celecoxib group due to a broken K-wire prior to K-wire removal. Additionally, one rabbit from the IA vehicle group, the IA vehicle with capsular release, and the IA celecoxib with capsular release group were each excluded from the capsular release data due to experimental error when running the dynamic load cell device. Despite this experimental attrition, our datasets were sufficiently robust to establish significant differences among relevant treatment groups. We did not complete any histological or genetic data to analyze fibrosis at the cellular and molecular levels, thus precluding augmentation of our findings using established biomarkers. Many previous studies have analyzed histological and molecular data regarding celecoxib treatment as well as capsular release.28,46 Given that our biomechanical findings are similar to these studies, it is likely that additional phenotyping via histological and molecular approaches would yield similar conclusions. Lastly, the rabbit model of arthrofibrosis is imperfect, as the trauma used on the rabbit joint is exaggerated (i.e. 45° hyperextension of the knee) compared to trauma from human arthroplasty. This exaggerated trauma may induce an unnecessarily severe contracture. However, this model has been carefully validated,28,30,31,48,49 and is currently the most reliable animal model for arthrofibrosis despite its limitations.
In conclusion, this study found that IA administration of celecoxib significantly improved extension in contracted rabbit knees compared to untreated controls. Additionally, this study found that IA celecoxib was superior to a capsular release in the treatment of arthrofibrosis in a rabbit model. Finally, IA celecoxib combined with a capsular release did not provide additive or synergistic effects in the improvement of extension in the contracted rabbit knee.
References
1. Ibrahim IO , Nazarian A , Rodriguez EK . Clinical management of arthrofibrosis: state of the art and therapeutic outlook . JBJS Rev . 2020 ; 8 ( 7 ): e1900223 . Crossref PubMed Google Scholar
2. Tibbo ME , Limberg AK , Salib CG , et al. Acquired idiopathic stiffness after total knee arthroplasty: a systematic review and meta-analysis . J Bone Joint Surg Am . 2019 ; 101-A ( 14 ): 1320 – 1330 . Crossref PubMed Google Scholar
3. Middleton AH , Perlewitz MA , Edelstein AI , Vetter CS . Knee Arthrofibrosis following Tibial Plateau Fracture Treated with Arthroscopic Lysis of Adhesions with Manipulation . J Knee Surg . 2020; Epub ahead of print . Crossref PubMed Google Scholar
4. Cregar WM , Khazi ZM , Lu Y , Forsythe B , Gerlinger TL . Lysis of adhesion for arthrofibrosis after total knee arthroplasty is associated with increased risk of subsequent revision total knee arthroplasty . J Arthroplasty . 2021 ; 36 ( 1 ): 339 – 344 . Crossref PubMed Google Scholar
5. Bodendorfer BM , Keeling LE , Michaelson EM , et al. Predictors of knee arthrofibrosis and outcomes after arthroscopic lysis of adhesions following ligamentous reconstruction: a retrospective case-control study with over two years’ average follow-up . J Knee Surg . 2019 ; 32 ( 6 ): 536 – 543 . Google Scholar
6. Volchenko E , Schwarzman G , Robinson M , Chmell SJ , Gonzalez MH . Arthroscopic lysis of adhesions with manipulation under anesthesia versus manipulation alone in the treatment of arthrofibrosis after TKA: a matched cohort study . Orthopedics . 2019 ; 42 ( 3 ): 163 – 167 . Crossref PubMed Google Scholar
7. Cohen JS , Gu A , Kapani N , et al. Efficacy of arthroscopic arthrolysis in the treatment of arthrofibrosis: a systematic review . J Knee Surg . 2021 ; 34 ( 12 ): 1349 – 1354 . Crossref PubMed Google Scholar
8. Biggs-Kinzer A , Murphy B , Shelbourne KD , Urch S . Perioperative rehabilitation using a knee extension device and arthroscopic debridement in the treatment of arthrofibrosis . Sports Health . 2010 ; 2 ( 5 ): 417 – 423 . Crossref PubMed Google Scholar
9. Aspinall SK , Bamber ZA , Hignett SM , Godsiff SP , Wheeler PC , Fong DTP . Medical stretching devices are effective in the treatment of knee arthrofibrosis: A systematic review . J Orthop Translat . 2021 ; 27 : 119 – 131 . Crossref PubMed Google Scholar
10. Formby PM , Donohue MA , Cannova CJ , Caulfield JP . Hydraulic distension of the knee: a novel treatment for arthrofibrosis after total knee replacement (case series) . ANZ J Surg . 2016 ; 86 ( 6 ): 480 – 482 . Crossref PubMed Google Scholar
11. Jerosch J , Aldawoudy AM . Arthroscopic treatment of patients with moderate arthrofibrosis after total knee replacement . Knee Surg Sports Traumatol Arthrosc . 2007 ; 15 ( 1 ): 71 – 77 . Crossref PubMed Google Scholar
12. Lindenfeld TN , Wojtys EM , Husain A . Surgical treatment of arthrofibrosis of the knee . Instr Course Lect . 2000 ; 49 : 211 – 221 . PubMed Google Scholar
13. Pariente GM , Lombardi AV , Berend KR , Mallory TH , Adams JB . Manipulation with prolonged epidural analgesia for treatment of TKA complicated by arthrofibrosis . Surg Technol Int . 2006 ; 15 : 221 – 224 . PubMed Google Scholar
14. Sassoon AA , Adigweme OO , Langford J , Koval KJ , Haidukewych GJ . Manipulation under anesthesia: a safe and effective treatment for posttraumatic arthrofibrosis of the knee . J Orthop Trauma . 2015 ; 29 ( 12 ): e464 - 8 . Crossref PubMed Google Scholar
15. Stiefel EC , McIntyre L . Arthroscopic lysis of adhesions for treatment of post-traumatic arthrofibrosis of the knee joint . Arthrosc Tech . 2017 ; 6 ( 4 ): e939 – e944 . Crossref PubMed Google Scholar
16. Wang JH , Zhao JZ , He YH . A new treatment strategy for severe arthrofibrosis of the knee. A review of twenty-two cases . J Bone Joint Surg Am . 2006 ; 88-A ( 6 ): 1245 – 1250 . Crossref PubMed Google Scholar
17. Wang JH , Zhao JZ , He YH . A new treatment strategy for severe arthrofibrosis of the knee. Surgical technique . J Bone Joint Surg Am . 2007 ; 89 Suppl 2 Pt.1 : 93 – 102 . Crossref PubMed Google Scholar
18. Evans KN , Lewandowski L , Pickett A , Strauss JE , Gordon WT . Outcomes of manipulation under anesthesia versus surgical management of combat-related arthrofibrosis of the knee . J Surg Orthop Adv . 2013 ; 22 ( 1 ): 36 – 41 . Crossref PubMed Google Scholar
19. Bingham JS , Bukowski BR , Wyles CC , Pareek A , Berry DJ , Abdel MP . Rotating-hinge revision total knee arthroplasty for treatment of severe arthrofibrosis . J Arthroplasty . 2019 ; 34 ( 7S ): S271 – S276 . Crossref PubMed Google Scholar
20. Stephenson JJ , Quimbo RA , Gu T . Knee-attributable medical costs and risk of re-surgery among patients utilizing non-surgical treatment options for knee arthrofibrosis in a managed care population . Curr Med Res Opin . 2010 ; 26 ( 5 ): 1109 – 1118 . Crossref PubMed Google Scholar
21. Blessing WA , Okajima SM , Cubria MB , et al. Intraarticular injection of relaxin-2 alleviates shoulder arthrofibrosis . Proc Natl Acad Sci U S A . 2019 ; 116 ( 25 ): 12183 – 12192 . Crossref PubMed Google Scholar
22. Arsoy D , Salib CG , Trousdale WH , et al. Joint contracture is reduced by intra-articular implantation of rosiglitazone-loaded hydrogels in a rabbit model of arthrofibrosis . J Orthop Res . 2018 ; 36 ( 11 ): 2949 – 2955 . Crossref PubMed Google Scholar
23. Barlow JD , Morrey ME , Hartzler RU , et al. Effectiveness of rosiglitazone in reducing flexion contracture in a rabbit model of arthrofibrosis with surgical capsular release: a biomechanical, histological, and genetic analysis . Bone Joint Res . 2016 ; 5 ( 1 ): 11 – 17 . Crossref PubMed Google Scholar
24. Limberg AK , Tibbo ME , Salib CG , et al. Reduction of arthrofibrosis utilizing a collagen membrane drug-eluting scaffold with celecoxib and subcutaneous injections with ketotifen . J Orthop Res . 2020 ; 38 ( 11 ): 2474 – 2483 . Crossref PubMed Google Scholar
25. Salib CG , Reina N , Trousdale WH , et al. Inhibition of COX-2 pathway as a potential prophylaxis against arthrofibrogenesis in a rabbit model of joint contracture . J Orthop Res . 2019 ; 37 ( 12 ): 2609 – 2620 . Crossref PubMed Google Scholar
26. Tibbo ME , Limberg AK , Salib CG , et al. Anti-fibrotic effects of the antihistamine ketotifen in a rabbit model of arthrofibrosis . Bone Joint Res . 2020 ; 9 ( 6 ): 302 – 310 . Crossref PubMed Google Scholar
27. Li F , He B , Liu S , Fan C . Celecoxib effectively inhibits the formation of joint adhesions . Exp Ther Med . 2013 ; 6 ( 6 ): 1507 – 1511 . Crossref PubMed Google Scholar
28. Barlow JD , Hartzler RU , Abdel MP , et al. Surgical capsular release reduces flexion contracture in a rabbit model of arthrofibrosis . J Orthop Res . 2013 ; 31 ( 10 ): 1529 – 1532 . Crossref PubMed Google Scholar
29. Abdel MP , Morrey ME , Grill DE , et al. Effects of joint contracture on the contralateral unoperated limb in a rabbit knee contracture model: a biomechanical and genetic study . J Orthop Res . 2012 ; 30 ( 10 ): 1581 – 1585 . Crossref PubMed Google Scholar
30. Abdel MP , Morrey ME , Barlow JD , et al. Intra-articular decorin influences the fibrosis genetic expression profile in a rabbit model of joint contracture . Bone Joint Res . 2014 ; 3 ( 3 ): 82 – 88 . Crossref PubMed Google Scholar
31. Morrey ME , Sanchez-Sotelo J , Lewallen EA , et al. Intra-articular injection of a substance P inhibitor affects gene expression in a joint contracture model . J Cell Biochem . 2018 ; 119 ( 2 ): 1326 – 1336 . Crossref PubMed Google Scholar
32. Reina N , Trousdale WH , Salib CG , et al. Validation of a dynamic joint contracture measuring device in a live rabbit model of arthrofibrosis . J Orthop Res . 2018; Epub ahead of print . Crossref PubMed Google Scholar
33. Trousdale WH , Salib CG , Reina N , et al. A drug eluting scaffold for the treatment of arthrofibrosis . Tissue Eng Part C Methods . 2018 ; 24 ( 9 ): 514 – 523 . Crossref PubMed Google Scholar
34. Nesterenko S , Morrey ME , Abdel MP , et al. New rabbit knee model of posttraumatic joint contracture: indirect capsular damage induces a severe contracture . J Orthop Res . 2009 ; 27 ( 8 ): 1028 – 1032 . Crossref PubMed Google Scholar
35. Keeney JA , Clohisy JC , Curry M , Maloney WJ . Revision total knee arthroplasty for restricted motion . Clin Orthop Relat Res . 2005 ; 440 : 135 – 140 . Crossref PubMed Google Scholar
36. Kim J , Nelson CL , Lotke PA . Stiffness after total knee arthroplasty. Prevalence of the complication and outcomes of revision . J Bone Joint Surg Am . 2004 ; 86-A ( 7 ): 1479 – 1484 . PubMed Google Scholar
37. Cheuy VA , Foran JRH , Paxton RJ , Bade MJ , Zeni JA , Stevens-Lapsley JE . Arthrofibrosis Associated With Total Knee Arthroplasty . J Arthroplasty . 2017 ; 32 ( 8 ): 2604 – 2611 . Crossref PubMed Google Scholar
38. Aspinall SK , Wheeler PC , Godsiff SP , Hignett SM , Fong DTP . The STAK tool: evaluation of a new device to treat arthrofibrosis and poor range of movement following total knee arthroplasty and major knee surgery . Bone Jt Open . 2020 ; 1 ( 8 ): 465 – 473 . Crossref PubMed Google Scholar
39. Fitzsimmons SE , Vazquez EA , Bronson MJ . How to treat the stiff total knee arthroplasty?: a systematic review . Clin Orthop Relat Res . 2010 ; 468 ( 4 ): 1096 – 1106 . Crossref PubMed Google Scholar
40. Sunil Kumar KH , Mamarelis G , Pettit M , Khanduja V . Management of stiffness following total knee arthroplasty: international survey on surgeon preferences . SICOT J . 2021 ; 7 : 30 . Crossref PubMed Google Scholar
41. Mamarelis G , Sunil-Kumar KH , Khanduja V . Timing of manipulation under anaesthesia for stiffness after total knee arthroplasty . Ann Transl Med . 2015 ; 3 ( 20 ): 316 . Crossref PubMed Google Scholar
42. Tjoumakaris FP , Tucker BC , Post Z , Pepe MD , Orozco F , Ong AC . Arthroscopic lysis of adhesions for the stiff total knee: results after failed manipulation . Orthopedics . 2014 ; 37 ( 5 ): e482 - 7 . Crossref PubMed Google Scholar
43. Middleton AM , Ziegele MJ , Vetter CS , Edelstein AI . Arthroscopic lysis of adhesions with manipulation for management of late-presenting stiffness after total knee arthroplasty . Arthroplast Today . 2020 ; 6 ( 4 ): 761 – 765 . Crossref PubMed Google Scholar
44. Gomes JLE , Leie MA , de Freitas Soares A , Ferrari MB , Sánchez G . Posterior capsulotomy of the knee: treatment of minimal knee extension deficit . Arthrosc Tech . 2017 ; 6 ( 5 ): e1535 – e1539 . Crossref PubMed Google Scholar
45. Leie MA , de Castro JV , Gomes JE . Posterior knee capsulotomy for the relief of patellofemoral joint pain: long-term follow-up . J Knee Surg . 2021 ; 34 ( 2 ): 164 – 170 . Crossref PubMed Google Scholar
46. Limberg AK , Tibbo ME , Salib CG , et al. Reduction of arthrofibrosis utilizing a collagen membrane drug-eluting scaffold with celecoxib and subcutaneous injections with ketotifen . J Orthop Res . 2020 ; 38 ( 11 ): 2474 – 2483 . Crossref PubMed Google Scholar
47. Murray IR , Gonzalez ZN , Baily J , et al. αv integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis . Nat Commun . 2017 ; 8 ( 1 ): 1118 . Crossref PubMed Google Scholar
48. Abdel MP , Morrey ME , Barlow JD , et al. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture . J Orthop Res . 2012 ; 30 ( 5 ): 713 – 719 . Crossref PubMed Google Scholar
49. Nesterenko S , Morrey ME , Abdel MP , et al. New rabbit knee model of posttraumatic joint contracture: indirect capsular damage induces a severe contracture . J Orthop Res . 2009 ; 27 ( 8 ): 1028 – 1032 . Crossref PubMed Google Scholar
Author contributions
W. H. Trousdale: Conceptualization, Investigation, Writing – original draft.
A. K. Limberg: Data curation, Formal analysis, Writing – original draft.
N. Reina: Conceptualization, Data curation, Writing – review & editing.
C. G. Salib: Conceptualization, Data curation, Writing – review & editing.
R. Thaler: Formal analysis, Writing – review & editing.
A. Dudakovic: Formal analysis, Data curation, Methodology, Writing – review & editing.
D. J. Berry: Conceptualization, Investigation, Methodology, Writing – review & editing.
M. E. Morrey: Conceptualization, Investigation, Methodology, Writing – review & editing.
J. Sanchez-Sotelo: Conceptualization, Investigation, Methodology, Writing – review & editing.
A. van Wijnen: Conceptualization, Investigation, Methodology, Writing – review & editing.
M. P. Abdel: Investigation, Conceptualization, Writing – original, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under Award Number AR072597-01A1 and the Anna-Maria and Stephen Kellen Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
J. Sanchez-Sotelo reports a grant, royalties, and consulting fees from Stryker, consulting fees from Exactech and Alumed, stock or stock options from PSI and Precision OS, publishing royalties from Elsevier and Oxford University Press, and is the associate editor of the Journal of Shoulder and Elbow Surgery. N. Reina reports consulting fees from Adler, B. Braun, and Stryker, and board participation for Digikare. D. J. Berry reports consulting fees and royalties from DePuy, consulting fees and stock/stock options from Bodycad, royalties from Wolters Kluwer and Elsevier, and patents from DePuy. D. J. Berry is also a trustee on the board for the Journal of Bone and Joint Surgery, President-Elect of the International Hip Society, a steering committee member of the International Society of Arthroplasty Registries, a trustee on the board for the Orthopaedic Research and Education Foundation, and the Senior Director of Current Concepts in Joint Replacement. M. P. Abdel reports royalties from Stryker, and sits on the Board of Directors for the American Academy of Orthopaedic Surgeons.
Ethical review statement
Institutional Review Board Approval was obtained prior to study initiation.
Open access funding
The authors confirm that the open access fee for this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under Award Number AR072597-01A1 and the Anna-Maria and Stephen Kellen Foundation.
Supplementary material
An ARRIVE checklist is included to show that the ARRIVE guidelines were adhered to in this study.









