Abstract
Aims
We wished to assess the feasibility of a future randomised controlled trial of parathyroid hormone (PTH) supplements to aid healing of trochanteric fractures of the hip, by an open label prospective feasibility and pilot study with a nested qualitative sub study. This aimed to inform the design of a future powered study comparing the functional recovery after trochanteric hip fracture in patients undergoing standard care, versus those who undergo administration of subcutaneous injection of PTH for six weeks.
Patients and Methods
We undertook a pilot study comparing the functional recovery after trochanteric hip fracture in patients 60 years or older, admitted with a trochanteric hip fracture, and potentially eligible to be randomised to either standard care or the administration of subcutaneous PTH for six weeks. Our desired outcomes were functional testing and measures to assess the feasibility and acceptability of the study.
Results
A total of 724 patients were screened, of whom 143 (20%) were eligible for recruitment. Of these, 123 were approached and 29 (4%) elected to take part. However, seven patients did not complete the study. Compliance with the injections was 11 out of 15 (73%) showing the intervention to be acceptable and feasible in this patient population.
Take home message: Only 4% of patients who met the inclusion criteria were both eligible and willing to consent to a study involving injections of PTH, so delivering this study on a large scale would carry challenges in recruitment and retention. Methodological and sample size planning would have to take this into account. PTH administration to patients to enhance fracture healing should still be considered experimental.
Cite this article: Bone Joint J 2016;98-B:840–5.
In the United Kingdom, an estimated 70 000 people are admitted to hospital with a hip fracture every year.1 Recovery from a hip fracture requires substantial resources from health and social services, and has implications on the quality of life and social support network for the individual.1 These patients rarely recover their pre-injury state.2,3 Parathyroid hormone (PTH) is licensed for the treatment of osteoporosis, however, a number of animal studies suggest it can also accelerate fracture healing.4,5 Its effectiveness in patients who have sustained hip fractures is currently unknown. A short course of PTH has the potential to improve the rate of functional recovery and consequently reduce the length of hospital stay and period of dependence on health and social care services. Two published human studies investigating PTH in fracture healing (osteoporotic wrist fractures and pelvic fractures)6,7 have shown encouraging results, demonstrating an increased proportion of early unions in the intervention groups. A number of methodological concerns exist, however, including the use of daily injections in older acutely injured patients. The potential impact of this treatment on patients and their recovery is currently unclear.
The aim of this study was to establish the feasibility of daily injections of PTH in a population of patients with trochanteric hip fractures and to pilot the study design in terms of eligibility, acceptability, more specifically the acceptability of the intervention and outcome measures, consent and defining the standard care for the comparator arm. This was to assess the potential to deliver a randomised controlled trial with the hypothesis that intermittent PTH injections could improve functional recovery after a trochanteric hip fracture through the mechanism of accelerated fracture healing.
Our objectives were to establish:
- the potential recruitment, compliance with the intervention and the protocol and to inform methodological planning for the full study, including;
- to assess the acceptability and validity from the patients’ perspective of the outcome measures intended for use in the full study;
- to clarify and define ‘standard’ drug therapy for osteoporotic fractures received by the comparison group;
- to establish the feasibility and acceptability of injection therapy over six weeks in this population.
Patients and Methods
The design was an open label, prospective feasibility and pilot study with randomised treatment allocation, comparator controlled with blinded outcome assessment and a nested qualitative sub-study as previously published by the study group.8 The study was approved by South West 2 Research Ethics Committee (10/H0206/34) and registered with EudraCT (2010-020081-22) and ISRCTN (03362357). Funding was provided by a National Institute for Health Research (NIHR) Research for Patient Benefit grant (PB-PG-0408-16292) and sponsored by North Bristol NHS Trust (R& I 2185).
Recruitment was undertaken from a continuous sample of patients screened to meet the inclusion criteria from admissions to the trauma and orthopaedic services at six National Health Service teaching hospitals in the United Kingdom. Patients aged 60 years or older admitted with a trochanteric hip fracture were included. Exclusion criteria included contraindications to the study medication, lack of capacity to give informed consent or the inability to comply with the study protocol. Consenting participants were randomised between the intervention group and standard care using an internet-based, computer-generated random allocation provided by Sealed Envelope (London, United Kingdom) on a 1:1 ratio in blocks of ten. Randomisation was performed by the research associate at the recruiting site following the consent of the participant.8 The intervention group was prescribed a daily subcutaneous injection of PTH 1-34 (Teriparatide) for 42 days, commencing within ten days of surgery. Other medications related to bone-health (except vitamin D and calcium), including bisphosphonates and denosumab, were withheld for the duration of the intervention in this group. Participants were taught by the study team to self-administer the injections, with assistance from relatives or carers if required. The standard care group received treatment according to National Institute for Health and Clinical Excellence (NICE) guidance.9 Adherence to this guidance was monitored as part of the study.
Standardised data collection was undertaken at screening, on recruitment to the study, on discharge from the acute hospital stay and at six and 12 weeks, and six months after surgery. Hospital systems were checked for occurrence of any further fractures within 12 months of surgery. Outcome measures including the Short Physical Performance Battery (SPPB),10-12 the Short Form (SF)-36,13 EuroQol 5-D (EQ-5D)14 general health questionnaires and visual analogue scales (VAS)15 for pain on bearing weight (0 to 10 scale where 10 was the worst pain imaginable and 0 was no pain) were completed with participants by an assessor blinded to treatment allocation, at outpatient appointments at six and 12 weeks post-operatively. The general health questionnaires were repeated in a telephone consultation with participants at six months. Pre- and post-operative radiographs were used to classify the fracture according to AO/OTA system,16 ascertain the type of operative intervention, measure the amount of collapse as measured on the anteroposterior final post-operative radiograph, assess union and identify any failure of fixation.
As this was a pilot study to determine the appropriateness of the proposed methodology for a main study with adequate power, no formal sample size calculations were performed and groups of 20 participants were deemed suitable.
On this basis, statistical comparison of outcomes in the intervention and comparison group were not carried out. Instead, we report measures assessing the feasibility and acceptability of the study (such as eligibility, retention, compliance and data completeness).
Results
Of the 724 patients screened to meet the inclusion criteria, 143 (20%) were eligible for the study; of these, 123 were approached, and 29 (20%) of those eligible consented to participate (4% of the total patients screened) (Fig. 1). These numbers were significantly lower than the 50% of the population originally predicted to be eligible. Lack of capacity precluded 314 of the 581 (54%) of those screened as eligible from giving informed consent. Recruitment took place between March 2012 and March 2013.
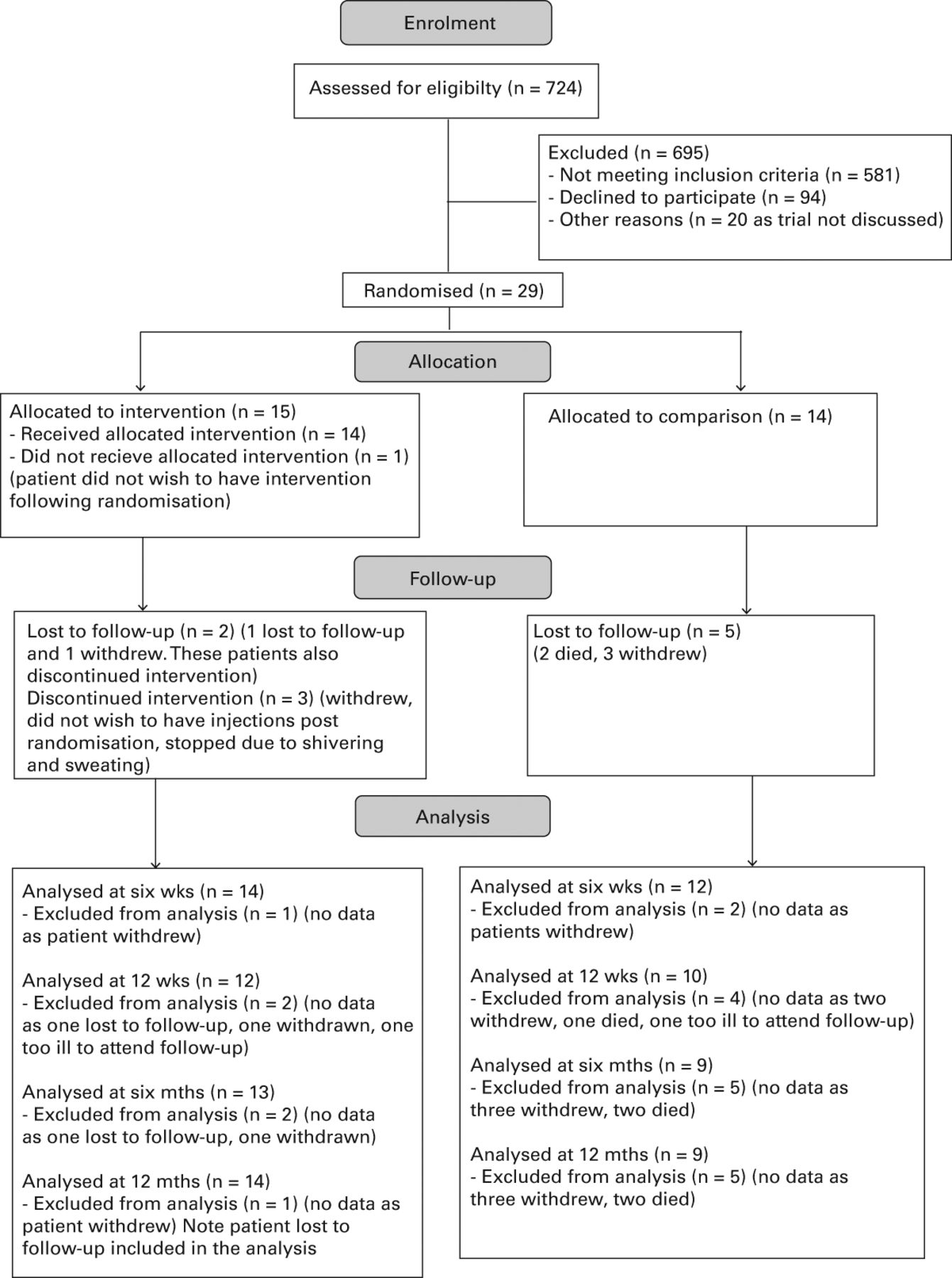
Fig. 1
Consolidated Standards of Reporting Trials (CONSORT) diagram.
In total, 192 patients (33%) of the population were excluded due to contraindications to the intervention, such as renal failure, cancer or pre-existing metabolic bone disease. Inability to comply with the study schedule excluded 272 patients (47%) but 129 of these were already excluded for other reasons. The demographics of those screened and consented are shown in Table I.
Table I
Demographics of patients randomised
| Intervention group (n = 15) | Comparison group (n = 14) | |
|---|---|---|
| Mean age at randomisation (standard deviation) | 80.6 (8.8) | 78.6 (9.3) |
| Gender | ||
| n (%) Male | 8 (53) | 11 (79) |
| n (%) Female | 6 (40) | 3 (21) |
| n (%) Indeteterminate | 1 (7) | 0 (0) |
| Type of surgical fixation | ||
| n (%) Sliding hip screw | 12 (80) | 13 (93) |
| n (%) Cephalomedullary nail | 3 (20) | 1 (7) |
A total of seven patients (24%) failed to complete the study. Of these, two patients died, contact was lost with one and four withdrew quoting “stress of comorbidities,” being “fed up”, “not wanting to participate further” and the burden of recovery as reasons. Only two of these withdrawals were by patients in the intervention group. The timing of withdrawal varied from two to 214 days after enrolment. A total of 26 participants (90%) completed the six week follow-up visit and 22 (76%) the 12-week and six-month visits (Fig. 1).
All patients remaining in the study at the relevant point completed the SPPB at six (n = 26) and 12 weeks (n = 22). Full completion of the EQ-5D was performed by all patients remaining at six weeks, by 21 of 26 (96%) at 12 weeks and 20 of 21 (91%) at six months. The SF-36 was fully completed by 20 of 26 (77%) patients at the six week follow-up and 17 of 22 (77%) at 12 weeks, which may reflect the more complicated nature and greater length of the questionnaire. This improved, however, to 18 of 22 patients (92%) at the six month follow-up, when this was generally completed via telephone consultation. The results at 12 weeks are reported in Table II.
Table II
Results
| All (n = 29) | Intervention group (n = 15) | Comparison group (n = 14) | |
|---|---|---|---|
| Median (min to max) length of stay (days) | 15 (6 to 62) | 15 (6 to 51) | 13.5 (6 to 62) |
| n (%) discharged to pre-fracture residence | 18 (62) | 9 (60) | 9 (64) |
| n (%) attending 12-week appointment | 22 (76) | 12 (80) | 10 (71) |
| n (%) fully completing EQ-5D | 21(72) | 12 (100) | 9 (90) |
| Mean (sd) of EQ-5D | 0.57 (0.26) | 0.63 (0.18) | |
| n (%) completing EQ-5D VAS score | 12 (100) | 9 (90) | |
| Mean (standard deviation) of EQ-5D VAS score | 66.9 (26.3) | 75.4 (14.0) | |
| n (%) fully completed SPPB | 12 (100) | 10 (100) | |
| Mean stand score (sd) | 2.75 (1.60) | 2.80 (1.62) | |
| Mean walk score (sd) | 1.83 (1.12) | 1.60 (1.08) | |
| Mean chair stands (sd) | 0.75 (1.06) | 1.30 (1.49) | |
| Mean SPPB score (sd) | 5.33 (3.20) | 5.70 (3.27) | |
-
SPPB, Short Physical Performance Battery, EQ-5D, EuroQol 5D; VAS, visual analogue score; sd, standard deviation
Of the 15 patients randomised to the intervention group, 11 completed the course of injections. The injections were predominantly self-administered - two patients required assistance from family to deliver the injection and others reported reminders or assistance in accessing the medication, provided by their family. Of those not compliant in the intervention group, one withdrew following randomisation, one stopped with potentially related side effects (night sweats and shivering) but continued in the trial, one withdrew from the study entirely on discharge from hospital and one started the injections late and therefore received a reduced treatment duration, due to misunderstanding the instructions.
Adverse events were recorded for patients in both groups. In all 15 events were reported as being serious according to ICH-GCP definitions.17 In all, eight of these were in the intervention group and seven in the comparison group. None of the serious adverse events were related to the study intervention and all may be expected in this patient population.
A total of six patients sustained AO/OTA A1 fractures, 15 A2 fractures and four A3 fractures. The implants used were 25 sliding hip screws and four cephalomedullary nails. There were no follow-up radiographs for four patients. Radiographs were available for the other 25 patients at six weeks and 21 had further images at 12 weeks. All fractures had united at 12 weeks with ten patients demonstrating no collapse of the fracture, nine up to 5 mm collapse and six up to 10 mm collapse. No fracture had collapsed more than 10 mm. There were no complications reported regarding fracture fixation.
A total of 13 of the 14 patients in the comparison group were managed in line with NICE guidelines,9 while patient 14 died one month into the study. Five of these participants were already taking osteoporosis medications on admission. At six weeks, 17 of 26 (65%) of patients reported taking their calcium and vitamin D supplements as prescribed. Six of eight patients (75%) under 75 years of age on entry to the study were referred for a Dual-energy X-ray absorptiometry scan in line with NICE guidance. One of the remaining two had undergone a recent scan and the other died a month into the study.
Discussion
PTH has been shown to improve bone mineral density (BMD) and reduce subsequent fractures in those with osteoporosis.18 Despite evidence of benefit from animal studies in the treatment of fractures19-23 the dose, duration and cost effectiveness of treatment remain in question.24 Subcutaneous injection remains the only licensed route of administration23 and although other delivery systems are under investigation, they are not yet licensed.25 While it is increasingly frequently prescribed, evidence from human studies is lacking.6
Patients with trochanteric fractures were chosen, as hip fractures represent the most costly fracture with large socio-economic costs.26 Assessing union and measuring an impact on the recovery of function in lower limb fractures is very difficult27 and so this study tested the option of a surrogate measure such as the SPPB.10-12 This requires patient contact and can be influenced by other physical comorbidities, which are common in this frail group of patients.
This study has highlighted important challenges that should be considered when designing research studies in patients with hip fractures. These challenges could be extrapolated to any frail, elderly trauma patient population where the combination of age, comorbidities and an acute injury requiring surgery present a unique situation in which to conduct research. The greatest challenge of delivering this study was that of identifying a sufficient number of eligible patients and gaining informed consent in this elderly population, with only 4% of those 724 patients who met the inclusion criteria sustaining trochanteric fractures being both eligible and willing to consent.
If a patient was recruited, the protocol has been shown to be deliverable across multiple sites. Once enrolled and taught the injection technique, the compliance and adherence to the injection intervention was good. Data completion to six weeks was good but dropped at the later time points.
As a non-blinded study it was important to define the care received by the comparison group. Since the design of the study, the implementation of a national tariff through the National Hip Fracture Database has standardised the care of this patient group.26 Surrogate markers of the outcome of rehabilitation, such as discharge destination and length of stay were successfully recorded. It was difficult, however, to obtain measures of other aspects of rehabilitation such as the intensity of therapy and social support required, due to lack of access to and detail of recorded data.
Limitations of the study included its pilot nature, and the small groups which precluded objective assessment of the value of PTH in this injury. Recruitment was only open to those with no cognitive impairment, no contraindications to PTH and the ability to comply with the study protocol, which excluded 80% (581) of the patients screened. Follow-up of these patients was difficult and seven (24%) failed to complete the study. This makes the results difficult to generalise to the whole population.
With these difficulties in mind, expansion into a large multicentre trial should be undertaken with caution. Any definitive study recruiting a representative cohort would need to include the population unable to give informed consent. Sample size calculations using the data from this study based on detecting a one-point change in the SPPB at 12 weeks as the primary outcome, assuming an 80% completion rate, would require 405 patients. Any design should, therefore, include strategies to maximise retention and completion such as follow-up in the community or by telephone.
If PTH is given to aid bone healing in trochanteric fractures, it should still be considered experimental.
1 Melton LJ III . Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res2003;18:1139–1141.CrossrefPubMed Google Scholar
2 Cree M , CarriereKC, SoskolneCL, Suarez-AlmazorM. Functional dependence after hip fracture. Am J Phys Med Rehabil2001;80:736–743.CrossrefPubMed Google Scholar
3 Magaziner J , FredmanL, HawkesW, et al.Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol2003;157:1023–1031.CrossrefPubMed Google Scholar
4 Barnes GL , KakarS, VoraS, et al.Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg [Am]2008;90:120–127.CrossrefPubMed Google Scholar
5 Chalidis B , TzioupisC, TsiridisE, GiannoudisPV. Enhancement of fracture healing with parathyroid hormone: preclinical studies and potential clinical applications. Expert Opin Investig Drugs2007;16:441–449.CrossrefPubMed Google Scholar
6 Aspenberg P , GenantHK, JohanssonT, et al.Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res2010;25:404–414.CrossrefPubMed Google Scholar
7 Peichl P , HolzerLA, MaierR, HolzerG. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg [Am]2011;93-A:1583–1587.CrossrefPubMed Google Scholar
8 Chesser T , FoxR, HardingK, et al.The administration of intermittent parathyroid hormone affects functional recovery from pertrochanteric fractured neck of femur: a protocol for a prospective mixed method pilot study with randomisation of treatment allocation and blinded assessment (FRACTT). BMJ Open2014;4:004389.CrossrefPubMed Google Scholar
9 No authors listed. Nice Technology Appraisal Guidence.http://www.nice.org.uk/Guidance/TA160 (date last accessed 12 January 2016). Google Scholar
10 Ostir GV , VolpatoS, FriedLP, ChavesP, GuralnikJM. ; Women’s Health and Aging Study. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol2002;55:916–921. Google Scholar
11 Perera S , ModySH, WoodmanRC, StudenskiSA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc2006;54:743–749.CrossrefPubMed Google Scholar
12 Guralnik JM , SimonsickEM, FerrucciL, et al.A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol1994;49:M85–M94.CrossrefPubMed Google Scholar
13 Ware JE , SherbourneCD. The MOS 36-Item Short-Form Heatlh Survey (SF36). I. Conceptual Framework and Item selection. Med Care1992;30:473–483. Google Scholar
14 Brooks R . Euroqol: the current state of play. Health Policy1996;337:53–72.CrossrefPubMed Google Scholar
15 Kahl C , ClelandJ. Visual Analogue Scale, Numeric Pain Rating Scale and the McGill pain questionnaire: An overview of psychometric properties. Physical Therapy Reviews2005;10:123–128. Google Scholar
16 Marsh JL , SlongoTF, AgelJ, et al.Fracture and dislocation classification compendium - 2007: orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma2007;21:S1–S133.CrossrefPubMed Google Scholar
17 No authors listed. ICH 1994 Clinical Safety Data Management definitions and standards for expedited reporting. E2A. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf (last accessed 06/04/2016). Google Scholar
18 Barnes GL , EinhornTA. Enhancement of fracture healing with parathyroid hormone. Clin Rev Bone Miner Metab2006;4:269–275.CrossrefPubMed Google Scholar
19 Andreassen TT , FledeliusC, EjerstedC, OxlundH. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand2001;72:304–307.CrossrefPubMed Google Scholar
20 Holzer G , MajeskaRJ, LundyMW, HartkeJR, EinhornTA. Parathyroid hormone enhances fracture healing. A preliminary report. Clin Orthop Relat Res1999;366:258–263.CrossrefPubMed Google Scholar
21 Skripitz R , AspenbergP. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg [Br]2001;83-B:437–440.CrossrefPubMed Google Scholar
22 Alkhiary YM , GerstenfeldLC, KrallE, et al.Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg [Am]2005;87-A:731–741.CrossrefPubMed Google Scholar
23 Morley P . Delivery of parathyroid hormone for the treatment of osteoporosis. Expert Opin Drug Deliv2005;2:993–1002.CrossrefPubMed Google Scholar
24 Jahng JS , KimHW. Effect of intermittent administration of parathyroid hormone on fracture healing in ovariectomized rats. Orthopedics2000;23:1089–1094.CrossrefPubMed Google Scholar
25 Cosman F , LaneNE, BologneseMA, et al.Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab2010;95:151–158.CrossrefPubMed Google Scholar
26 No authors listed. The National Hip Fracture Database. http://www.nhfd.co.uk (date last accessed 12 January 2016). Google Scholar
27 Chiavaras MM , BainsS, ChoudurH, et al.The Radiographic Union Score for Hip (RUSH): the use of a checklist to evaluate hip fracture healing improves agreement between radiologists and orthopedic surgeons. Skeletal Radiol2013;42:1079–1088.CrossrefPubMed Google Scholar
Author contributions:
T. J. S. Chesser: Study design, Study co-ordination, Data collection, Data analysis, Writing the paper.
R. Fox: Study Design, Study co-ordination, Data collection, Data analysis, Writing the paper.
K. Harding: Study design, Study co-ordination, Data collection, Writing the paper.
R. Halliday: Study design, Study co-ordination, Data collection, Data analysis, Writing the paper.
S. Barnfield: Study Design, Study co-ordination, Data collection, Data analysis, Writing the paper.
K. Willett: Study design, and analysis, Contributed to writing the paper.
S. Lamb: Study design, Contributed to writing the paper.
C. Yau: Recruitment of patients, Contributed to writing the paper.
M. K. Javaid: Study design, Data analysis, Writing the paper.
A. Gray: Recruitment of patients, Contributed to writing the paper.
J. Young: Recruitment of patients, Contributed to writing the paper.
H. Taylor: Data analysis.
K. Shah: Recruitment of patients, Contributed to writing the paper.
R. Greenwood: Study design, Statistical analysis.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
This article was primary edited by P. Page and first proof edited by G. Scott.









